Visualization of Cell Membrane Tension Regulated by the Microfilaments as a “Shock Absorber” in Micropatterned Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrate with Micropatterns
2.2. Culture of Patterned Cells and Plasmids
2.3. The Cytoskeletal Filamemts Staining and FAs Localization
2.4. Microscope Image Acquisition
2.5. Image Snalysis and Statistical Analysis
2.6. Calculation of Angular Information Entropy of Cytoskeleton and Membrane Tension Information Entropy
3. Results
3.1. Cell Patterning Alters the Arrangement of Cytoskeletal Filaments and the Distribution of FAs
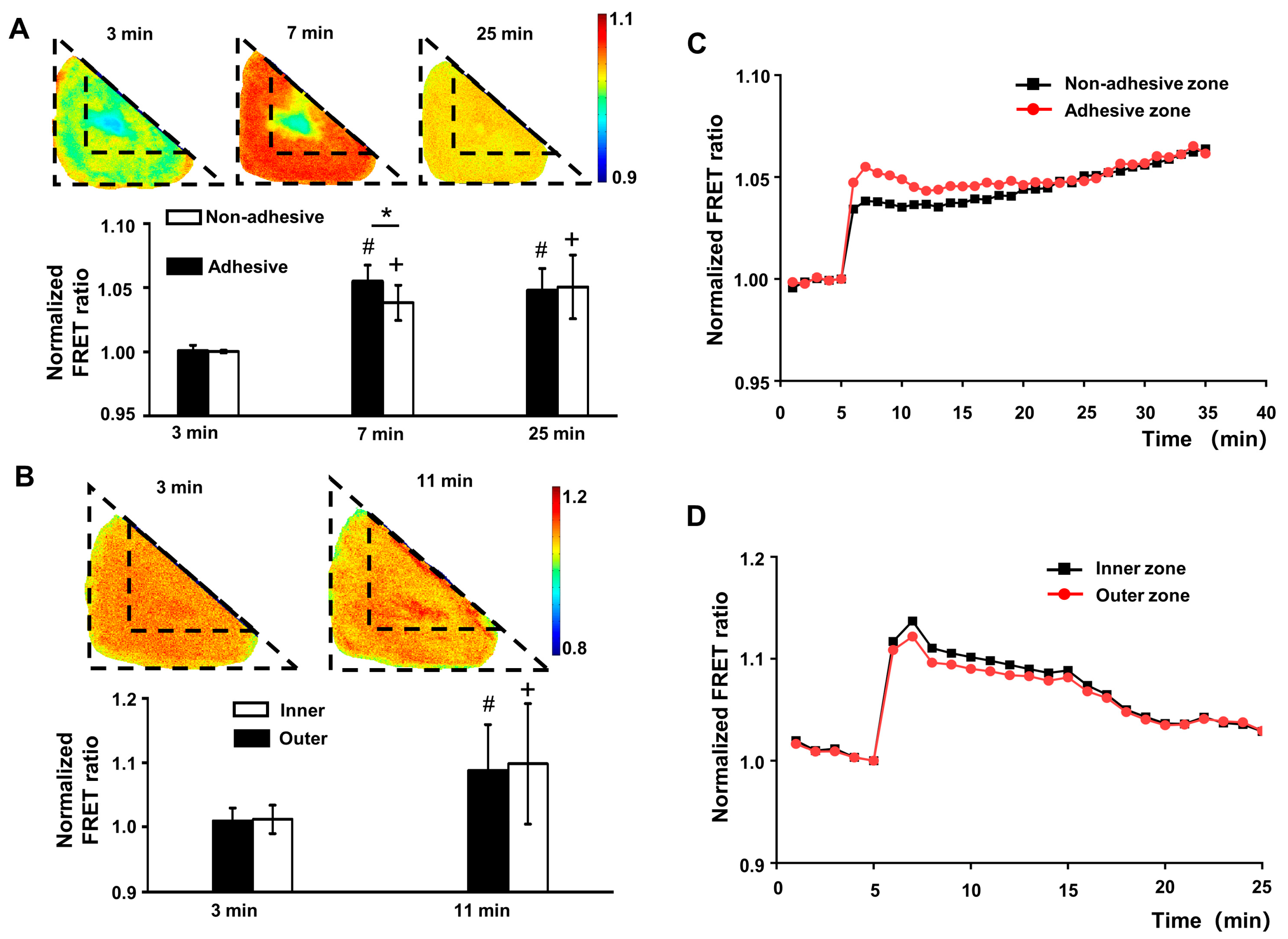
3.2. Cell Membrane Tension Is Independent of Cytoskeleton and FAs’ Distribution under Static Conditions
3.3. The Arrangement of Cytoskeletal Filaments Directly Affects the Changing Pattern of Membrane Tension
3.4. The FAs Functions in Regualting the Memrbane Tension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Z.; Zhi-Ping, Y.A.N.; Lun-Jie, M.A.; Xiao-Heng, L.I.U. Research Progress of Cell Autophagy Induced by Mechanical Stress. Prog. Biochem. Biophys. 2019, 46, 555–564. [Google Scholar]
- Lee, S.G.; Lee, S.N.; Baek, J.; Yoon, J.H.; Lee, H. Mechanical compression enhances ciliary beating through cytoskeleton remodeling in human nasal epithelial cells. Acta Biomater. 2021, 128, 346–356. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Miura, S.; Sato, K.; Kato-Negishi, M.; Teshima, T.; Takeuchi, S. Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nat. Commun. 2015, 6, 8871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.K.; Kim, J.H.; Huang, C.L. Flow-induced activation of TRPV5 and TRPV6 channels stimulates Ca2+-activated K+ channel causing membrane hyperpolarization. Biochim. Biophys. Acta 2013, 1833, 3046–3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeddoust, M.; Shamloo, A. A model for cell density effect on stress fiber alignment and collective directional migration. Phys. Biol. 2015, 12, 066023. [Google Scholar] [CrossRef]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef] [Green Version]
- Poh, Y.C.; Na, S.; Chowdhury, F.; Ouyang, M.; Wang, Y.; Wang, N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS ONE 2009, 4, e7886. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Zhang, B.; Xu, W.; Liao, X.; Liu, B.J.B. Membrane fluidity regulates high shear stress-induced FAK activation at different subcellular compartments. Biocell 2018, 41, 45–53. [Google Scholar] [CrossRef]
- Houk, A.R.; Jilkine, A.; Mejean, C.O.; Boltyanskiy, R.; Dufresne, E.R.; Angenent, S.B.; Altschuler, S.J.; Wu, L.F.; Weiner, O.D. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 2012, 148, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, N.C.; Fardin, M.A.; Roca-Cusachs, P.; Sheetz, M.P. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA 2011, 108, 14467–14472. [Google Scholar] [CrossRef] [Green Version]
