Composition and Long-Term Variation Characteristics of Coral Reef Fish Species in Yongle Atoll, Xisha Islands, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Feeding Habits
2.3. Individual Size
2.4. Conservation Status
2.5. Data Analyses
3. Results
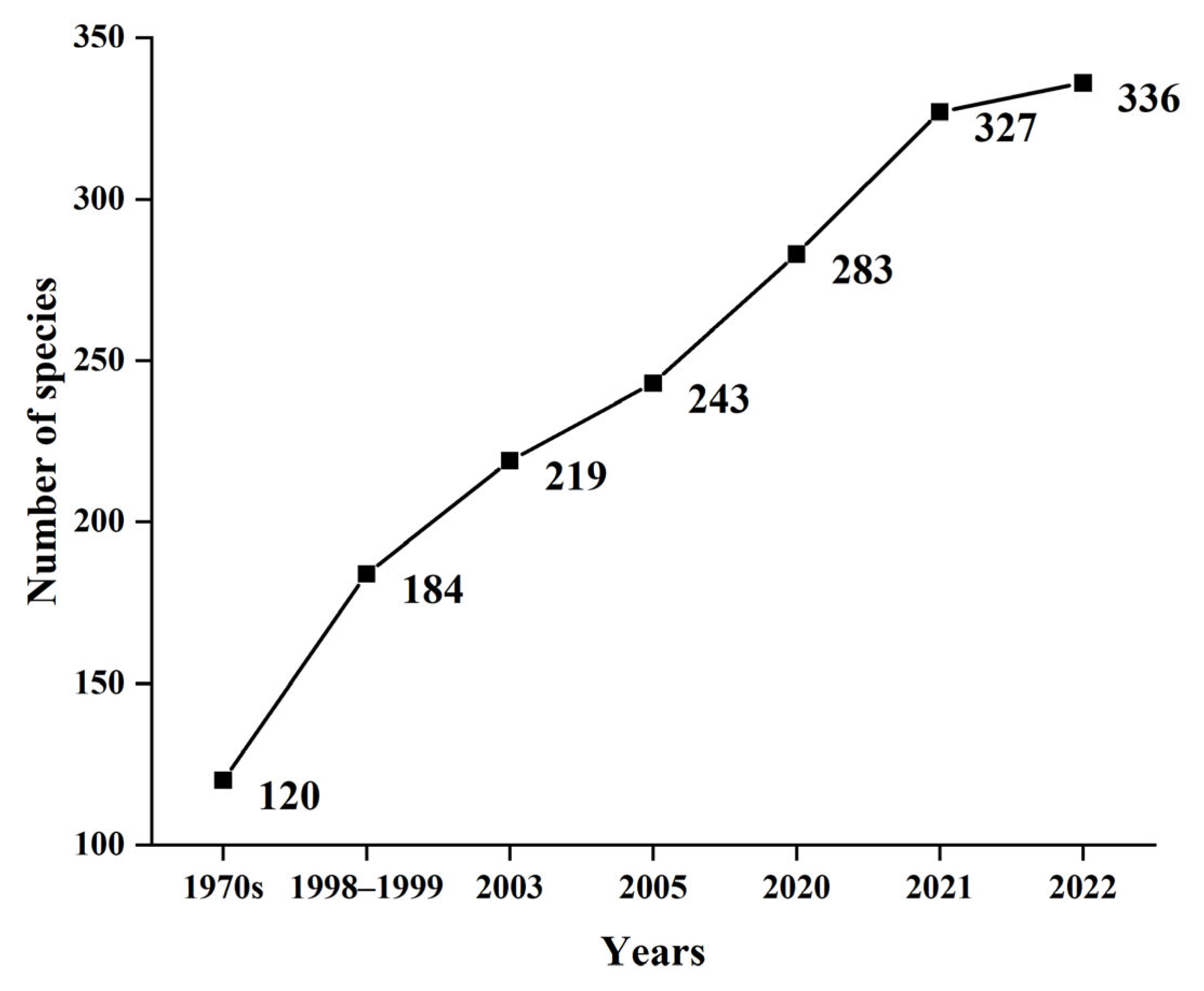
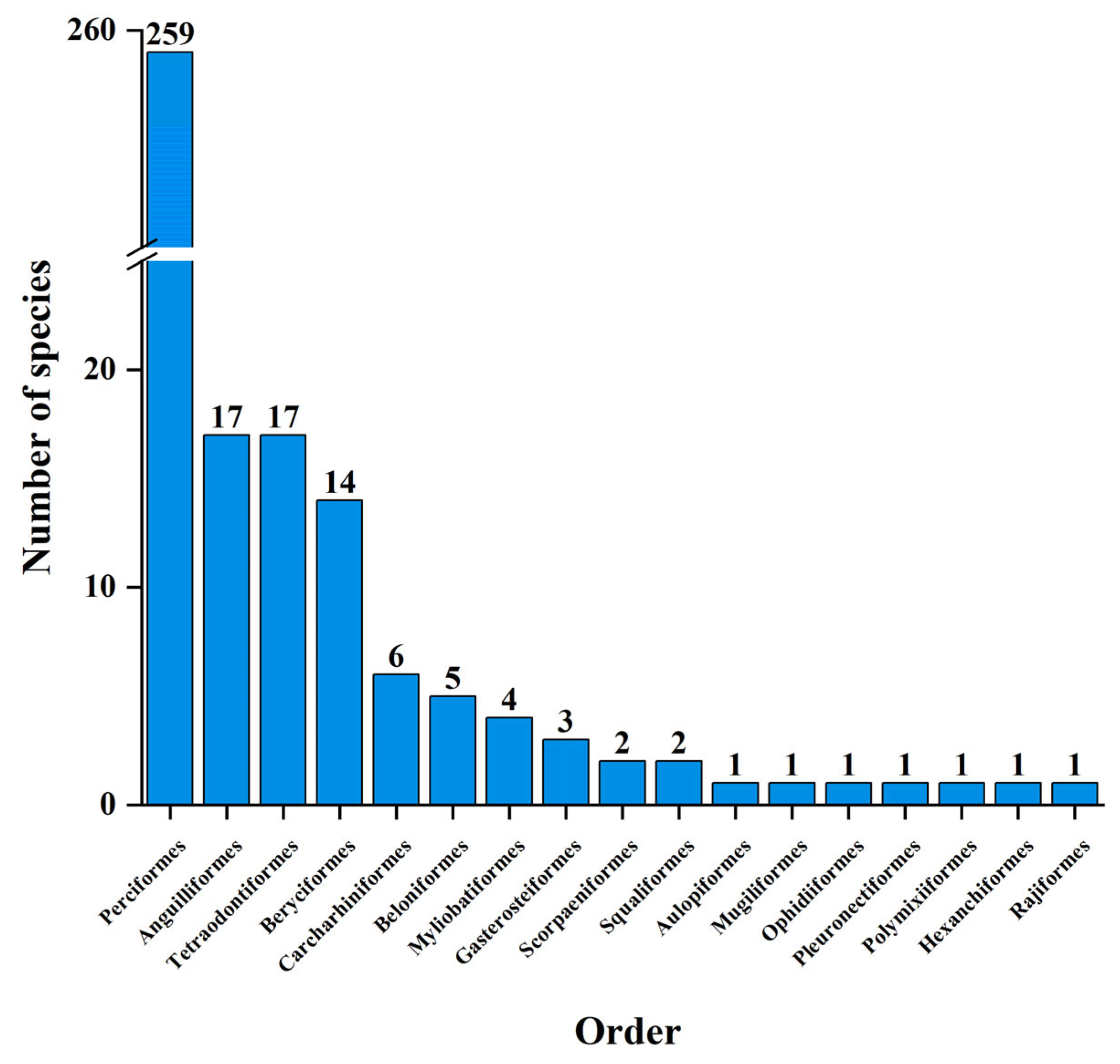
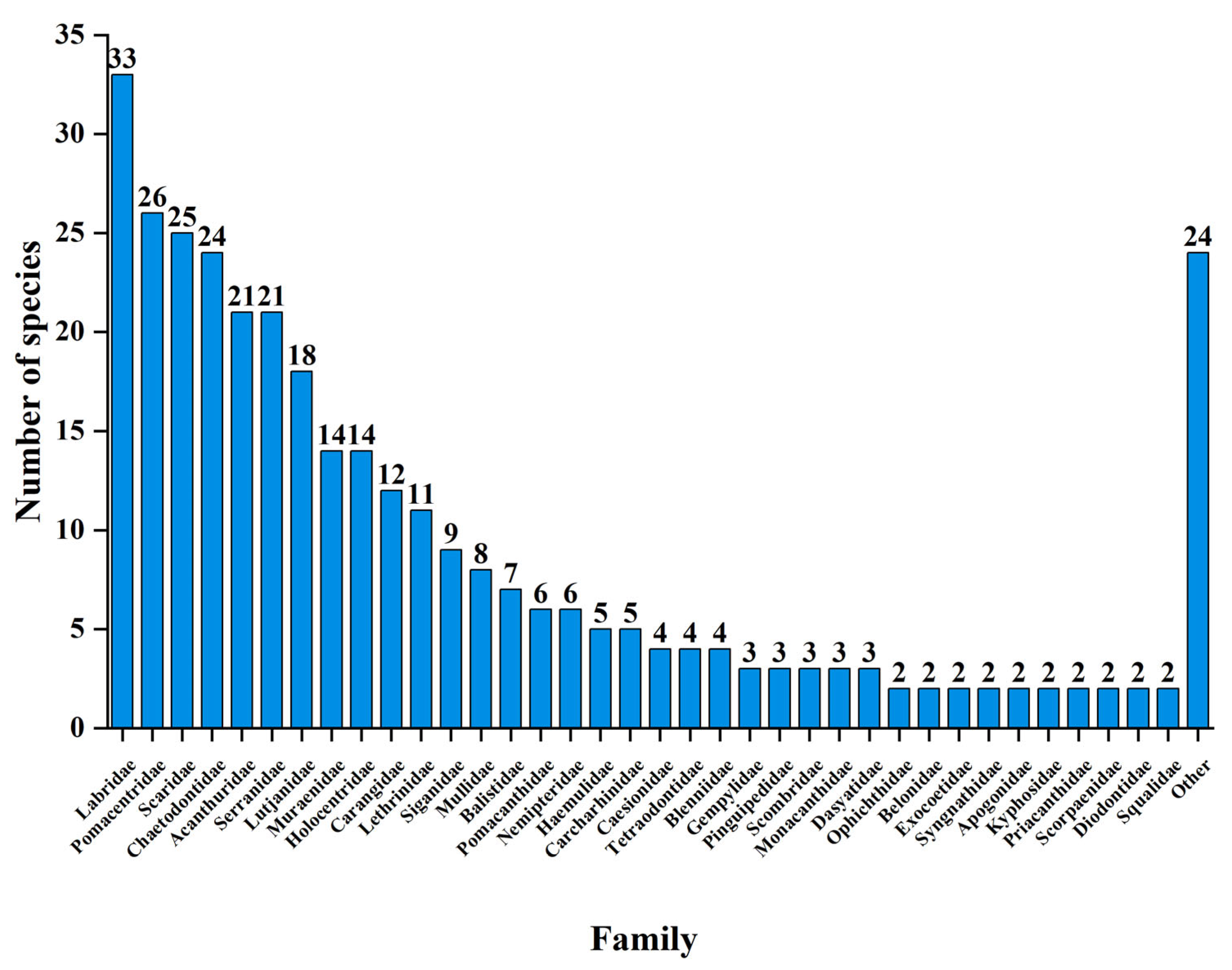
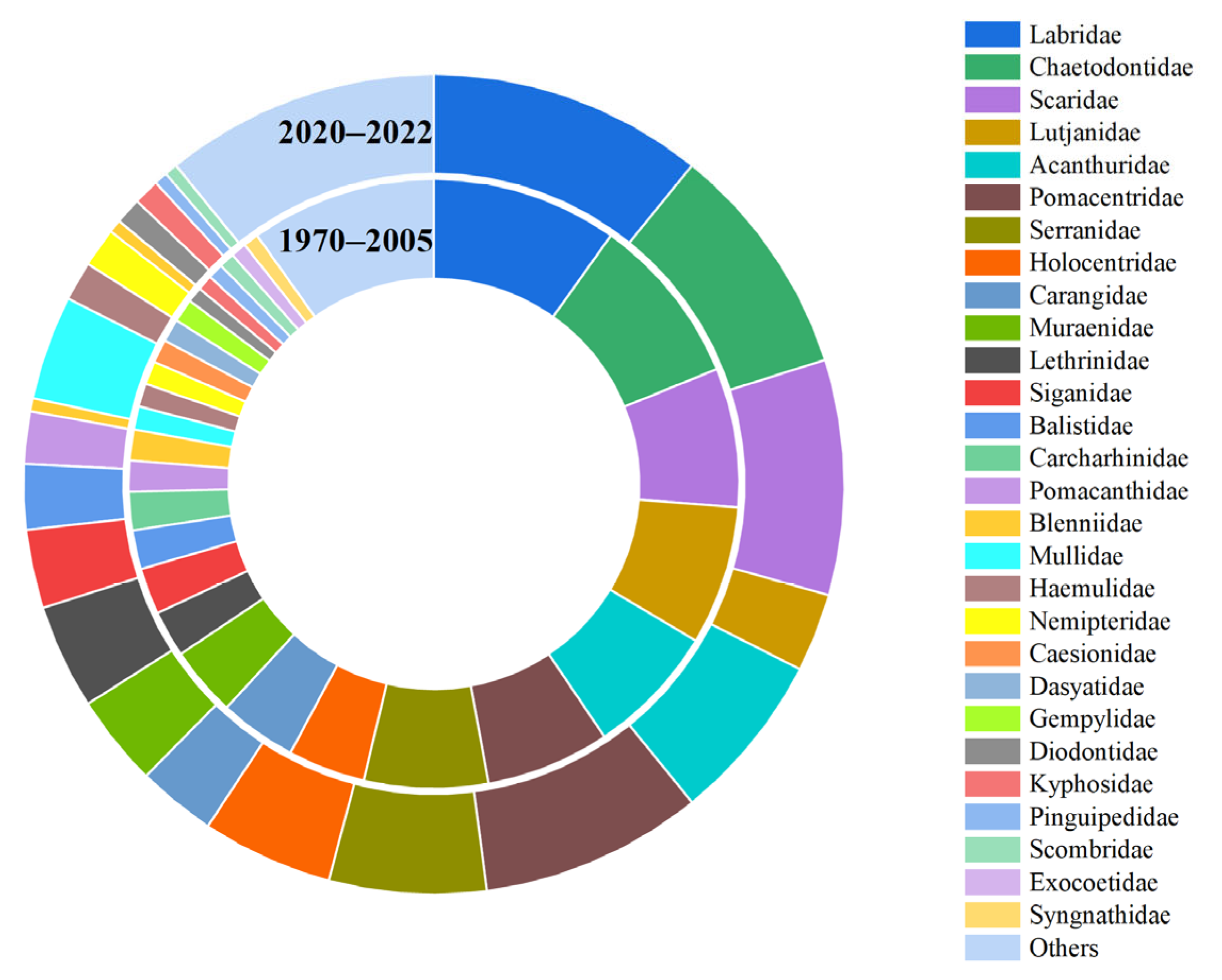
3.1. Fish Species Composition
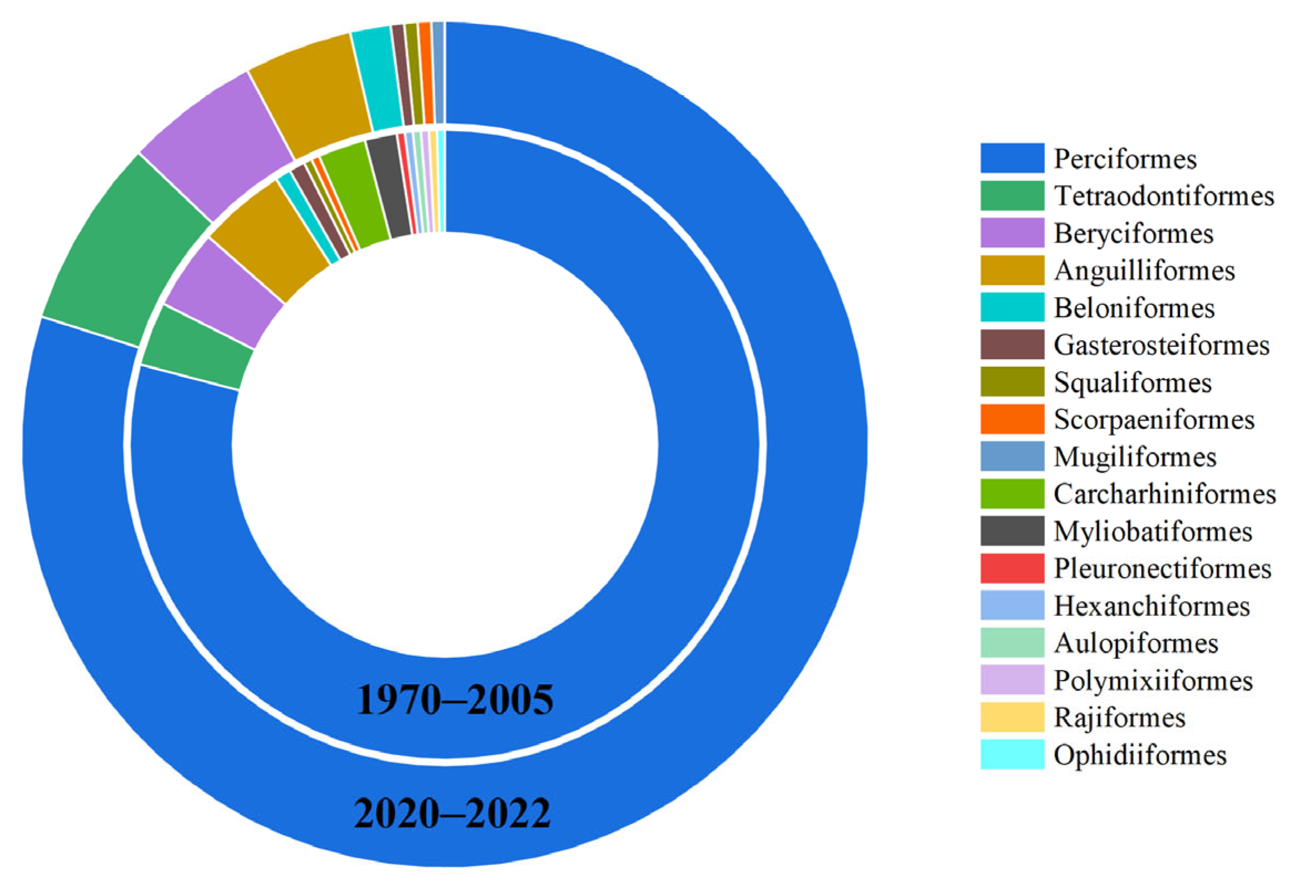
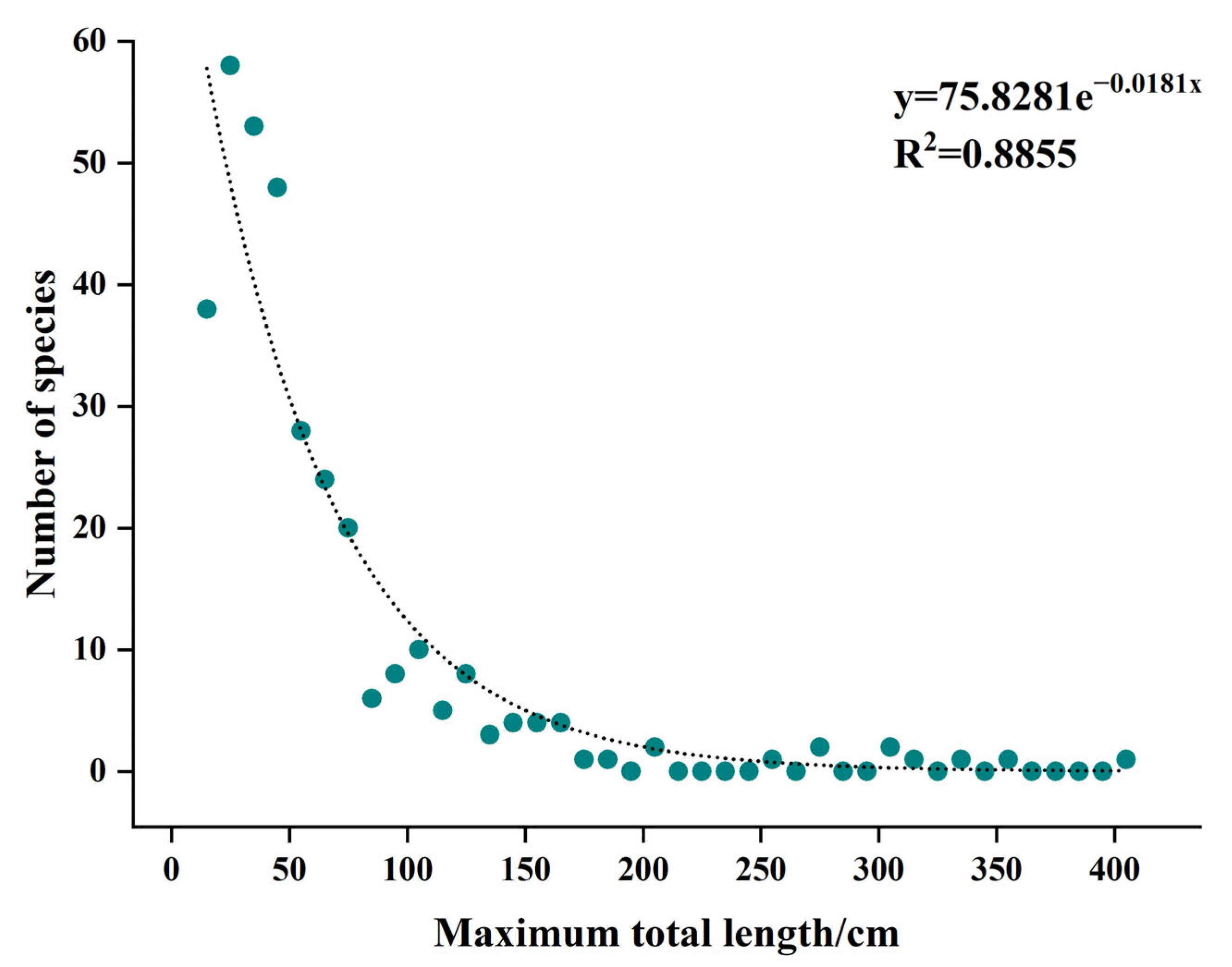
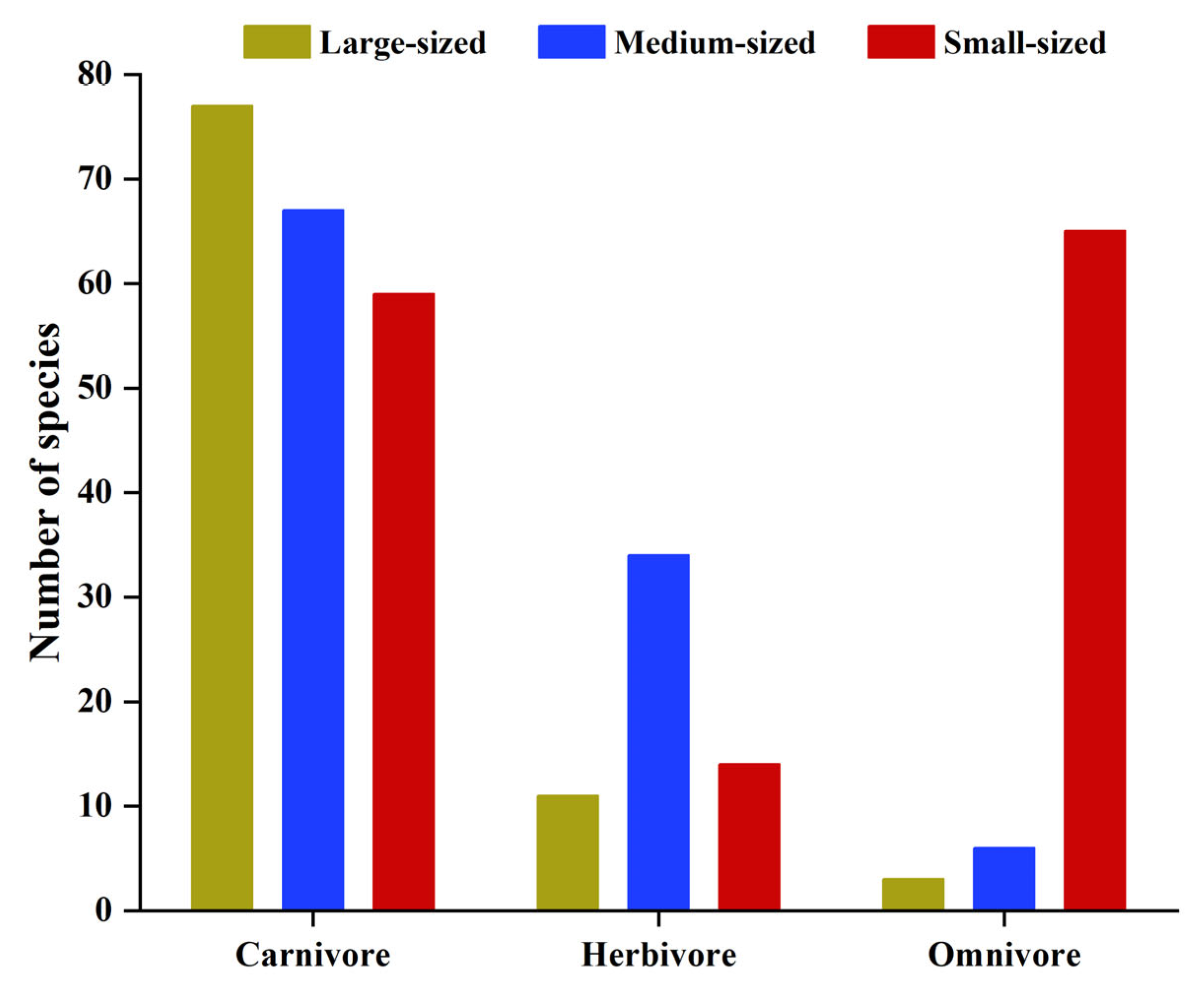
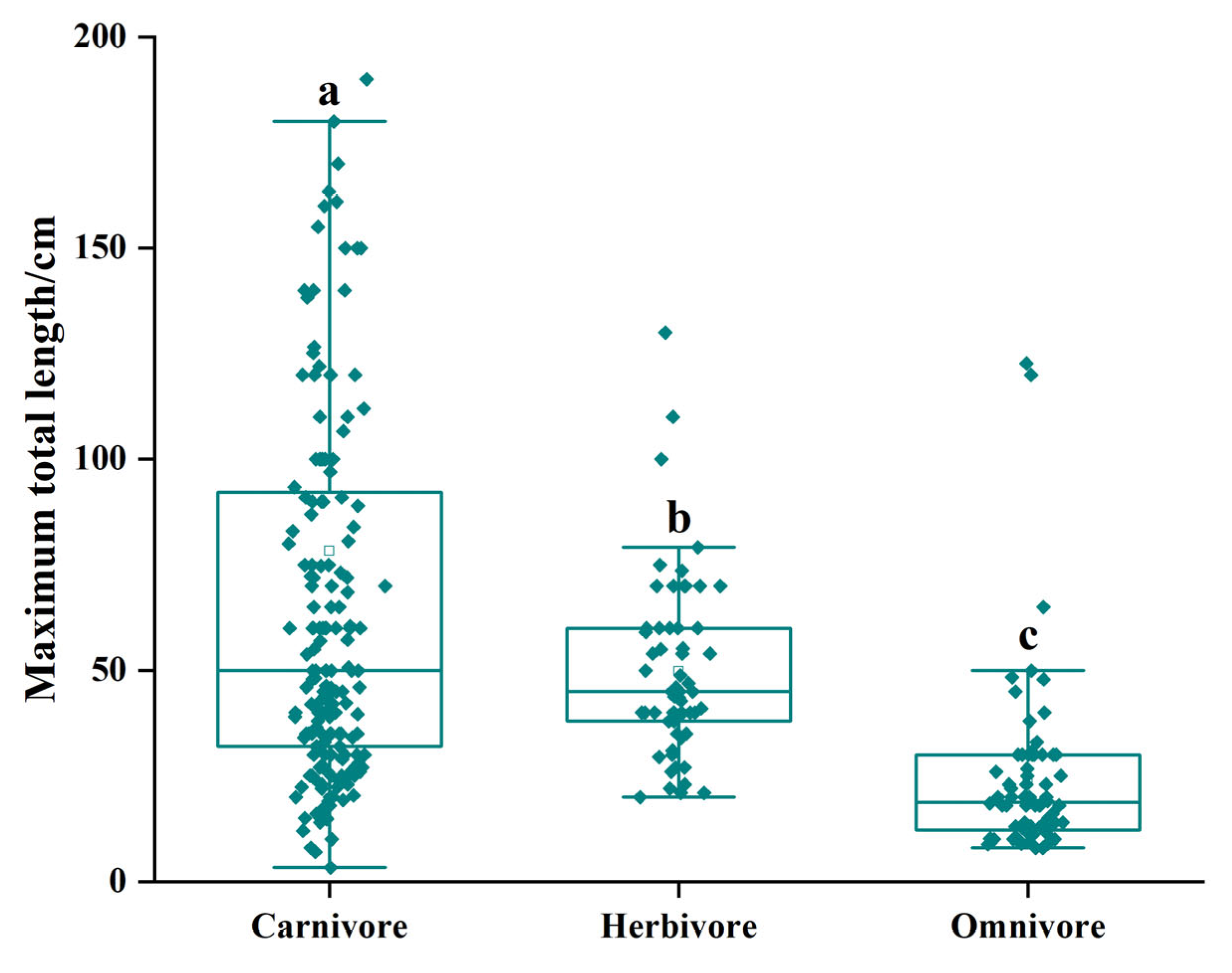
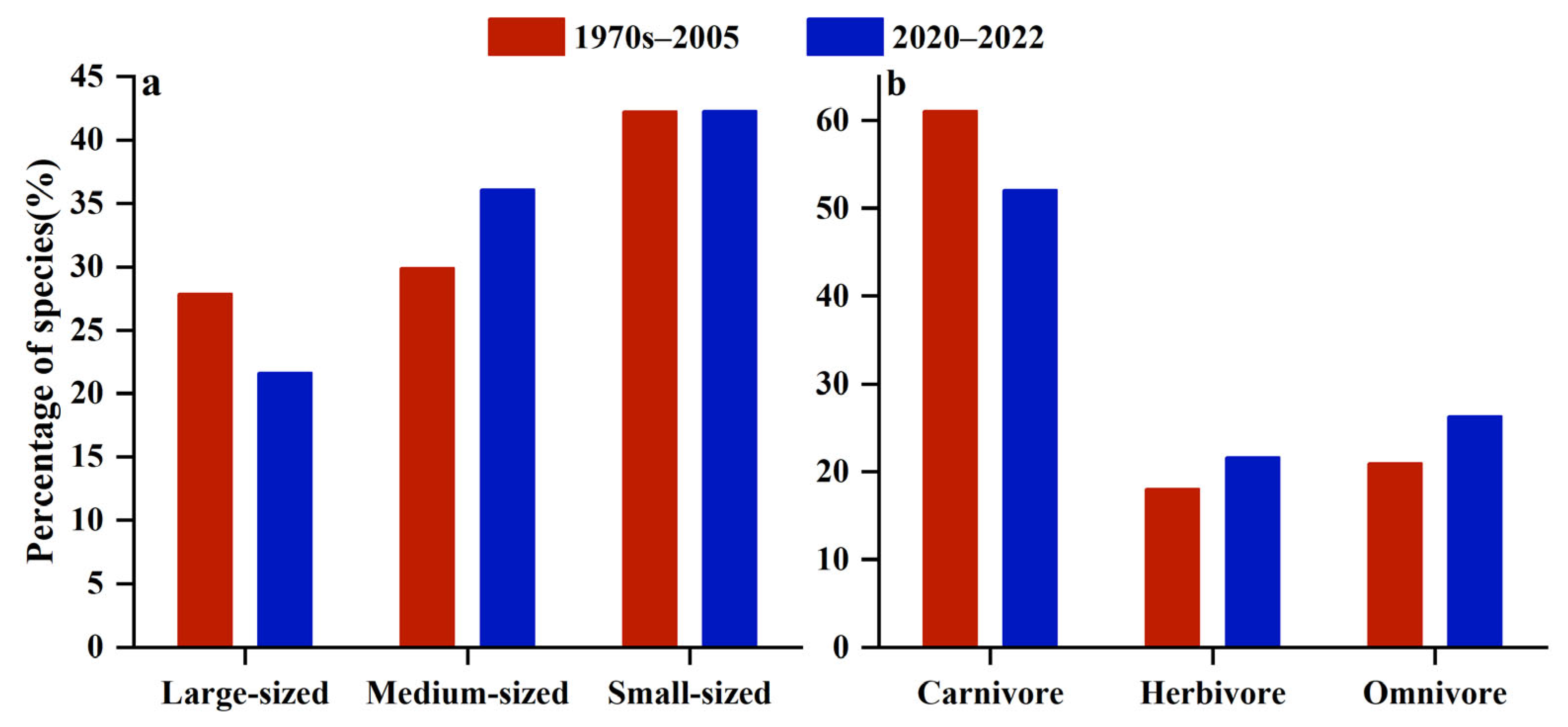
3.2. Fish Community Structure and Changes
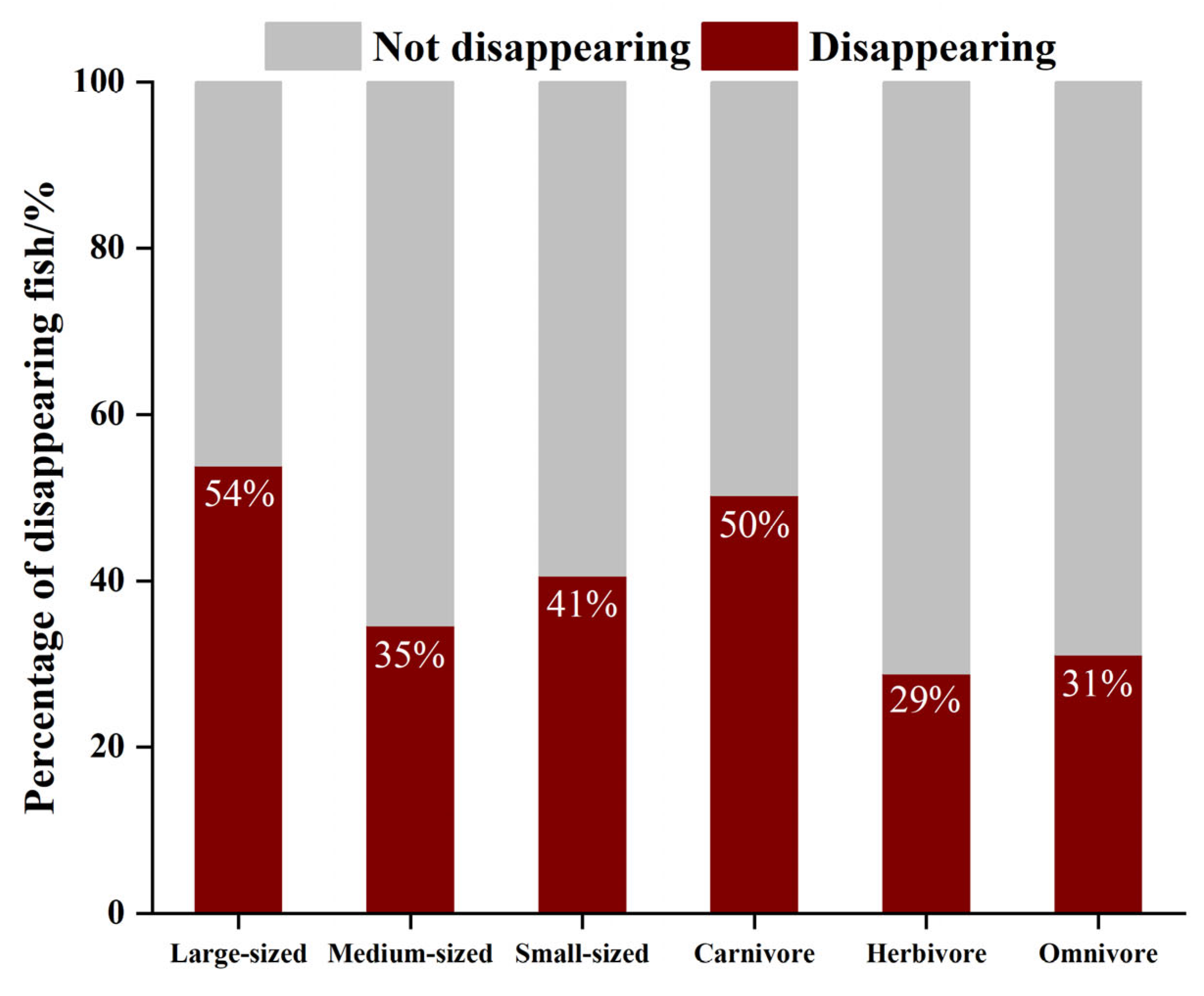
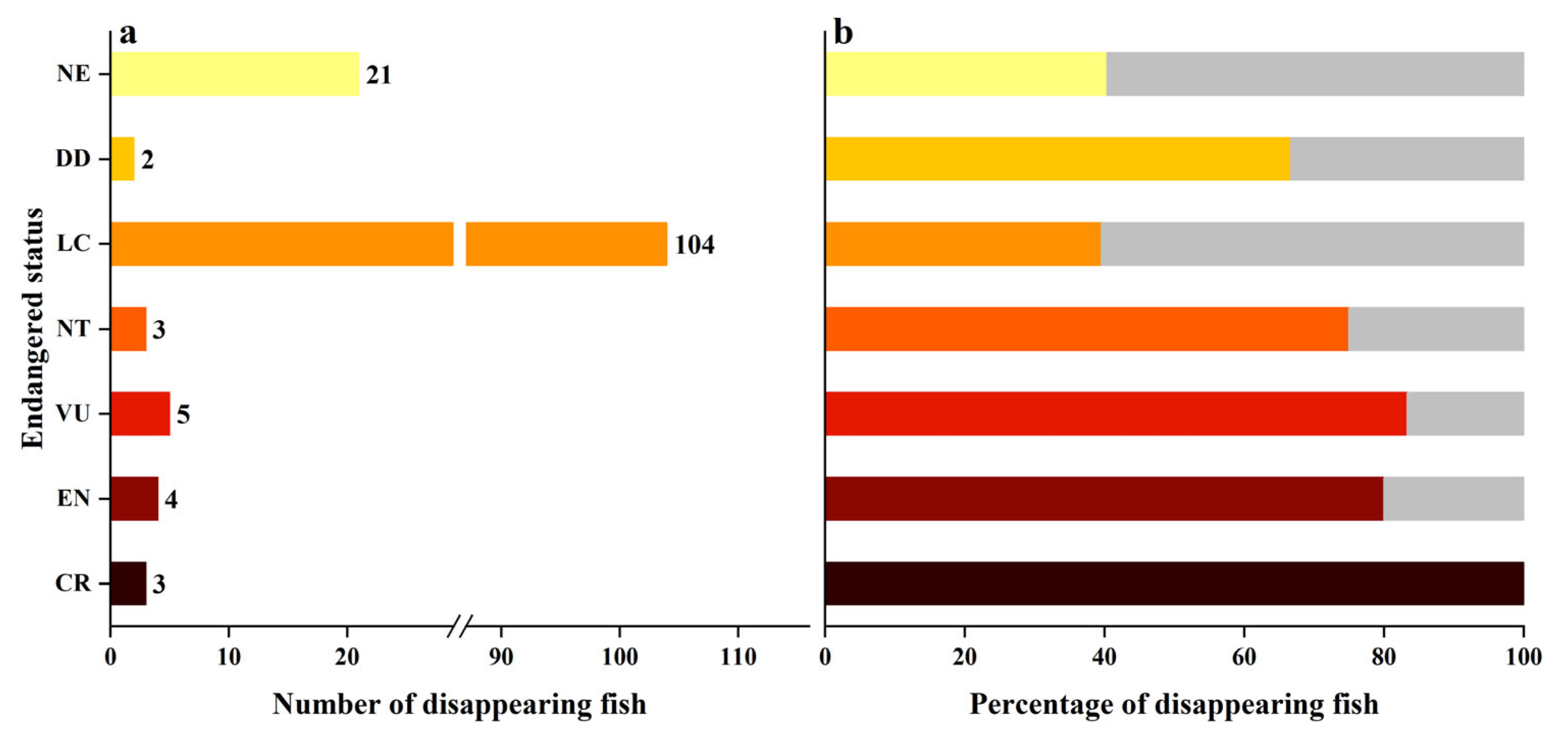
3.3. Disappearing Fish Community Structure
4. Discussion
4.1. Causes of Changes in Fish Species Composition
4.2. Causes of Changes in Fish Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, R.; O’Leary, R.A.; Low-Choy, S.; Mengersen, K.; Knowlton, N.; Brainard, R.E.; Caley, M.J. Species Richness on Coral Reefs and the Pursuit of Convergent Global Estimates. Curr. Biol. 2015, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.P.W.; Wilson, S.K.; Robinson, J.; Gerry, C.; Lucas, J.; Assan, C.; Govinden, R.; Jennings, S.; Graham, N.A.J. Productive instability of coral reef fisheries after climate-driven regime shifts. Nat. Ecol. Evol. 2019, 3, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, C.H.; Wang, T.; Zhao, J.F.; Liu, Y.; Xiao, Y.Y. Distribution Pattern of Coral Reef Fishes in China. Sustainability 2022, 14, 15107. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Alvarez-Noriega, M.; Alvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Cheal, A.J.; Macneil, M.A.; Emslie, M.J.; Sweatman, H. The threat to coral reefs from more intense cyclones under climate change. Glob. Change Biol. 2017, 23, 1511–1524. [Google Scholar] [CrossRef]
