Gestational Age-Dependent Regulation of Transthyretin in Mice during Pregnancy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Real-Time Quantitative Polymerase Chain Reaction
2.3. Serum Preparation and Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Immunofluorescence Staining
2.5. Immunoblotting
2.6. Statistical Analysis
3. Results
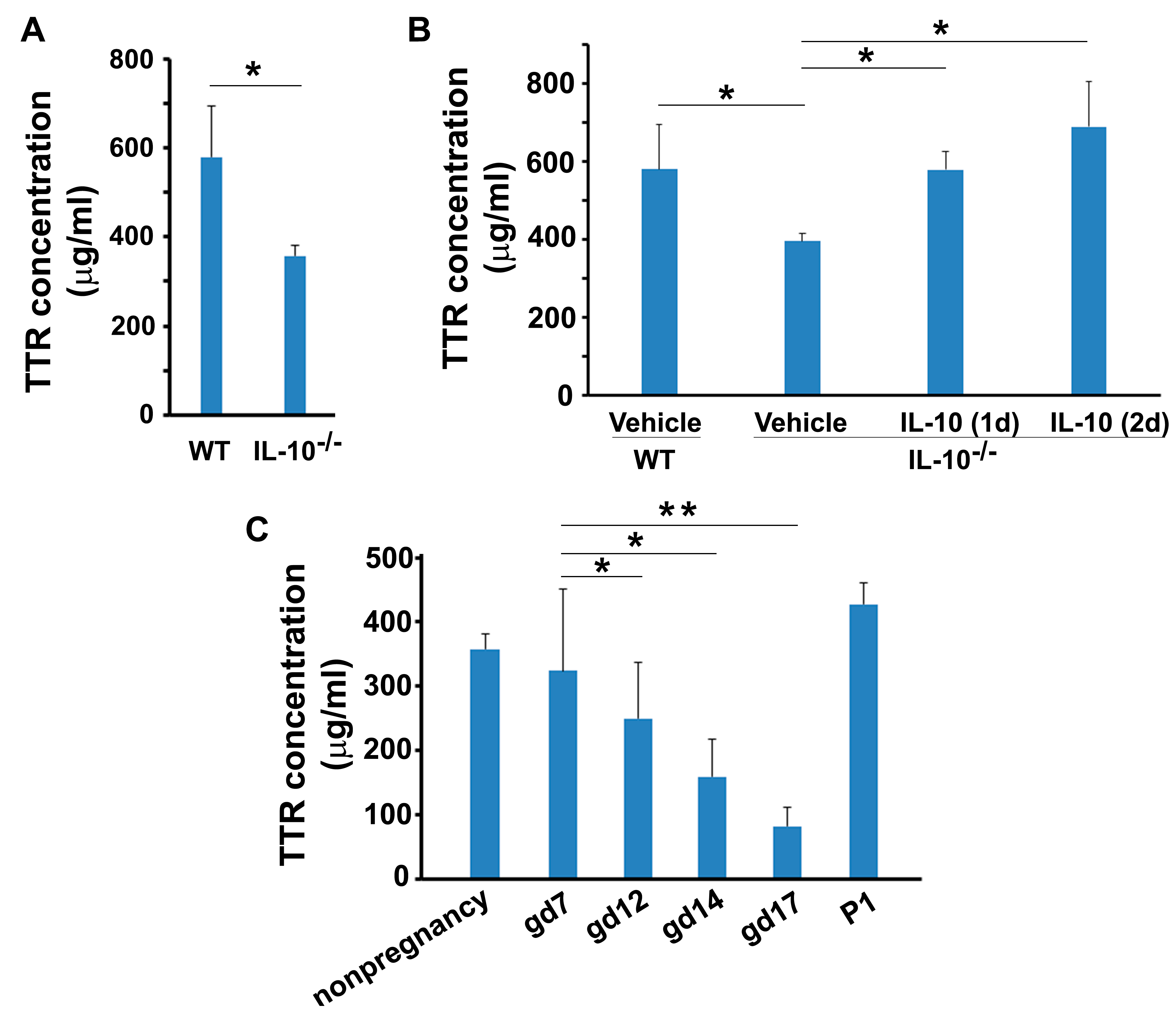
3.1. Temporal Changes in Circulating TTR during Mouse Pregnancy
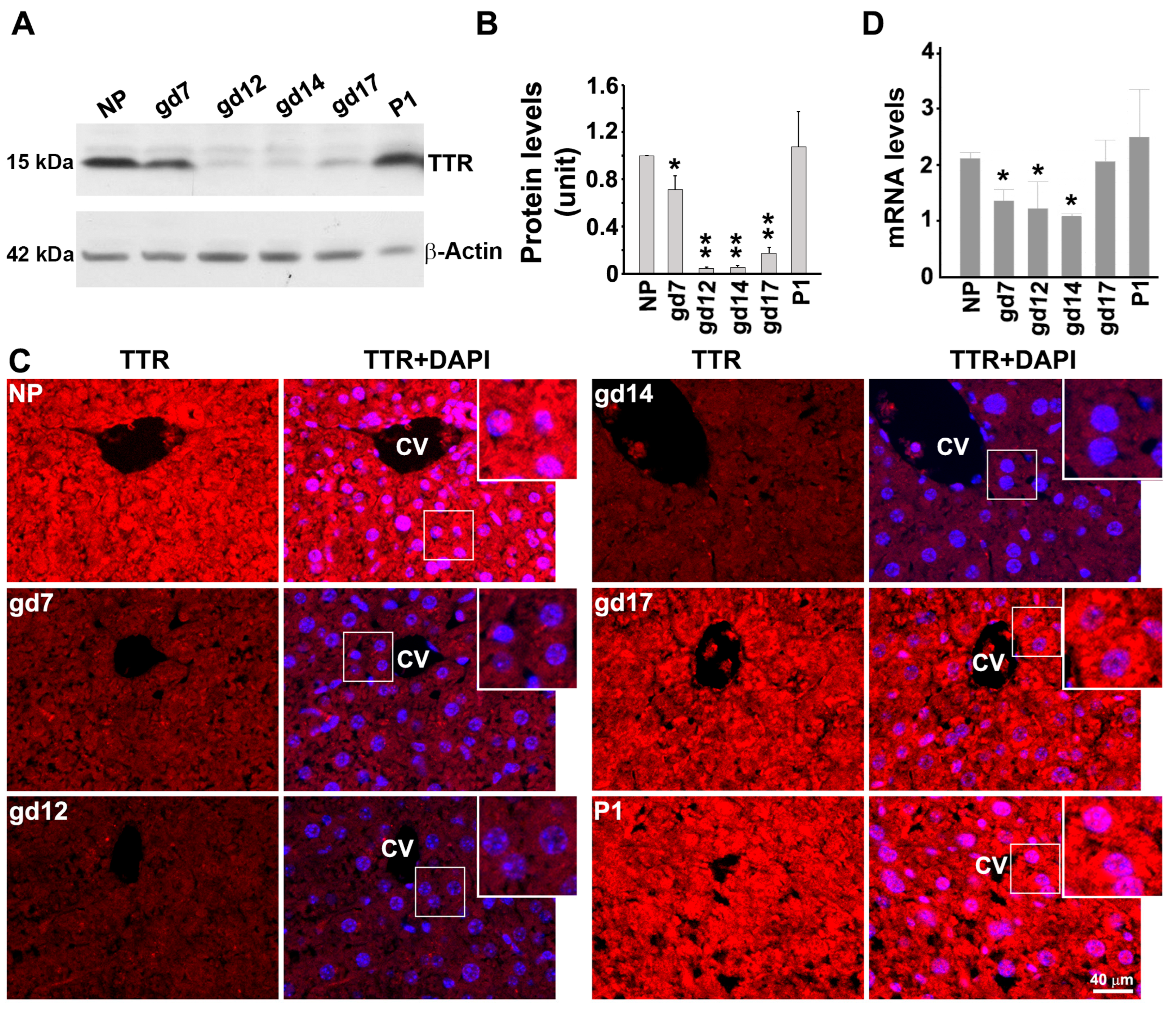
3.2. Expression Pattern of TTR in the Mouse Liver at Protein and mRNA Levels during Gestation
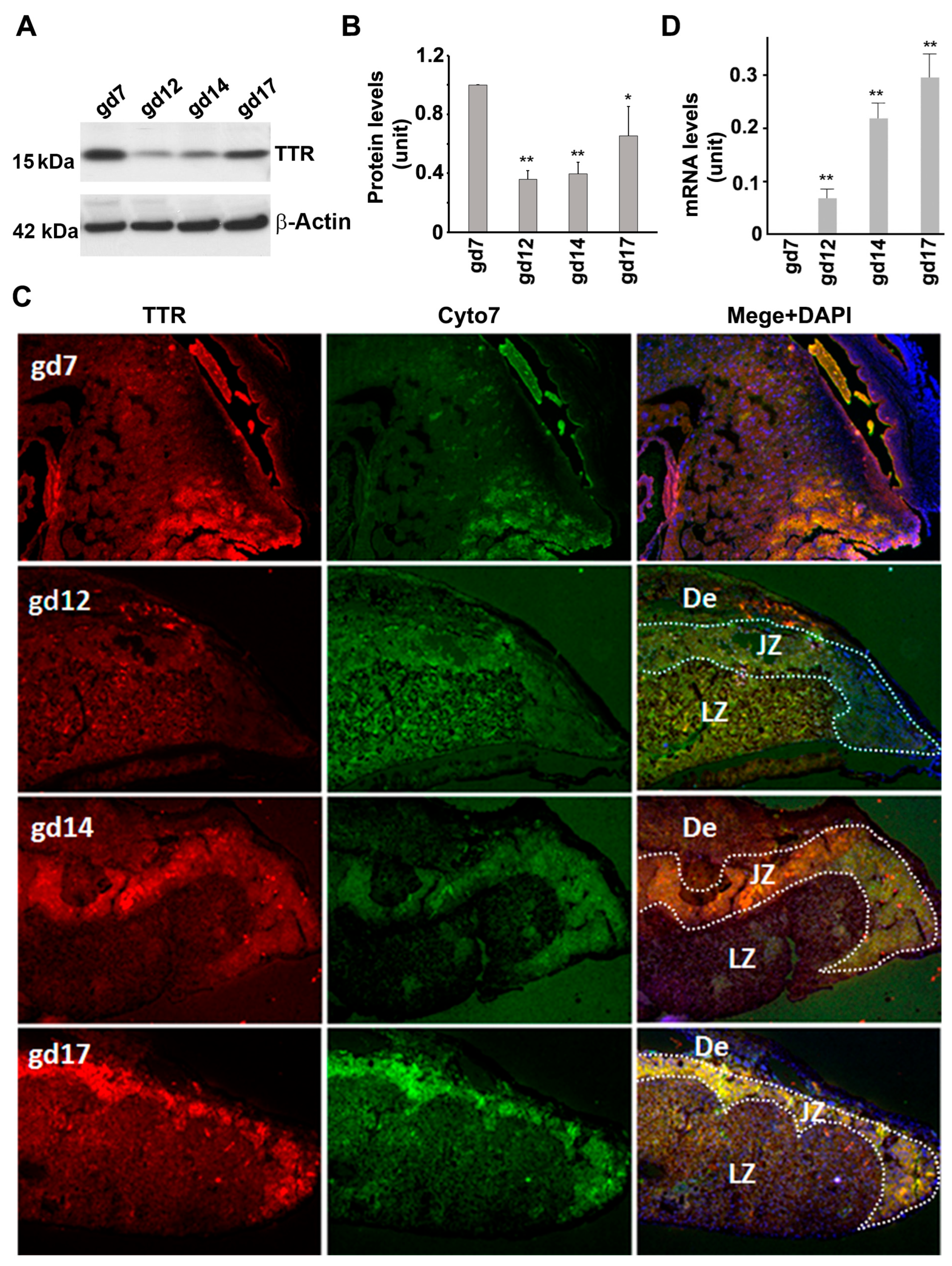
3.3. TTR Protein and mRNA Expression in the Placenta
3.4. Presence of TTR in Sera from Non-Pregnant and Pregnant IL-10−/− Mice
3.5. Overexpression of Human TTR in Transgenic Mice Disturbed Normal Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, M.R.; Naylor, S.L.; Kluve-Beckerman, B.; Long, G.L.; McDonald, L.; Shows, T.B.; Benson, M.D. Localization of the human prealbumin gene to chromosome 18. Biochem. Biophys. Res. Commun. 1985, 129, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Landers, K.A.; Li, H.; Mortimer, R.H.; Richard, K. Ontogenic changes in placental transthyretin. Placenta 2011, 32, 817–822. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.; Li, H.; Richard, K.; Mortimer, R. Synthesis of thyroid hormone binding proteins transthyretin and albumin by human trophoblast. J. Clin. Endocrinol. Metab. 2005, 90, 6714–6720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Chakraborty, S.; Bhattacharya, A.; Biswas, A.; Ain, R. MicroRNA regulation of transthyretin in trophoblast differentiation and intra-uterine growth restriction. Sci. Rep. 2017, 7, 16548. [Google Scholar] [CrossRef] [Green Version]