- Raucher, D.; Sheetz, M.P. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J. Cell Biol. 2000, 148, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togo, T.; Krasieva, T.B.; Steinhardt, R.A. A decrease in membrane tension precedes successful cell-membrane repair. Mol. Biol. Cell 2000, 11, 4339–4346. [Google Scholar] [CrossRef] [Green Version]
- Osborn, E.A.; Rabodzey, A.; Dewey, C.F., Jr.; Hartwig, J.H. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am. J. Physiol. Cell Physiol. 2006, 290, C444–C452. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Yu, L.; Li, Y.; Pan, Y.; Zhang, Q.; Wang, F.; Chen, J.; Zhu, X. Nudel and FAK as antagonizing strength modulators of nascent adhesions through paxillin. PLoS Biol. 2009, 7, e1000116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thottacherry, J.J.; Kosmalska, A.J.; Kumar, A.; Vishen, A.S.; Elosegui-Artola, A.; Pradhan, S.; Sharma, S.; Singh, P.P.; Guadamillas, M.C.; Chaudhary, N.; et al. Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nat. Commun. 2018, 9, 4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda-Yamahara, M.; Rogg, M.; Frimmel, J.; Trachte, P.; Helmstaedter, M.; Schroder, P.; Schiffer, M.; Schell, C.; Huber, T.B. FERMT2 links cortical actin structures, plasma membrane tension and focal adhesion function to stabilize podocyte morphology. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 68–69, 263–279. [Google Scholar] [CrossRef]
- Pires, R.H.; Shree, N.; Manu, E.; Guzniczak, E.; Otto, O. Cardiomyocyte mechanodynamics under conditions of actin remodelling. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2019, 374, 20190081. [Google Scholar] [CrossRef] [Green Version]
- Lehtimäki, J.I.; Rajakylä, E.K.; Tojkander, S.; Lappalainen, P. Generation of stress fibers through myosin-driven reorganization of the actin cortex. eLife 2021, 10, e60710. [Google Scholar] [CrossRef]
- Svitkina, T.M. Ultrastructure of the actin cytoskeleton. Curr. Opin. Cell Biol. 2018, 54, 623–635. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Calderwood, D.A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Fenix, A.M.; Burnette, D.T. Assembly of myosin II filament arrays: Network Contraction versus Expansion. Cytoskeleton 2018, 75, 545–549. [Google Scholar] [CrossRef]