- Lamb, J.B.; Willis, B.L.; Fiorenza, E.A.; Couch, C.S.; Howard, R.; Rader, D.N.; True, J.D.; Kelly, L.A.; Ahmad, A.; Jompa, J.; et al. Plastic waste associated with disease on coral reefs. Science 2018, 359, 460–462. [Google Scholar] [CrossRef]
- McCallen, E.; Knott, J.; Nunez-Mir, G.; Taylor, B.; Jo, I.; Fei, S.L. Trends in ecology: Shifts in ecological research themes over the past four decades. Front. Ecol. Environ. 2019, 17, 109–116. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Cumming, G.S. Managing cross-scale dynamics in marine conservation: Pest irruptions and lessons from culling of crown-of-thorns starfish (Acanthaster spp.). Biol. Conserv. 2019, 238, 108211. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.T.; Liu, Y.; Li, C.H.; Lin, L.; Xiao, Y.Y.; Wu, P.; Li, C.R. Reproductive biological characteristics of Chlorurus sordidus from the Yongle Atoll and Meiji Reef. J. Fish. Sci. China 2022, 29, 1366–1374. [Google Scholar]
- Zhao, M.X.; Yu, K.F.; Shi, Q.; Yang, H.Q.; Riegl, B.; Zhang, Q.M.; Yan, H.Q.; Chen, T.R.; Liu, G.H.; Lin, Z.Y. The coral communities of Yongle atoll: Status, threats and conservation significance for coral reefs in South China Sea. Mar. Freshw. Res. 2016, 67, 1888–1896. [Google Scholar] [CrossRef]
- South China Sea Fisheries Research Institute of the State Administration of Fisheries; Xiamen Fisheries College; South China Sea Institute of Oceanography, Chinese Academy of Sciences; South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. Ichthyology of the South China Sea Islands; Science Press: Beijing, China, 1979. [Google Scholar]
