In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Skin Fibroblasts
2.2. Direct Conversion of Fibroblasts to Induced Neurons
2.3. Neuronal Morphology Analysis
2.4. Western Blot
2.5. Digital Droplet PCR
3. Results
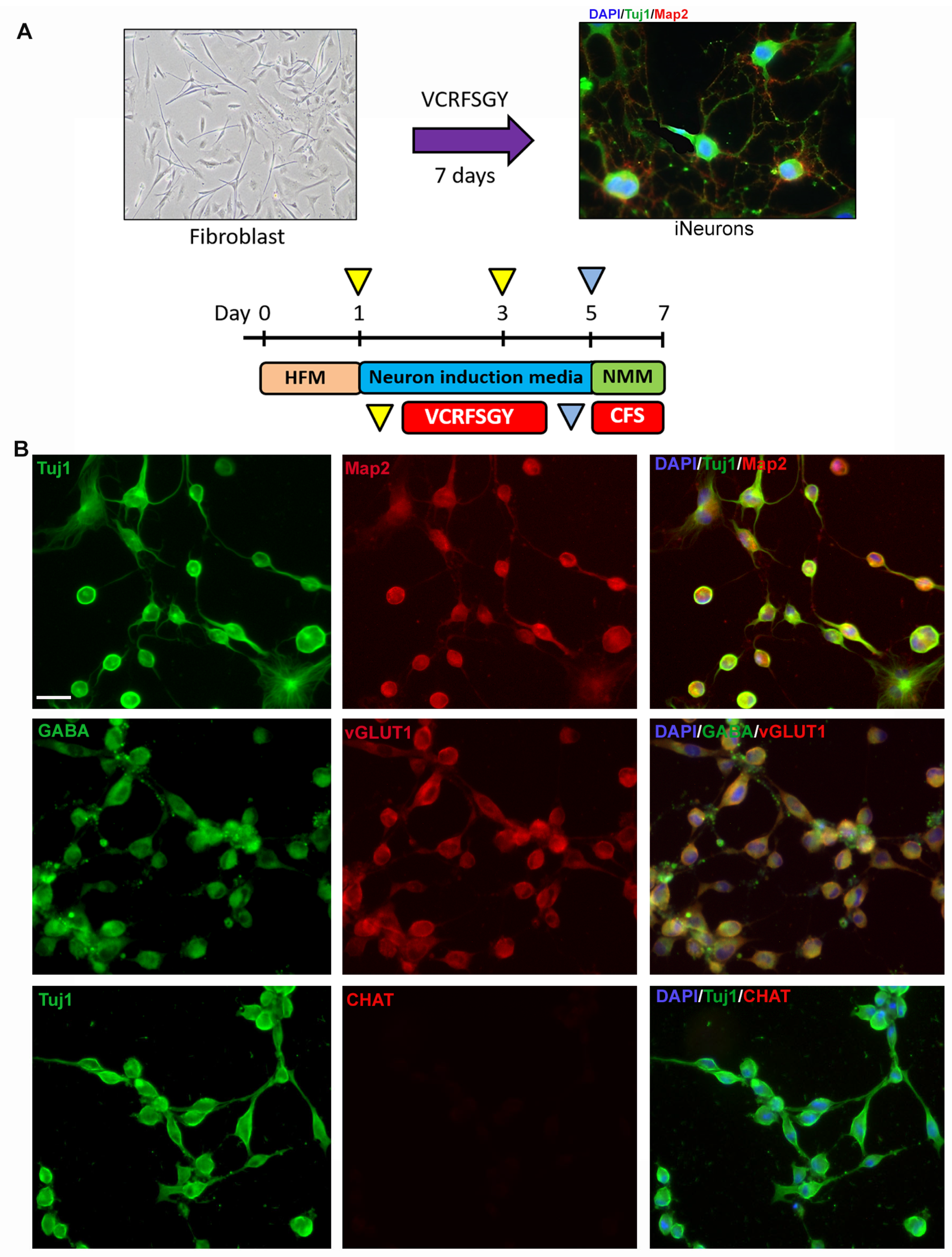
3.1. Direct Conversion of Fibroblast Allows for Rapid Differentiation into Induced Neurons (iNs)
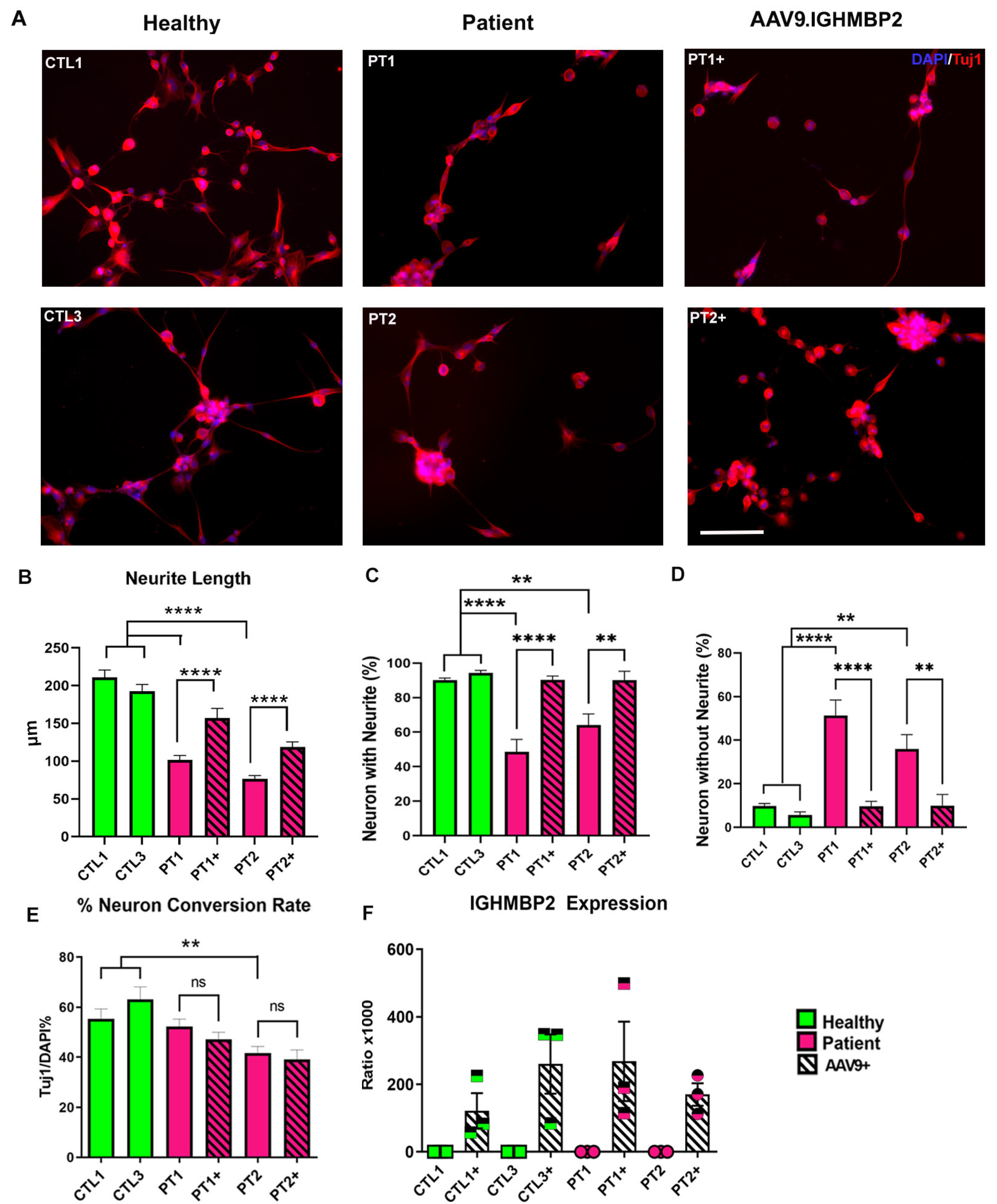
3.2. Neurons of SMA Patients Show Abnormal Morphology In Vitro
3.3. AAV9.IGHMBP2 Treatment Rescues the Disease Phenotype of SMARD1/CMT2S iNs
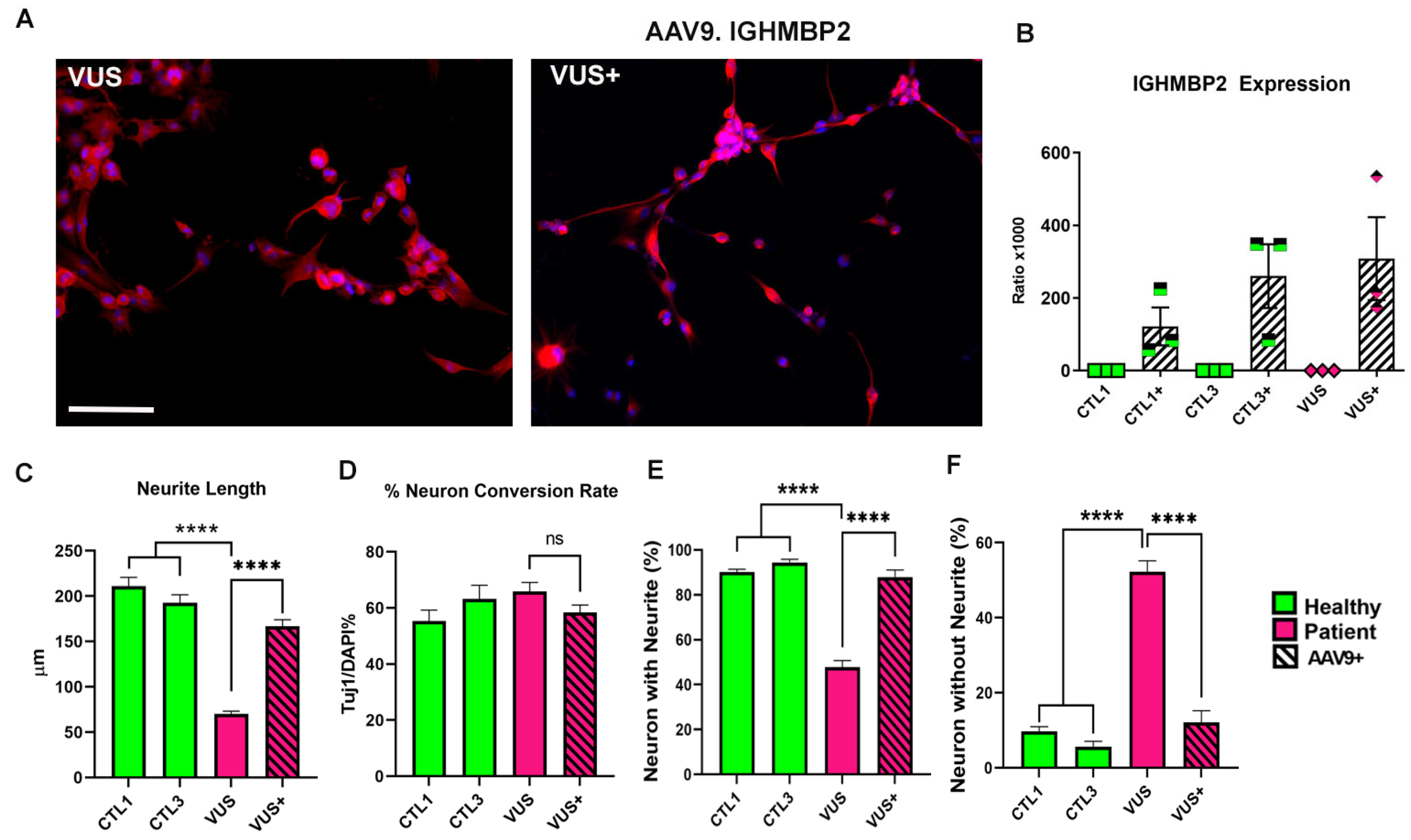
3.4. iN Can Help Identify Variants of Uncertain Significance according to Phenotype and Response to Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, M.; Likhite, S.; Meyer, K. Onasemnogene abeparvovec-xioi: A gene replacement strategy for the treatment of infants diagnosed with spinal muscular atrophy. Drugs Today 2021, 57, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal Muscular Atrophy: Mutations, Testing, and Clinical Relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurster, C.D.; Ludolph, A.C. Nusinersen for spinal muscular atrophy. Ther. Adv. Neurol. Disord. 2018, 11, 1756285618754459. [Google Scholar] [CrossRef] [Green Version]
