The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
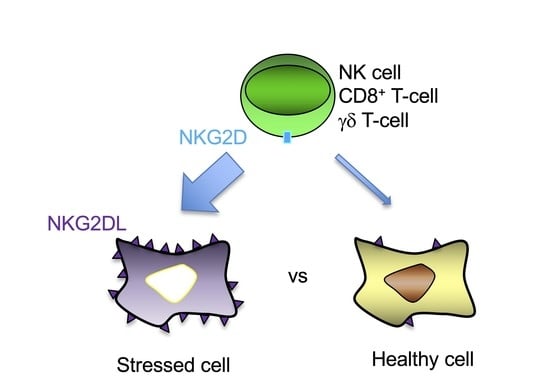
2. NKG2D-Mediated Immune Response
3. Expression of NKG2DL in Healthy Tissues
4. Induction and Regulation of NKG2DL Expression in Cancer
| Stimulus | Subtype/Notes | References |
|---|---|---|
| DNA-damaging agents | Radiation | [49,50,51] |
| Mitomycin C | [50] | |
| Hydroxyurea | [50] | |
| 5-Fluorouracil | [50,52] | |
| Cisplatin | [50] | |
| Temozolomide | [51] | |
| Doxorubicin | [53] | |
| Melphalan | [53] | |
| Etoposide | [53] | |
| Gemcitabine | [54] | |
| Docetaxel | [54] | |
| Vincristine | [55] | |
| STING pathway | [56,57] | |
| Cell cycle inhibition | Mediated via DNA damage response | [50] |
| DNA polymerase inhibitor | [50] | |
| Oxidative stress | [58,59] | |
| Heat shock | [32,36,49] but not [50] | |
| Transcription factors | E2F | [60] |
| KLF4 | [61] | |
| ATF4 | [62] | |
| Oncogenic pathways | BCR/ABL | [63] |
| c-myc | [64,65] | |
| Ras | [66] | |
| ErbB signalling | [67,68] | |
| Cancer-associated metabolic alterations | [69,70] | |
| Cancer-associated inflammation | See text |
5. Tumour Evasion of NKG2D-Mediated Immune Surveillance
6. Role of the NKG2D/NKG2DL Axis in Animal Models of Cancer
7. Pharmacological Regulation of NKG2DL Expression
8. Clinical Significance of the NKG2D/NKG2DL System in Human Cancer
| Tumour | Number | MICA/B | ULBP1 | ULBP2 | ULBP3 | ULBP4 | ULBP5 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Breast (all subgroups) | 677 | 50% | 90% | 99% | 100% | 26% | 90% | [173] |
| Breast (all subgroups) | 530 | 97% | [172] | |||||
| Breast (no TNBC) | 31 | 91% † | 74%† | 78% ***† | 68% † | |||
| Breast (ductal) | 5 | 40% | 60% | 80% | 60% | 60% | 40% | [167] |
| Breast | 16 | 100% | [37] | |||||
| TNBC | Not provided | 93% † | 85% † | 85% ***† | 85% † | [100] ††† | ||
| Colorectal | 462 * | 100% | >50% | >50% | >50% | >50% | >50% | [168,169] |
| Colorectal | 25 | 100% † | 57% † | 72% ***† | 92% † | [100] ††† | ||
| Colorectal | 42 | 48% | [184] | |||||
| Colorectal | 5 | 100% | 100% | 80% | 100% | 80% | 80% | [167] |
| Colorectal | 86 | 85% (predominantly cytoplasmic) | [170] | |||||
| Colorectal | 13 | 100% (cytoplasmic) | [37] | |||||
| AML | 104 | 70% ¶ | [185] | |||||
| AML | 50 | 55% | [186] | |||||
| AML | 30 | Low-level expression seen | [42] | |||||
| AML | 25 | 0% | 16% | 4% | 16% | 0% | ND | [187] |
| AML | 66 | Preferential expression on monocytic subtypes | [188] | |||||
| AML | 14 | 0% 36% 64% 36% 14% | ND | [189] | ||||
| AML/CML/CLL | 25 | 56% expressed at least one ligand | [190] | |||||
| ALL | 11 | 0% | 9% | 18% | 0% | 0% | [187] | |
| ALL | 30 | 67%¶ | [185] | |||||
| CML | 11 | 82%¶ | [185] | |||||
| CLL | 3 | 0% | 0% | 0% | 0% | 0% | [187] | |
| CLL | 60 | 85%¶ | [185] | |||||
| CLL | 51 | Elevated MICA MFI on CLL cells | [178] | |||||
| AML | 50 | 55% ¶¶ | [186] | |||||
| T-ALL | 6 | 5/6 ¶¶¶ | [191] | |||||
| GBM § | 20 | 94/82% | 93% | 84% | 89% | [104] | ||
| GBM | 18 | 88.9% | 23.5% | 0% | 0% | [192] | ||
| Paediatric brain | 125 | Increased ULBP4 in low-grade gliomas only | [193] | |||||
| Neuroblastoma | 12–22 | 0%/86% ‡ 0% 50% 0% | [194] | |||||
| CCA | 82 | 96% | 100% | 77% *** | [195] | |||
| CCA | 5 | 40% | 80% | 60% | 60% | 0% | 20% | [167] |
| Bile duct | 5 | 20% | 40% | 40% | 60% | 40% | 40% | [167] |
| Ovarian | 82 | 80% | 83% | [176] | ||||
| Ovarian | 357 | 88% | 63% | 60% | 59% | 68% | 85% | [175] |