- Zhao, J.F.; Liu, Y.; Li, C.H.; Wang, T.; Shi, J.; Xiao, Y.Y.; Wu, P.; Song, X.Y. Study on species composition and distribution of fish eggs in Y ongle Atoll and Dongdao Island by high-throughput sequencing technology. J. Trop. Oceanogr. 2023, 1–10. [Google Scholar] [CrossRef]
- Fishbase Database. Available online: www.fishbase.se/search.php (accessed on 26 October 2022).
- Peter, F.S. The Ecology of Fishes on Coral Reefs; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Wang, T.; Liu, Y.; Quan, Q.M.; Xiao, Y.Y.; Wu, P.; Li, C.H. Species composition characteristics analysis of Qilianyu reef fishes of Xisha Islands. J. Fish. Sci. China 2022, 29, 102–117. [Google Scholar]
- IUCN. Available online: www.iucnredlist.org (accessed on 29 October 2022).
- Mouillot, D.; Laune, J.; Tomasini, J.A.; Aliaume, C.; Brehmer, P.; Dutrieux, E.; Chi, T.D. Assessment of coastal lagoon quality with taxonomic diversity indices of fish, zoobenthos and macrophyte communities. Hydrobiologia 2005, 550, 121–130. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Zintzen, V.; Anderson, M.J.; Roberts, C.D.; Diebel, C.E. Increasing variation in taxonomic distinctness reveals clusters of specialists in the deep sea. Ecography 2011, 34, 306–317. [Google Scholar] [CrossRef]
- Allen, G.R. Reef and shore fishes of Milne Bay Province, Papua New Guinea. In A Rapid Biodiversity Assessment of the Coral Reefs of Milne Bay Province, Papua New Guinea; Werner, T.B., Allen, G.R., Eds.; RAP working papers; Conservation International: Arlington County, VA, USA, 1988. [Google Scholar]
- Wang, T.; Li, C.H.; Zhao, J.F.; Shi, J.; Yu, Y.F.; Xiao, Y.Y.; Liu, Y. The characteristica of species composition and succession of coral reef fishes in Xisha East Island. Acta Hydrobiol. Sin. 2023, 47, 1456–1463. [Google Scholar]