- Ratni, H.; Scalco, R.S.; Stephan, A.H. Risdiplam, the First Approved Small Molecule Splicing Modifier Drug as a Blueprint for Future Transformative Medicines. ACS Med. Chem. Lett. 2021, 12, 874–877. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Saladini, M.; Nizzardo, M.; Govoni, A.; Taiana, M.; Bresolin, N.; Comi, G.P.; Corti, S. Spinal muscular atrophy with respiratory distress type 1: Clinical phenotypes, molecular pathogenesis and therapeutic insights. J. Cell. Mol. Med. 2020, 24, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Taiana, M.; Govoni, A.; Salani, S.; Kleinschmidt, N.; Galli, N.; Saladini, M.; Ghezzi, S.B.; Melzi, V.; Bersani, M.; Del Bo, R.; et al. Molecular analysis of SMARD1 patient-derived cells demonstrates that nonsense-mediated mRNA decay is impaired. J. Neurol. Neurosurg. Psychiatry 2022, 93, 908–910. [Google Scholar] [CrossRef]
- Stalpers, X.L.; Verrips, A.; Poll-The, B.T.; Cobben, J.-M.; Snoeck, I.N.; de Coo, I.F.; Brooks, A.; Bulk, S.; Gooskens, R.; Fock, A.; et al. Clinical and mutational characteristics of spinal muscular atrophy with respiratory distress type 1 in the Netherlands. Neuromuscul. Disord. 2013, 23, 461–468. [Google Scholar] [CrossRef]
- Grohmann, K.; Schuelke, M.; Diers, A.; Hoffmann, K.; Lucke, B.; Adams, C.; Bertini, E.; Leonhardt-Horti, H.; Muntoni, F.; Ouvrier, R.; et al. Mutations in the gene encoding immunoglobulin mu-binding protein 2 cause spinal muscular atrophy with respiratory distress type 1. Nat. Genet. 2001, 29, 75–77. [Google Scholar] [CrossRef]
- Lim, S.C.; Bowler, M.W.; Lai, T.F.; Song, H. The Ighmbp2 helicase structure reveals the molecular basis for disease-causing mutations in DMSA1. Nucleic Acids Res. 2012, 40, 11009–11022. [Google Scholar] [CrossRef] [Green Version]
- Cottenie, E.; Kochanski, A.; Jordanova, A.; Bansagi, B.; Zimon, M.; Horga, A.; Jaunmuktane, Z.; Saveri, P.; Rasic, V.M.; Baets, J.; et al. Truncating and missense mutations in IGHMBP2 cause Charcot-Marie Tooth disease type 2. Am. J. Hum. Genet. 