| Ovarian (HGSOC **) | 79 | 65% | 65% | 71% *** | 60% | [196] | ||
| Ovarian | 18 | 72% (cytoplasmic) | [37] | |||||
| Cervical | 5 | 20% | 20% | 40% | 100% | 80% | 40% | [167] |
| Cervical | 200 | 57% §§ | 42% §§ | 49% §§ | 56% §§ | 32% §§ | 43% §§ | [180] |
| Endometrial | 5 | 20% | 60% | 100% | 100% | 80% | 10% | [167] |
| Melanoma | 40/20 §§§ | 78/65% §§§§ | [183] | |||||
| Melanoma (metastases) | 16 | 75% | 50% | [177] | ||||
| Bladder | 23 | 91% † | 39% † | 87% ***† | 78% † | [100] ††† | ||
| NSCLC | 91 | 31% | 48% | 50% †† | 22% | 69% | [197] | |
| NSCLC | 10 | 100% (cytoplasmic) | [37] | |||||
| NSCLC | 40 | 27.5% | [198] | |||||
| NSCLC | 222 | 98.2% | [174] | |||||
| Lung AdCa | 5 | 20% | 60% | 20% | 40% | 20% | 60% | [167] |
| Lung squamous | 5 | 0% | 20% | 20% | 0% | 0% | 0% | [167] |
| Lung (unknown subtype) | 6 | 100% (cytoplasmic) | [37] | |||||
| Oesophageal | 5 | 20% | 20% | 20% | 20% | 40% | 0% | [167] |
| Gastric | 5 | 60% | 80% | 60% | 80% | 80% | 60% | [167] |
| Gastric | 23 | 57%/50% ‡ | [199] | |||||
| Gastric | 98 | 71% | [200] | |||||
| Gastric | 11 | 100% (cytoplasmic) | [37] | |||||
| Prostate | 5 | 0% | 80% | 20% | 20% | 20% | 0% | [167] |
| Prostate | 12 | 92% (cytoplasmic) | [37] | |||||
| Prostate | 165 | 65% (with 85% stromal staining which increased in Gleason stage) | [201] | |||||
| Renal cell | 5 | 0% | 20% | 20% | 0% | 0% | 20% | [167] |
| Renal (clear cell) | 71 | 42% | [202] | |||||
| Urothelial | 5 | 20% | 60% | 80% | 100% | 80% | 60% | [167] |
| Tongue | 5 | 0% | 0% | 0% | 0% | 60% | 0% | [167] |
| Larynx | 5 | 20% | 20% | 0% | 0% | 40% | 60% | [167] |
| Nasopharyngeal | 111 | ULBP4 only measured and was reduced in tumour versus normal tissue | [181] | |||||
| Thyroid papillary | 5 | 60% | 80% | 60% | 80% | 80% | 10% | [167] |
| Thyroid follicular | 5 | 33% | 100% | 100% | 67% | 0% | 10% | [167] |
| Skin | 5 | 20% | 0% | 0% | 0% | 40% | 20% | [167] |
| Thymoma | 36 | Widespread expression of all ligands; % not provided | [203] | |||||
| HCC | 5 | 40% | 100% | 60% | 40% | 60% | 10% | [167] |
| HCC | 10 | 60% (RT-PCR) | [204] | |||||
| HCC | 96 | 78% (not detected in surrounding noncancer tissue) ¶¶¶¶ | [161] | |||||
| HCC | 54 | †††† 46% 0% 0% 0% | [157] | |||||
| HCC | 6 | 50% (cytoplasmic) | [37] | |||||
| HCC | 143 | 100% MICA-positive but levels lower than in adjacent noncancer tissue | [205] | |||||
| Panc. AdCa | 25 | 63% (85% if patients had received neoadjuvant gemcitabine) | [154] | |||||
| Panc. AdCa | 103 | 89.3% (lower expression if poorly differentiated)**** | [206] | |||||
| Panc. AdCa | 9 | 88% (cytoplasmic) | [37] | |||||
| Panc. AdCa | 22 | 77% (more pronounced in poorly differentiated tumours) | [207] | |||||
| Panc. AdCa | 5 | 0% | 0% | 0% | 20% | 0% | 20% | [167] |
| Panc. AdCa | 22 | 100% † | 80% † | 87% ***† | 47% † | [100] ††† | ||
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Groh, V.; Bruhl, A.; El-Gabalawy, H.; Nelson, J.L.; Spies, T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2003, 100, 9452–9457. [Google Scholar] [CrossRef]
- Groh, V.; Smythe, K.; Dai, Z.; Spies, T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat. Immunol. 2006, 7, 755–762. [Google Scholar] [CrossRef]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Jelencic, V.; Polic, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef]