- Landers, K.A.; Mortimer, R.H.; Richard, K. Transthyretin and the human placenta. Placenta 2013, 34, 513–517. [Google Scholar] [CrossRef]
- Nunes, R.J.; de Oliveira, P.; Lages, A.; Becker, J.D.; Marcelino, P.; Barroso, E.; Perdigoto, R.; Kelly, J.W.; Quintas, A.; Santos, S.C. Transthyretin proteins regulate angiogenesis by conferring different molecular identities to endothelial cells. J. Biol. Chem. 2013, 288, 31752–31760. [Google Scholar] [CrossRef] [Green Version]
- Fleming, C.E.; Mar, F.M.; Franquinho, F.; Saraiva, M.J.; Sousa, M.M. Transthyretin internalization by sensory neurons is megalin mediated and necessary for its neuritogenic activity. J. Neurosci. 2009, 29, 3220–3232. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.C.; Marques, F.; Dias-Ferreira, E.; Cerqueira, J.J.; Sousa, N.; Palha, J.A. Transthyretin influences spatial reference memory. Neurobiol. Learn Mem. 2007, 88, 381–385. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Ye, Z.; Reixach, N.; Friske, L.; Levy, C.; Das, P.; Golde, T.; Masliah, E.; Roberts, A.R.; Bartfai, T. Transthyretin protects Alzheimer’s mice from the behavioral and biochemical effects of Abeta toxicity. Proc. Natl. Acad. Sci. USA 2008, 105, 2681–2686. [Google Scholar] [CrossRef]
- Garai, K.; Posey, A.E.; Li, X.; Buxbaum, J.N.; Pappu, R.V. Inhibition of amyloid beta fibril formation by monomeric human transthyretin. Protein Sci. 2018, 27, 1252–1261. [Google Scholar] [CrossRef] [Green Version]
- Reixach, N.; Deechongkit, S.; Jiang, X.; Kelly, J.W.; Buxbaum, J.N. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl. Acad. Sci. USA 2004, 101, 2817–2822. [Google Scholar] [CrossRef]