- Wang, T.; Liu, Y.; Li, C.H.; Xiao, Y.Y.; Lin, L.; Li, C.R.; Xie, Y.F.; Wu, P. Characteristics of fish community structure in coral reefs adjacent to yongxing island of xisha islands. Acta Hydrobiol. Sin. 2023, 47, 674–683. [Google Scholar]
- Gladstone, W. Selection of a spawning aggregation site by Chromis hypsilepis (Pisces: Pomacentridae): Habitat structure, transport potential, and food availability. Mar. Ecol. Prog. Ser. 2007, 351, 235–247. [Google Scholar] [CrossRef]
- Allen, G.R. Indo-Pacific Coral-Reef Fishes as Indicators of Conservation Hotspots. In Proceedings of the Ninth International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Ministry of Environment of the Republic of Indonesia: Jakarta, Indonesia, 2002; Volume 2, pp. 921–926. [Google Scholar] [CrossRef]
- Du, J.G.; Loh, K.H.; Hu, W.J.; Zheng, X.Q.; Affendi, Y.A.; Ooi, J.L.S.; Ma, Z.Y.; Rizman-Idid, M.; Chan, A.A. An updated checklist of the marine fish fauna of Redang Islands, Malaysia. Biodivers. Data J. 2019, 7, e47537. [Google Scholar] [CrossRef]
- Wickel, J.; Jamon, A.; Pinault, M.; Durville, P.; Chabanet, P. Species composition and structure of marine fish communities of Mayotte Island (south-western Indian Ocean). Cybium 2014, 38, 179–203. [Google Scholar]
- Kulbicki, M.; Williams, J.T. Checklist of the shorefishes of Ouvea Atoll New Caledonia. Atoll Res. Bull. 1997, 444, 1–26. [Google Scholar] [CrossRef]
- Barjau-Gonzalez, E.; Rodriguez-Romero, J.; Galvan-Magana, F.; Lopez-Martinez, J. Changes in the taxonomic diversity of the reef fish community of San Jos, Island, Gulf of California, Mexico. Biodivers. Conserv. 2012, 21, 3543–3554. [Google Scholar] [CrossRef]