2014, 95, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Kulshrestha, R.; Forrester, N.; Antoniadi, T.; Willis, T.; Sethuraman, S.K.; Samuels, M. Charcot Marie Tooth disease type 2S with late onset diaphragmatic weakness: An atypical case. Neuromuscul. Disord. 2018, 28, 1016–1021. [Google Scholar] [CrossRef]
- Joseph, S.; Robb, S.; Mohammed, S.; Lillis, S.; Simonds, A.; Manzur, A.; Walter, S.; Wraige, E. Interfamilial phenotypic heterogeneity in SMARD1. Neuromuscul. Disord. 2009, 19, 193–195. [Google Scholar] [CrossRef]
- De Planell-Saguer, M.; Schroeder, D.G.; Rodicio, M.C.; Cox, G.A.; Mourelatos, Z. Biochemical and genetic evidence for a role of IGHMBP2 in the translational machinery. Hum. Mol. Genet. 2009, 18, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- Vadla, G.P.; Hernandez, S.M.R.; Mao, J.; Garro-Kacher, M.O.; Lorson, Z.C.; Rice, R.P.; Hansen, S.A.; Lorson, C.L.; Singh, K.; Lorson, M.A. ABT1 modifies SMARD1 pathology via interactions with IGHMBP2 and stimulation of ATPase and helicase activity. JCI Insight 2023, 8, e164608. [Google Scholar] [CrossRef]
- Gene Therapy for IGHMBP2-Related Diseases. Available online: https://clinicaltrials.gov/ct2/show/NCT05152823 (accessed on 20 March 2023).