- Serwas, D.; Akamatsu, M.; Moayed, A.; Vegesna, K.; Vasan, R.; Hill, J.M.; Schöneberg, J.; Davies, K.M.; Rangamani, P.; Drubin, D.G. Mechanistic insights into actin force generation during vesicle formation from cryo-electron tomography. Dev. Cell 2022, 57, 1132–1145.e5. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, H.; Li, N.; Aziz, A.U.R.; Zhang, Z.; Liu, B. The raft cytoskeleton binding protein complexes personate functional regulators in cell behaviors. Acta Histochem. 2022, 124, 151859. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.C.; Nguyen, T.H. Direct monitoring of drug-induced mechanical response of individual cells by atomic force microscopy. JMR 2020, 33, e2847. [Google Scholar] [CrossRef] [Green Version]
- Luxenburg, C.; Zaidel-Bar, R. From cell shape to cell fate via the cytoskeleton–Insights from the epidermis. Exp. Cell Res. 2019, 378, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Deng, S.; Jiang, Q.; Zhang, H.; Zhang, Z.; Li, N.; Cong, F.; Tiihonen, T.; Liu, B. A DNA-encoded FRET biosensor for visualizing the tension across paxillin in living cells upon shear stress. Anal. Sens. 2022, 1, e202100061. [Google Scholar] [CrossRef]
- Liebman, C.; McColloch, A.; Rabiei, M.; Bowling, A.; Cho, M. Mechanics of the cell: Interaction mechanisms and mechanobiological models. Curr. Top. Membr. 2020, 86, 143–184. [Google Scholar] [CrossRef]
- Shi, Z.; Graber, Z.T.; Baumgart, T.; Stone, H.A.; Cohen, A.E. Cell Membranes Resist Flow. Cell 2018, 175, 1769–1779.e13. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.E.; Shi, Z. Do Cell Membranes Flow Like Honey or Jiggle Like Jello? BioEssays: News Rev. Mol. Cell. Dev. Biol. 2020, 42, e1900142. [Google Scholar] [CrossRef]
- Chugh, P.; Clark, A.G.; Smith, M.B.; Cassani, D.A.D.; Dierkes, K.; Ragab, A.; Roux, P.P.; Charras, G.; Salbreux, G.; Paluch, E.K. Actin cortex architecture regulates cell surface tension. Nat. Cell Biol. 2017, 19, 689–697. [Google Scholar] [CrossRef]
- Xia, S.; Lim, Y.B.; Zhang, Z.; Wang, Y.; Zhang, S.; Lim, C.T.; Yim, E.K.F.; Kanchanawong, P. Nanoscale Architecture of the Cortical Actin Cytoskeleton in Embryonic Stem Cells. Cell Rep. 2019, 28, 1251–1267.e7. [Google Scholar] [CrossRef] [Green Version]
- Freeman, S.A.; Vega, A.; Riedl, M.; Collins, R.F.; Ostrowski, P.P.; Woods, E.C.; Bertozzi, C.R.; Tammi, M.I.; Lidke, D.S.; Johnson, P.; et al. Transmembrane Pickets Connect Cyto- and Pericellular Skeletons Forming Barriers to Receptor Engagement. Cell 2018, 172, 305–317.e10. [Google Scholar] [CrossRef] [Green Version]
- Mandal, K.; Pogoda, K.; Nandi, S.; Mathieu, S.; Kasri, A.; Klein, E.; Radvanyi, F.; Goud, B.; Janmey, P.A.; Manneville, J.B. Role of a Kinesin Motor in Cancer Cell Mechanics. Nano Lett. 2019, 19, 7691–7702. [Google Scholar] [CrossRef]
- Datar, A.; Ameeramja, J.; Bhat, A.; Srivastava, R.; Mishra, A.; Bernal, R.; Prost, J.; Callan-Jones, A.; Pullarkat, P.A. The Roles of Microtubules and Membrane Tension in Axonal Beading, Retraction, and Atrophy. Biophys. J. 2019, 117, 880–891. [Google Scholar] [CrossRef]