- Cosman, D.; Mullberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Molfetta, R.; Fionda, C.; Soriani, A.; Paolini, R.; Cippitelli, M.; Cerboni, C.; Santoni, A. NKG2D and Its Ligands: “One for All, All for One”. Front. Immunol. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, O.; Lopez-Cobo, S.; Fernandez-Messina, L.; Pontes-Quero, S.; Pandolfi, R.; Reyburn, H.T.; Vales-Gomez, M. A GPI anchor explains the unique biological features of the common NKG2D-ligand allele MICA*008. Biochem. J. 2013, 454, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulpis, E.; Loconte, L.; Cassone, C.; Antonangeli, F.; Caracciolo, G.; Masuelli, L.; Fazio, F.; Petrucci, M.T.; Fionda, C.; Soriani, A.; et al. Cross-Dressing of Multiple Myeloma Cells Mediated by Extracellular Vesicles Conveying MIC and ULBP Ligands Promotes NK Cell Killing. Int. J. Mol. Sci. 2023, 24, 9467. [Google Scholar] [CrossRef]
- Fan, J.; Shi, J.; Zhang, Y.; Liu, J.; An, C.; Zhu, H.; Wu, P.; Hu, W.; Qin, R.; Yao, D.; et al. NKG2D discriminates diverse ligands through selectively mechano-regulated ligand conformational changes. EMBO J. 2022, 41, e107739. [Google Scholar] [CrossRef]
- Duan, S.; Guo, W.; Xu, Z.; He, Y.; Liang, C.; Mo, Y.; Wang, Y.; Xiong, F.; Guo, C.; Li, Y.; et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol. Cancer 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Garrity, D.; Call, M.E.; Feng, J.; Wucherpfennig, K.W. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc. Natl. Acad. Sci. USA 2005, 102, 7641–7646. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, J.L.; Arneson, L.N.; Schoon, R.A.; Dick, C.J.; Billadeau, D.D.; Leibson, P.J. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat. Immunol. 2006, 7, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.B.; Araki, M.; Hamerman, J.A.; Chen, T.; Yamamura, T.; Lanier, L.L. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J. Immunol. 2004, 173, 2470–2478. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8(+) T cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef]
- Wilton, K.M.; Overlee, B.L.; Billadeau, D.D. NKG2D-DAP10 signaling recruits EVL to the cytotoxic synapse to generate F-actin and promote NK cell cytotoxicity. J. Cell Sci. 2019, 133, jcs230508. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.C.; Dobbie, I.M.; Alakoskela, J.M.; Davis, I.; Davis, D.M. Super-resolution imaging of remodeled synaptic actin reveals different synergies between NK cell receptors and integrins. Blood 2012, 120, 3729–3740. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; Ljunggren, H.G.; Long, E.O. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 2009, 114, 2657–2666. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.S.; Richard, J.; Lee, W.S.; Vanderven, H.; Grant, M.D.; Finzi, A.; Kent, S.J. NKG2D Acts as a Co-Receptor for Natural Killer Cell-Mediated Anti-HIV-1 Antibody-Dependent Cellular Cytotoxicity. AIDS Res. Hum. Retrovir. 2016, 32, 1089–1096. [Google Scholar] [CrossRef]
- Inagaki, A.; Ishida, T.; Yano, H.; Ishii, T.; Kusumoto, S.; Ito, A.; Ri, M.; Mori, F.; Ding, J.; Komatsu, H.; et al. Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC. Int. J. Cancer 2009, 125, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lu, C.; Tian, W.; Wang, L.; Cui, B.; Jiao, Y.; Ma, C.; Ju, Y.; Zhu, L.; Shao, C.; et al. Possible association of decreased NKG2D expression levels and suppression of the activity of natural killer cells in patients with colorectal cancer. Int. J. Oncol. 2012, 40, 1285–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secchiari, F.; Nunez, S.Y.; Sierra, J.M.; Ziblat, A.; Regge, M.V.; Raffo Iraolagoitia, X.L.; Rovegno, A.; Ameri, C.; Secin, F.P.; Richards, N.; et al. The MICA-NKG2D axis in clear cell renal cell carcinoma bolsters MICA as target in immuno-oncology. Oncoimmunology 2022, 11, 2104991. [Google Scholar] [CrossRef] [PubMed]