- Dennys, C.N.; Sierra-Delgado, J.A.; Ray, S.S.; Hartlaub, A.M.; Roussel, F.S.; Rodriguez, Y.; Meyer, K. In vitro Modeling for Neurological Diseases using Direct Conversion from Fibroblasts to Neuronal Progenitor Cells and Differentiation into Astrocytes. J. Vis. Exp. 2021, 172, e62016. [Google Scholar]
- Traxler, L.; Edenhofer, F.; Mertens, J. Next-generation disease modeling with direct conversion: A new path to old neurons. FEBS Lett. 2019, 593, 3316–3337. [Google Scholar] [CrossRef] [Green Version]
- Vasan, L.; Park, E.; David, L.A.; Fleming, T.; Schuurmans, C. Direct Neuronal Reprogramming: Bridging the Gap Between Basic Science and Clinical Application. Front. Cell Dev. Biol. 2021, 9, 681087. [Google Scholar] [CrossRef]
- Parker, G.C.; Li, X.; Anguelov, R.A.; Toth, G.; Cristescu, A.; Acsadi, G. Survival motor neuron protein regulates apoptosis in anin vitro model of spinal muscular atrophy. Neurotox. Res. 2008, 13, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Yu, J.; Rose, F.F., Jr.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.-W.; Chen, H.; Liu, H.; Lu, J.; Qian, K.; Huang, C.-L.; Zhong, X.; Fan, F.; Zhang, S.-C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015, 6, 6626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohuchi, K.; Funato, M.; Kato, Z.; Seki, J.; Kawase, C.; Tamai, Y.; Ono, Y.; Nagahara, Y.; Noda, Y.; Kameyama, T.; et al. Established Stem Cell Model of Spinal Muscular Atrophy Is Applicable in the Evaluation of the Efficacy of Thyrotropin-Releasing Hormone Analog. Stem Cells Transl. Med. 2016, 5, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashnonejad, A.; Gunduz, C.; Susluer, S.Y.; Onay, H.; Durmaz, B.; Bandehpour, M.; Özkınay, F. In vitro gene manipulation of spinal muscular atrophy fibroblast cell line using gene-targeting fragment for restoration of SMN protein expression. Gene Ther. 2016, 23, 10–17. [Google Scholar] [CrossRef]
- Sison, S.L.; Patitucci, T.N.; Seminary, E.R.; Villalon, E.; Lorson, C.L.; Ebert, A.D. Astrocyte-produced miR-146a as a mediator of motor neuron loss in spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 3409–3420. [Google Scholar] [CrossRef] [Green Version]
- Ando, S.; Funato, M.; Ohuchi, K.; Kameyama, T.; Inagaki, S.; Seki, J.; Kawase, C.; Tsuruma, K.; Shimazawa, M.; Kaneko, H.; et al. Edaravone is a candidate agent for spinal muscular atrophy: In vitro analysis using a human induced pluripotent stem cells-derived disease model. Eur. J. Pharmacol. 2017, 814, 161–168. [Google Scholar] [CrossRef]
- Touznik, A.; Maruyama, R.; Yokota, T. In Vitro Evaluation of Antisense-Mediated Exon Inclusion for Spinal Muscular Atrophy. Methods Mol. Biol. 2018, 1828, 439–454. [Google Scholar]
- Son, Y.S.; Choi, K.; Lee, H.; Kwon, O.; Jung, K.B.; Cho, S.; Baek, J.; Son, B.; Kang, S.M.; Kang, M.; et al. A SMN2 Splicing Modifier Rescues the Disease Phenotypes in an In Vitro Human Spinal Muscular Atrophy Model. Stem Cells Dev. 2019, 28, 438–453. [Google Scholar] [CrossRef]
- De la Fuente, S.; Sansa, A.; Hidalgo, I.; Vivancos, N.; Romero-Guevara, R.; Garcera, A.; Soler, R.M. Calpain system is altered in survival motor neuron-reduced cells from in vitro and in vivo spinal muscular atrophy models. Cell Death Dis. 2020, 11, 487. [Google Scholar] [CrossRef]