- Jiang, X.; Labaudinière, R.; Buxbaum, J.N.; Monteiro, C.; Novais, M.; Coelho, T.; Kelly, J.W. A circulating, disease-specific, mechanism-linked biomarker for ATTR polyneuropathy diagnosis and response to therapy prediction. Proc. Natl. Acad. Sci. USA 2021, 118, e2016072118. [Google Scholar] [CrossRef]
- Teng, M.H.; Yin, J.Y.; Vidal, R.; Ghiso, J.; Kumar, A.; Rabenou, R.; Shah, A.; Jacobson, D.R.; Tagoe, C.; Gallo, G.; et al. Amyloid and nonfibrillar deposits in mice transgenic for wild-type human transthyretin: A possible model for senile systemic amyloidosis. Lab. Investig. 2001, 81, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Kalkunte, S.S.; Neubeck, S.; Norris, W.E.; Cheng, S.B.; Kostadinov, S.; Vu Hoang, D.; Ahmed, A.; von Eggeling, F.; Shaikh, Z.; Padbury, J.; et al. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am. J. Pathol. 2013, 183, 1425–1436. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Huang, Z.; Banerjee, S.; Jash, S.; Buxbaum, J.N.; Sharma, S. Evidence from Human Placenta, Endoplasmic Reticulum-Stressed Trophoblasts, and Transgenic Mice Links Transthyretin Proteinopathy to Preeclampsia. Hypertension 2022, 79, 1738–1754. [Google Scholar] [CrossRef]
- Nakashima, A.; Cheng, S.B.; Ikawa, M.; Yoshimori, T.; Huber, W.J.; Menon, R.; Huang, Z.; Fierce, J.; Padbury, J.F.; Sadovsky, Y.; et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy 2020, 16, 1771–1785. [Google Scholar] [CrossRef]