- Doubrovina, E.S.; Doubrovin, M.M.; Vider, E.; Sisson, R.B.; O’Reilly, R.J.; Dupont, B.; Vyas, Y.M. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol. 2003, 171, 6891–6899. [Google Scholar] [CrossRef] [Green Version]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Pernot, S.; Terme, M.; Radosevic-Robin, N.; Castan, F.; Badoual, C.; Marcheteau, E.; Penault-Llorca, F.; Bouche, O.; Bennouna, J.; Francois, E.; et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer 2020, 23, 73–81. [Google Scholar] [CrossRef]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vely, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra255. [Google Scholar] [CrossRef]
- Maasho, K.; Opoku-Anane, J.; Marusina, A.I.; Coligan, J.E.; Borrego, F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J. Immunol. 2005, 174, 4480–4484. [Google Scholar] [CrossRef] [Green Version]
- Groh, V.; Rhinehart, R.; Randolph-Habecker, J.; Topp, M.S.; Riddell, S.R.; Spies, T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001, 2, 255–260. [Google Scholar] [CrossRef]
- Rincon-Orozco, B.; Kunzmann, V.; Wrobel, P.; Kabelitz, D.; Steinle, A.; Herrmann, T. Activation of V gamma 9V delta 2 T cells by NKG2D. J. Immunol. 2005, 175, 2144–2151. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Kyle-Cezar, F.; Woolf, R.T.; Naceur-Lombardelli, C.; Owen, J.; Biswas, D.; Lorenc, A.; Vantourout, P.; Gazinska, P.; Grigoriadis, A.; et al. An innate-like Vdelta1(+) gammadelta T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med. 2019, 11, eaax9364. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Steinle, A.; Bauer, S.; Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998, 279, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Groh, V.; Spies, T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J. Immunol. 2002, 169, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Tosolini, M.; Pont, F.; Poupot, M.; Vergez, F.; Nicolau-Travers, M.L.; Vermijlen, D.; Sarry, J.E.; Dieli, F.; Fournie, J.J. Assessment of tumor-infiltrating TCRVgamma9Vdelta2 gammadelta lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology 2017, 6, e1284723. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Bahram, S.; Bauer, S.; Herman, A.; Beauchamp, M.; Spies, T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc. Natl. Acad. Sci. USA 1996, 93, 12445–12450. [Google Scholar] [CrossRef] [PubMed]
- Ghadially, H.; Brown, L.; Lloyd, C.; Lewis, L.; Lewis, A.; Dillon, J.; Sainson, R.; Jovanovic, J.; Tigue, N.J.; Bannister, D.; et al. MHC class I chain-related protein A and B (MICA and MICB) are predominantly expressed intracellularly in tumour and normal tissue. Br. J. Cancer 2017, 116, 1208–1217. [Google Scholar] [CrossRef] [Green Version]
- Hue, S.; Mention, J.J.; Monteiro, R.C.; Zhang, S.; Cellier, C.; Schmitz, J.; Verkarre, V.; Fodil, N.; Bahram, S.; Cerf-Bensussan, N.; et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004, 21, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Eagle, R.A.; Jafferji, I.; Barrow, A.D. Beyond Stressed Self: Evidence for NKG2D Ligand Expression on Healthy Cells. Curr. Immunol. Rev. 2009, 5, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.H.; Holm, T.L.; Krych, L.; Andresen, L.; Nielsen, D.S.; Rune, I.; Hansen, A.K.; Skov, S. Gut microbiota regulates NKG2D ligand expression on intestinal epithelial cells. Eur. J. Immunol. 2013, 43, 447–457. [Google Scholar] [CrossRef]