- Chang, T.; Zheng, W.; Tsark, W.; Bates, S.; Huang, H.; Lin, R.-J.; Yee, J.-K. Brief report: Phenotypic rescue of induced pluripotent stem cell-derived motoneurons of a spinal muscular atrophy patient. Stem Cells 2011, 29, 2090–2093. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.-J.; Qian, W.-J.; Zhang, Q.-J.; Wang, Z.-F.; Lu, Y.-Q.; Dong, E.-L.; He, J.; Wang, N.; Ma, L.-X.; et al. Modeling the differential phenotypes of spinal muscular atrophy with high-yield generation of motor neurons from human induced pluripotent stem cells. Oncotarget 2017, 8, 42030–42042. [Google Scholar] [CrossRef] [Green Version]
- Nizzardo, M.; Simone, C.; Rizzo, F.; Salani, S.; Dametti, S.; Rinchetti, P.; Del Bo, R.; Foust, K.; Kaspar, B.K.; Bresolin, N.; et al. Gene therapy rescues disease phenotype in a spinal muscular atrophy with respiratory distress type 1 (SMARD1) mouse model. Sci. Adv. 2015, 1, e1500078. [Google Scholar] [CrossRef] [Green Version]
- Simone, C.; Nizzardo, M.; Rizzo, F.; Ruggieri, M.; Riboldi, G.; Salani, S.; Bucchia, M.; Bresolin, N.; Comi, G.P.; Corti, S. iPSC-Derived neural stem cells act via kinase inhibition to exert neuroprotective effects in spinal muscular atrophy with respiratory distress type 1. Stem Cell Rep. 2014, 3, 297–311. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Qiu, B.; Guan, W.; Wang, Q.; Wang, M.; Li, W.; Gao, L.; Shen, L.; Huang, Y.; Xie, G.; et al. Direct Conversion of Normal and Alzheimer’s Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell 2015, 17, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Huh, C.J.; Zhang, B.; Victor, M.B.; Dahiya, S.; Batista, L.F.; Horvath, S.; Yoo, A.S. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. eLife 2016, 5, e18648. [Google Scholar] [CrossRef]
- Mertens, J.; Paquola, A.C.; Ku, M.; Hatch, E.; Böhnke, L.; Ladjevardi, S.; McGrath, S.; Campbell, B.; Lee, H.; Herdy, J.R.; et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell 2015, 17, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Liu, M.L.; Zang, T.; Zhang, C.L. Direct Reprogramming Rather than iPSC-Based Reprogramming Maintains Aging Hallmarks in Human Motor Neurons. Front. Mol. Neurosci. 2017, 10, 359. [Google Scholar] [CrossRef]
- Victor, M.B.; Richner, M.; Olsen, H.E.; Lee, S.W.; Monteys, A.M.; Ma, C.; Huh, C.J.; Zhang, B.; Davidson, B.L.; Yang, X.W.; et al. Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat. Neurosci. 2018, 21, 341–352. [Google Scholar] [CrossRef]
- Gatto, N.; Souza, C.D.S.; Shaw, A.C.; Bell, S.M.; Myszczynska, M.A.; Powers, S.; Meyer, K.; Castelli, L.M.; Karyka, E.; Mortiboys, H.; et al. Directly converted astrocytes retain the ageing features of the donor fibroblasts and elucidate the astrocytic contribution to human CNS health and disease. Aging Cell 2021, 20, e13281. [Google Scholar] [CrossRef]
- Liu, P.; Kaplan, A.; Yuan, B.; Hanna, J.H.; Lupski, J.R.; Reiner, O. Passage number is a major contributor to genomic structural variations in mouse iPSCs. Stem Cells 2014, 32, 2657–2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, I.M.; Borgonovo, T.; Kasai-Brunswick, T.H.; dos Santos, D.S.; Mesquita, F.C.P.; Vasques, J.F.; Gubert, F.; Rebelatto, C.L.K.; Senegaglia, A.C.; Brofman, P.R.S. Chromosomal aberrations after induced pluripotent stem cells reprogramming. Genet. Mol. Biol. 2021, 44, e20200147. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Dutta, D.; Dennys, C.; Powers, S.; Roussel, F.; Lisowski, P.; Glažar, P.; Zhang, X.; Biswas, P.; Caporale, J.R.; et al. Mechanisms of IRF2BPL-related disorders and identification of a potential therapeutic strategy. Cell Rep. 2022, 41, 111751. [Google Scholar]