- Shan, X.J.; Jin, X.S.; Yuan, W. Taxonomic diversity of fish assemblages in the Changjiang Estuary and its adjacent waters. Acta Oceanol. Sin. 2010, 29, 70–80. [Google Scholar] [CrossRef]
- Paz-Rios, C.E.; Sosa-Lopez, A.; Torres-Rojas, Y.E.; del Rio-Rodriguez, R.E. Long-term multiscale analysis of temporal variability in the fish community in Terminos Lagoon. Estuar. Coast. Shelf Sci. 2022, 277, 108066. [Google Scholar] [CrossRef]
- Kide, S.O.; Mante, C.; Demarcq, H.; Merigot, B. Groundfish assemblages diversity in upwelling ecosystems: Insights from the Mauritanian Exclusive Economic Zone. Biodivers. Conserv. 2021, 30, 2279–2304. [Google Scholar] [CrossRef]
- Arai, T. Diversity and conservation of coral reef fishes in the Malaysian South China Sea. Rev. Fish Biol. Fish. 2015, 25, 85–101. [Google Scholar] [CrossRef]
- Campbell, N.; Neat, F.; Burns, F.; Kunzlik, P. Species richness, taxonomic diversity, and taxonomic distinctness of the deep-water demersal fish community on the Northeast Atlantic continental slope (ICES Subdivision VIa). ICES J. Mar. Sci. 2011, 68, 365–376. [Google Scholar] [CrossRef]
- Valentine, J.F.; Heck, K.L. Perspective review of the impacts of overfishing on coral reef food web linkages. Coral Reefs 2005, 24, 209–213. [Google Scholar] [CrossRef]
- Mora, C. Ecology of Fishes on Coral Reefs; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Lindahl, U.; Ohman, M.C.; Schelten, C.K. The 1997/1998 mass mortality of corals: Effects on fish communities on a Tanzanian coral reef. Mar. Pollut. Bull. 2001, 42, 127–131. [Google Scholar] [CrossRef]