- Eagle, R.A.; Flack, G.; Warford, A.; Martinez-Borra, J.; Jafferji, I.; Traherne, J.A.; Ohashi, M.; Boyle, L.H.; Barrow, A.D.; Caillat-Zucman, S.; et al. Cellular expression, trafficking, and function of two isoforms of human ULBP5/RAET1G. PLoS ONE 2009, 4, e4503. [Google Scholar] [CrossRef]
- Nowbakht, P.; Ionescu, M.C.; Rohner, A.; Kalberer, C.P.; Rossy, E.; Mori, L.; Cosman, D.; De Libero, G.; Wodnar-Filipowicz, A. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood 2005, 105, 3615–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggi, A.; Prevosto, C.; Massaro, A.M.; Negrini, S.; Urbani, S.; Pierri, I.; Saccardi, R.; Gobbi, M.; Zocchi, M.R. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: Role of NKp30 and NKG2D receptors. J. Immunol. 2005, 175, 6352–6360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.B.; Rocco, A.; Lamb, L.S.; Friedman, G.K.; Hjelmeland, A.B. Regulation of NKG2D Stress Ligands and Its Relevance in Cancer Progression. Cancers 2022, 14, 2339. [Google Scholar] [CrossRef] [PubMed]
- Baragano Raneros, A.; Martin-Palanco, V.; Fernandez, A.F.; Rodriguez, R.M.; Fraga, M.F.; Lopez-Larrea, C.; Suarez-Alvarez, B. Methylation of NKG2D ligands contributes to immune system evasion in acute myeloid leukemia. Genes Immun. 2015, 16, 71–82. [Google Scholar] [CrossRef]
- Heinemann, A.; Zhao, F.; Pechlivanis, S.; Eberle, J.; Steinle, A.; Diederichs, S.; Schadendorf, D.; Paschen, A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012, 72, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Stern-Ginossar, N.; Gur, C.; Biton, M.; Horwitz, E.; Elboim, M.; Stanietsky, N.; Mandelboim, M.; Mandelboim, O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat. Immunol. 2008, 9, 1065–1073. [Google Scholar] [CrossRef]
- Guerra, N.; Lanier, L.L. Editorial: Emerging Concepts on the NKG2D Receptor-Ligand Axis in Health and Diseases. Front. Immunol. 2020, 11, 562. [Google Scholar] [CrossRef]
- Kim, J.Y.; Son, Y.O.; Park, S.W.; Bae, J.H.; Chung, J.S.; Kim, H.H.; Chung, B.S.; Kim, S.H.; Kang, C.D. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp. Mol. Med. 2006, 38, 474–484. [Google Scholar] [CrossRef] [Green Version]
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190. [Google Scholar] [CrossRef] [Green Version]
- Weiss, T.; Schneider, H.; Silginer, M.; Steinle, A.; Pruschy, M.; Polic, B.; Weller, M.; Roth, P. NKG2D-Dependent Antitumor Effects of Chemotherapy and Radiotherapy against Glioblastoma. Clin. Cancer Res. 2018, 24, 882–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wang, W.J.; Zhang, J.N.; Zhang, X.Y. 5-Fluorouracil and interleukin-2 immunochemotherapy enhances immunogenicity of non-small cell lung cancer A549 cells through upregulation of NKG2D ligands. Asian Pac. J. Cancer Prev. 2014, 15, 4039–4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriani, A.; Zingoni, A.; Cerboni, C.; Iannitto, M.L.; Ricciardi, M.R.; Di Gialleonardo, V.; Cippitelli, M.; Fionda, C.; Petrucci, M.T.; Guarini, A.; et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009, 113, 3503–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okita, R.; Wolf, D.; Yasuda, K.; Maeda, A.; Yukawa, T.; Saisho, S.; Shimizu, K.; Yamaguchi, Y.; Oka, M.; Nakayama, E.; et al. Contrasting Effects of the Cytotoxic Anticancer Drug Gemcitabine and the EGFR Tyrosine Kinase Inhibitor Gefitinib on NK Cell-Mediated Cytotoxicity via Regulation of NKG2D Ligand in Non-Small-Cell Lung Cancer Cells. PLoS ONE 2015, 10, e0139809. [Google Scholar] [CrossRef] [Green Version]