- Sierra-Delgado, J.A.; Likhite, S.; Bautista, P.K.; Gómez-Ochoa, S.A.; Echeverría, L.E.; Guío, E.; Vargas, C.; Serrano, N.C.; Meyer, K.C.; Rincon, M.Y. Prevalence of Neutralizing Antibodies against Adeno-Associated Virus Serotypes 1, 2, and 9 in Non-Injected Latin American Patients with Heart Failure—ANVIAS Study. Int. J. Mol. Sci. 2023, 24, 5579. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zuo, X.; Jing, J.; Ma, Y.; Wang, J.; Liu, D.; Zhu, J.; Du, X.; Xiong, L.; Du, Y.; et al. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell 2015, 17, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, H.; Zhao, A.-D.; Sun, M.-L.; Ma, K.; Fu, X.-B. Direct conversion of human fibroblasts into dopaminergic neuron-like cells using small molecules and protein factors. Mil. Med. Res. 2020, 7, 52. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, R.; Wu, X.; Zhao, Y.; Fan, Y.; Xiao, Z.; Han, J.; Sun, L.; Wang, X.; Dai, J. Rapid and Efficient Conversion of Human Fibroblasts into Functional Neurons by Small Molecules. Stem Cell Rep. 2019, 13, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.-S.; Chang, M.-Y.; Kim, S.-M.; Yi, S.-H.; Suh-Kim, H.; Jung, S.J.; Kim, M.J.; Kim, J.H.; Lee, Y.-S.; Lee, S.Y.; et al. Generation of Dopamine Neurons from Rodent Fibroblasts through the Expandable Neural Precursor Cell Stage. J. Biol. Chem. 2015, 290, 17401–17414. [Google Scholar] [CrossRef] [Green Version]
- Pilotto, F.; Schmitz, A.; Maharjan, N.; Diab, R.; Odriozola, A.; Tripathi, P.; Yamoah, A.; Scheidegger, O.; Oestmann, A.; Dennys, C.N.; et al. PolyGA targets the ER stress-adaptive response by impairing GRP75 function at the MAM in C9ORF72-ALS/FTD. Acta Neuropathol. 2022, 144, 939–966. [Google Scholar] [CrossRef]
- Hedgecoe, A.; Job, K.; Clarke, A. Reflexive standardization and the resolution of uncertainty in the genomics clinic. Soc. Stud. Sci. 2023, 53, 358–378. [Google Scholar] [CrossRef]
- Morales, A.; Hershberger, R.E. Variants of Uncertain Significance: Should We Revisit How They Are Evaluated and Disclosed? Circ. Genom Precis Med. 2018, 11, e002169. [Google Scholar] [CrossRef]
- Zimmermann, J.; Herman, M.A.; Rosenmund, C. Co-release of glutamate and GABA from single vesicles in GABAergic neurons exogenously expressing VGLUT3. Front. Synaptic Neurosci. 2015, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Zell, V.; Gutierrez-Reed, N.; Wu, J.; Ressler, R.; Shenasa, M.A.; Johnson, A.B.; Fife, K.H.; Faget, L.; Hnasko, T.S. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 2016, 7, 13697. [Google Scholar] [CrossRef] [Green Version]
- Tritsch, N.; Granger, A.; Sabatini, A.J.G.B.L. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 2016, 17, 139–145. [Google Scholar] [CrossRef]
- Setien, M.B.; Smith, K.R.; Howard, K.; Williams, K.; Suhr, S.T.; Purcell, E.K. Differentiation and characterization of neurons derived from rat iPSCs. J. Neurosci. Methods 2020, 338, 108693. [Google Scholar] [CrossRef]