- Cheal, A.J.; Wilson, S.K.; Emslie, M.J.; Dolman, A.M.; Sweatman, H. Responses of reef fish communities to coral declines on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2008, 372, 211–223. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Gill, J.A.; Dulvy, N.K. Complex reef architecture supports more small-bodied fishes and longer food chains on Caribbean reefs. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Milne, R.; Bauch, C.T.; Anand, M. Local Overfishing Patterns Have Regional Effects on Health of Coral, and Economic Transitions Can Promote Its Recovery. Bull. Math. Biol. 2022, 84, 46. [Google Scholar] [CrossRef]
- Shin, Y.J.; Rochet, M.J.; Jennings, S.; Field, J.G.; Gislason, H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystrom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Brandl, S.J.; Goatley, C.H.R.; Bellwood, D.R.; Tornabene, L. The hidden half: Ecology and evolution of cryptobenthic fishes on coral reefs. Biol. Rev. 2018, 93, 1846–1873. [Google Scholar] [CrossRef]
- Ansell, A.; Gibson, R.; Barnes, M.; Press, U. The ecological implications of small body size among coral-reef fishes. Oceanogr. Mar. Biol. Annu. Rev. 1998, 36, 373–411. [Google Scholar]
- Li, Z.L.; Shan, X.J.; Jin, X.S.; Dai, F.Q. Long-term variations in body length and age at maturity of the small yellow croaker (Larimichthys polyactis Bleeker, 1877) in the Bohai Sea and the Yellow Sea, China. Fish. Res. 2011, 110, 67–74. [Google Scholar] [CrossRef]
- Coker, D.J.; Wilson, S.K.; Pratchett, M.S. Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish. 2014, 24, 89–126. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Sweatman, H.; Delean, S. Recovery from disturbance of coral and reef fish communities on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2008, 371, 177–190. [Google Scholar] [CrossRef]