- Cheng, S.B.; Nakashima, A.; Sharma, S. Understanding pre-eclampsia using Alzheimer’s etiology: An intriguing viewpoint. Am. J. Reprod. Immunol. 2016, 75, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Cheng, S.B.; Chen, Q.; DeSousa, J.; Stone, P.R.; James, J.L.; Chamley, L.W.; Sharma, S. Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci. Rep. 2017, 7, 6694. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Banerjee, S.; Daiello, L.A.; Nakashima, A.; Jash, S.; Huang, Z.; Drake, J.D.; Ernerudh, J.; Berg, G.; Padbury, J.; et al. Novel blood test for early biomarkers of preeclampsia and Alzheimer’s disease. Sci. Rep. 2021, 11, 15934. [Google Scholar] [CrossRef]
- Kalkunte, S.; Boij, R.; Norris, W.; Friedman, J.; Lai, Z.; Kurtis, J.; Lim, K.H.; Padbury, J.F.; Matthiesen, L.; Sharma, S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am. J. Pathol. 2010, 177, 2387–2398. [Google Scholar] [CrossRef]
- Banerjee, S.; Huang, Z.; Wang, Z.; Nakashima, A.; Saito, S.; Sharma, S.; Cheng, S. Etiological value of sterile inflammation in preeclampsia: Is it a non-infectious pregnancy complication? Front. Cell Infect. Microbiol. 2021, 11, 694298. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Davis, S.; Sharma, S. Maternal-fetal cross talk through cell-free fetal DNA, telomere shortening, microchimerism, and inflammation. Am. J. Reprod. Immunol. 2018, 79, e12851. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Nakashima, A.; Huber, W.J.; Davis, S.; Banerjee, S.; Huang, Z.; Saito, S.; Sadovsky, Y.; Sharma, S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019, 10, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.B.; Sharma, S. Interleukin-10: A pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Rennick, D.; Davidson, N.; Berg, D. Interleukin-10 gene knock-out mice: A model of chronic inflammation. Clin. Immunol. Immunopathol. 1995, 76 Pt 2, S174–S178. [Google Scholar] [CrossRef]