- Orlowska, A.; Perera, P.T.; Al Kobaisi, M.; Dias, A.; Nguyen, H.K.D.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Ivanova, E.P. The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells. Materials 2017, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Adnan, N.; Motazedian, A.; Akter, F.; Hossain, S.; Kutsuzawa, K.; Nag, K.; Kobatake, E.; Akaike, T. An Engineered N-Cadherin Substrate for Differentiation, Survival, and Selection of Pluripotent Stem Cell-Derived Neural Progenitors. PLoS ONE 2015, 10, e0135170. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, L.A.; Rebaza, L.M.; Derzic, S.; Schwartz, P.H.; Monuki, E.S. Regulation of human neural precursor cells by laminin and integrins. J. Neurosci. Res. 2006, 83, 845–856. [Google Scholar] [CrossRef] [Green Version]
- Crawford, T.O.; Paushkin, S.V.; Kobayashi, D.T.; Forrest, S.J.; Joyce, C.L.; Finkel, R.S.; Kaufmann, P.; Swoboda, K.J.; Tiziano, D.; Lomastro, R.; et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE 2012, 7, e33572. [Google Scholar] [CrossRef] [Green Version]

| SMA Type | Onset | SMN2 Copy Number | Clinical Phenotype |
|---|---|---|---|
| Type 0 | At birth | 0 | Most severe: death before 1 month of age |
| Type 1 | 6 months | 1–2 | Severe: failure to sit unaided, respiratory failure before the age of 2 |
| Type 2 | 6–18 months | 2–3 | Intermediate: patients are able to sit but not walk unaided |
| Type 3 | 3a: before 3 years | 3–4 | Mild: patients are able to walk unassisted, eventually become wheelchair-bound |
| 3b: after 3 years | |||
| Type 4 | 2nd-3rd decade of life. | 4+ | Mildest phenotype: normal life expectancy |
| Patient ID | Age at Donation (Months) | Mutation | SMA Type |
|---|---|---|---|
| SMA-1 | 12 | Homozygous for deletions of exons 7 and 8 in SMN1 | Type II (3 copies of SMN2) |
| SMA-2 | 36 | Homozygous deletion of exons 7 and 8 in SMN1. | Type II (3 copies of SMN2) |
| Patient ID | Gene | Mutation |
|---|---|---|
| PT1 | IGHMBP2 | Compound heterozygous: c.1432G > A(p.Val478Net) and c.1082T > C (p.Leu361Pro) |
| PT2 | IGHMBP2 | Compound heterozygous: c.1488C > A(pCys496 *) and C.1478C > T(p.Thr493Ile) |
| Mutations ON IGHMBP2 PATIENT VUS | ||
|---|---|---|
| Allele 1 | c.1478C > T | PATHOGENIC |
| Allele 2 | c.1126G > A | VUS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Delgado, J.A.; Sinha-Ray, S.; Kaleem, A.; Ganjibakhsh, M.; Parvate, M.; Powers, S.; Zhang, X.; Likhite, S.; Meyer, K. In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders. Biology 2023, 12, 867. https://doi.org/10.3390/biology12060867
Sierra-Delgado JA, Sinha-Ray S, Kaleem A, Ganjibakhsh M, Parvate M, Powers S, Zhang X, Likhite S, Meyer K. In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders. Biology. 2023; 12(6):867. https://doi.org/10.3390/biology12060867
Chicago/Turabian StyleSierra-Delgado, Julieth Andrea, Shrestha Sinha-Ray, Abuzar Kaleem, Meysam Ganjibakhsh, Mohini Parvate, Samantha Powers, Xiaojin Zhang, Shibi Likhite, and Kathrin Meyer. 2023. "In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders" Biology 12, no. 6: 867. https://doi.org/10.3390/biology12060867
APA StyleSierra-Delgado, J. A., Sinha-Ray, S., Kaleem, A., Ganjibakhsh, M., Parvate, M., Powers, S., Zhang, X., Likhite, S., & Meyer, K. (2023). In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders. Biology, 12(6), 867. https://doi.org/10.3390/biology12060867