- LI, Y.C.; Wu, Z.J.; Liang, J.L.; Chen, S.Q.; Zhao, J.M. Analysis on the outbreak period and cause of Acanthaster planci in Xisha Islands in recent 15 years. Chin. Sci. Bull. 2019, 64, 3478–3484. [Google Scholar]
- Coleman, F.C.; Williams, S.L. Overexploiting marine ecosystem engineers: Potential consequences for biodiversity. Trends Ecol. Evol. 2002, 17, 40–44. [Google Scholar] [CrossRef]
- Du, J.G.; Chen, B.; Lu, Z.B.; Song, P.Q.; Xu, Z.C.; Yu, W.W.; Song, X.K. Changes of fish diversity and trophic levels in Quanzhou Bay. Biodivers. Sci. 2010, 18, 420–427. [Google Scholar]
- Planque, B.; Fromentin, J.M.; Cury, P.; Drinkwater, K.F.; Jennings, S.; Perry, R.I.; Kifani, S. How does fishing alter marine populations and ecosystems sensitivity to climate? J. Mar. Syst. 2010, 79, 403–417. [Google Scholar] [CrossRef]
- Fenner, D. Fishing down the largest coral reef fish species. Mar. Pollut. Bull. 2014, 84, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rnnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Rummer, J.L.; Munday, P.L. Climate change and the evolution of reef fishes: Past and future. Fish Fish. 2017, 18, 22–39. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, Y.C.; Du, J.G.; Qiu, S.T.; Xie, B.; Chen, W.L.; Wang, J.J.; Hu, W.J.; Wu, Z.J.; Chen, B. Effects of ocean warming and fishing on the coral reef ecosystem: A case study of Xisha Islands, South China Sea. Front. Mar. Sci. 2022, 9, 1046106. [Google Scholar] [CrossRef]
- Zuo, X.L.; Su, F.Z.; Wu, W.Z.; Chen, Z.K.; Shi, W. Spatial and temporal variability of thermal stress to China’s coral reefs in South China Sea. Chin. Geogr. Sci. 2015, 25, 159–173. [Google Scholar] [CrossRef]
- Halford, A.; Cheal, A.J.; Ryan, D.; Williams, D.M. Resilience to large-scale disturbance in coral and fish assemblages on the Great Barrier Reef. Ecology 2004, 85, 1892–1905. [Google Scholar] [CrossRef]
- Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Resistance and resilience of a coral reef fish community to changes in coral cover. Mar. Ecol. Prog. Ser. 2008, 371, 263–271. [Google Scholar] [CrossRef]
- Eddy, T.D.; Lam, V.W.Y.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W.L. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]

| Years | 1998–2005 | 2020–2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| Order | Family | Genus | Species | Order | Family | Genus | Species | |
| 1970s | 0.17 | 0.22 | 0.12 | 0.10 | 0.25 | 0.29 | 0.28 | 0.22 |
| 1998–2005 | / | / | / | / | 0.27 | 0.31 | 0.24 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Li, C.; Wang, T.; Shi, J.; Song, X.; Liu, Y. Composition and Long-Term Variation Characteristics of Coral Reef Fish Species in Yongle Atoll, Xisha Islands, China. Biology 2023, 12, 1062. https://doi.org/10.3390/biology12081062
Zhao J, Li C, Wang T, Shi J, Song X, Liu Y. Composition and Long-Term Variation Characteristics of Coral Reef Fish Species in Yongle Atoll, Xisha Islands, China. Biology. 2023; 12(8):1062. https://doi.org/10.3390/biology12081062
Chicago/Turabian StyleZhao, Jinfa, Chunhou Li, Teng Wang, Juan Shi, Xiaoyu Song, and Yong Liu. 2023. "Composition and Long-Term Variation Characteristics of Coral Reef Fish Species in Yongle Atoll, Xisha Islands, China" Biology 12, no. 8: 1062. https://doi.org/10.3390/biology12081062
APA StyleZhao, J., Li, C., Wang, T., Shi, J., Song, X., & Liu, Y. (2023). Composition and Long-Term Variation Characteristics of Coral Reef Fish Species in Yongle Atoll, Xisha Islands, China. Biology, 12(8), 1062. https://doi.org/10.3390/biology12081062