- Lai, Z.; Kalkunte, S.; Sharma, S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension 2011, 57, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.P.; Fast, L.D.; Hanna, N.N.; Sharma, S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J. Immunol. 2005, 175, 4084–4090. [Google Scholar] [CrossRef] [Green Version]
- Bazel, S.; Andrejko, K.M.; Chen, J.; Deutschman, C.S. Hepatic gene expression and cytokine responses to sterile inflammation: Comparison with cecal ligation and puncture sepsis in the rat. Shock 1999, 11, 347–355. [Google Scholar] [CrossRef]
- Morgado, I.; Santos, C.R.; Jacinto, R.; Power, D.M. Regulation of transthyretin by thyroid hormones in fish. Gen. Comp. Endocrinol. 2007, 152, 189–197. [Google Scholar] [CrossRef]
- Palm, M.; Axelsson, O.; Wernroth, L.; Larsson, A.; Basu, S. Involvement of inflammation in normal pregnancy. Acta. Obstet. Gynecol. Scand. 2013, 92, 601–605. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Huang, Z.; Nakashima, A.; Sharma, S. Gestational Age-Dependent Regulation of Transthyretin in Mice during Pregnancy. Biology 2023, 12, 1048. https://doi.org/10.3390/biology12081048
Cheng S, Huang Z, Nakashima A, Sharma S. Gestational Age-Dependent Regulation of Transthyretin in Mice during Pregnancy. Biology. 2023; 12(8):1048. https://doi.org/10.3390/biology12081048
Chicago/Turabian StyleCheng, Shibin, Zheping Huang, Akitoshi Nakashima, and Surendra Sharma. 2023. "Gestational Age-Dependent Regulation of Transthyretin in Mice during Pregnancy" Biology 12, no. 8: 1048. https://doi.org/10.3390/biology12081048