- Barger, S.R.; Reilly, N.S.; Shutova, M.S.; Li, Q.; Maiuri, P.; Heddleston, J.M.; Mooseker, M.S.; Flavell, R.A.; Svitkina, T.; Oakes, P.W.; et al. Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat. Commun. 2019, 10, 1249. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.S.; Nowak, R.B.; Zhou, S.; Giannetto, M.; Gokhin, D.S.; Papoin, J.; Ghiran, I.C.; Blanc, L.; Wan, J.; Fowler, V.M. Correction for Smith et al., Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc. Natl. Acad. Sci. USA 2018, 115, E6385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontes, B.; Monzo, P.; Gole, L.; Le Roux, A.L.; Kosmalska, A.J.; Tam, Z.Y.; Luo, W.; Kan, S.; Viasnoff, V.; Roca-Cusachs, P.; et al. Membrane tension controls adhesion positioning at the leading edge of cells. J. Cell Biol. 2017, 216, 2959–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brückner, B.R.; Janshoff, A. Elastic properties of epithelial cells probed by atomic force microscopy. Biochim. Biophys. Acta 2015, 1853, 3075–3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcioglu, H.E.; Harkes, R.; Danen, E.H.J.; Schmidt, T. Substrate rigidity modulates traction forces and stoichiometry of cell-matrix adhesions. J. Chem. Phys. 2022, 156, 085101. [Google Scholar] [CrossRef]
- Tanaka, M.; Fujii, Y.; Hirano, K.; Higaki, T.; Nagasaki, A.; Ishikawa, R.; Okajima, T.; Katoh, K. Fascin in lamellipodia contributes to cell elasticity by controlling the orientation of filamentous actin. Genes Cells: Dev. Mol. Cell. Mech. 2019, 24, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitani, W.S.; Saif, T.A.; Wang, Y. Calcium signaling in live cells on elastic gels under mechanical vibration at subcellular levels. PLoS ONE 2011, 6, e26181. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, N.; Zhang, Z.; Qin, K.; Zhang, H.; Shao, S.; Liu, B. Visualization of Cell Membrane Tension Regulated by the Microfilaments as a “Shock Absorber” in Micropatterned Cells. Biology 2023, 12, 889. https://doi.org/10.3390/biology12060889
Wang X, Li N, Zhang Z, Qin K, Zhang H, Shao S, Liu B. Visualization of Cell Membrane Tension Regulated by the Microfilaments as a “Shock Absorber” in Micropatterned Cells. Biology. 2023; 12(6):889. https://doi.org/10.3390/biology12060889
Chicago/Turabian StyleWang, Xianmeng, Na Li, Zhengyao Zhang, Kairong Qin, Hangyu Zhang, Shuai Shao, and Bo Liu. 2023. "Visualization of Cell Membrane Tension Regulated by the Microfilaments as a “Shock Absorber” in Micropatterned Cells" Biology 12, no. 6: 889. https://doi.org/10.3390/biology12060889
APA StyleWang, X., Li, N., Zhang, Z., Qin, K., Zhang, H., Shao, S., & Liu, B. (2023). Visualization of Cell Membrane Tension Regulated by the Microfilaments as a “Shock Absorber” in Micropatterned Cells. Biology, 12(6), 889. https://doi.org/10.3390/biology12060889