- Soriani, A.; Borrelli, C.; Ricci, B.; Molfetta, R.; Zingoni, A.; Fionda, C.; Carnevale, S.; Abruzzese, M.P.; Petrucci, M.T.; Ricciardi, M.R.; et al. p38 MAPK differentially controls NK activating ligands at transcriptional and post-transcriptional level on multiple myeloma cells. Oncoimmunology 2017, 6, e1264564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bert, N.; Lam, A.R.; Ho, S.S.; Shen, Y.J.; Liu, M.M.; Gasser, S. STING-dependent cytosolic DNA sensor pathways regulate NKG2D ligand expression. Oncoimmunology 2014, 3, e29259. [Google Scholar] [CrossRef]
- Lam, A.R.; Bert, N.L.; Ho, S.S.; Shen, Y.J.; Tang, L.F.; Xiong, G.M.; Croxford, J.L.; Koo, C.X.; Ishii, K.J.; Akira, S.; et al. RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res. 2014, 74, 2193–2203. [Google Scholar] [CrossRef] [Green Version]
- Borchers, M.T.; Harris, N.L.; Wesselkamper, S.C.; Vitucci, M.; Cosman, D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L222–L231. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fujiyama, Y.; Andoh, A.; Bamba, T.; Okabe, H. Oxidative stress increases MICA and MICB gene expression in the human colon carcinoma cell line (CaCO2). Biochim. Biophys. Acta 2001, 1526, 10–12. [Google Scholar] [CrossRef]
- Jung, H.; Hsiung, B.; Pestal, K.; Procyk, E.; Raulet, D.H. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J. Exp. Med. 2012, 209, 2409–2422. [Google Scholar] [CrossRef] [Green Version]
- Alkhayer, R.; Ponath, V.; Frech, M.; Adhikary, T.; Graumann, J.; Neubauer, A.; von Strandmann, E.P. KLF4-mediated upregulation of the NKG2D ligand MICA in acute myeloid leukemia: A novel therapeutic target identified by enChIP. Cell Commun. Signal. 2023, 21, 94. [Google Scholar] [CrossRef]
- Gowen, B.G.; Chim, B.; Marceau, C.D.; Greene, T.T.; Burr, P.; Gonzalez, J.R.; Hesser, C.R.; Dietzen, P.A.; Russell, T.; Iannello, A.; et al. A forward genetic screen reveals novel independent regulators of ULBP1, an activating ligand for natural killer cells. Elife 2015, 4, e08474. [Google Scholar] [CrossRef] [PubMed]
- Boissel, N.; Rea, D.; Tieng, V.; Dulphy, N.; Brun, M.; Cayuela, J.M.; Rousselot, P.; Tamouza, R.; Le Bouteiller, P.; Mahon, F.X.; et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J. Immunol. 2006, 176, 5108–5116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unni, A.M.; Bondar, T.; Medzhitov, R. Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proc. Natl. Acad. Sci. USA 2008, 105, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Nanbakhsh, A.; Pochon, C.; Mallavialle, A.; Amsellem, S.; Bourhis, J.H.; Chouaib, S. c-Myc regulates expression of NKG2D ligands ULBP1/2/3 in AML and modulates their susceptibility to NK-mediated lysis. Blood 2014, 123, 3585–3595. [Google Scholar] [CrossRef]
- Liu, X.V.; Ho, S.S.; Tan, J.J.; Kamran, N.; Gasser, S. Ras activation induces expression of Raet1 family NK receptor ligands. J. Immunol. 2012, 189, 1826–1834. [Google Scholar] [CrossRef] [Green Version]
- Okita, R.; Mougiakakos, D.; Ando, T.; Mao, Y.; Sarhan, D.; Wennerberg, E.; Seliger, B.; Lundqvist, A.; Mimura, K.; Kiessling, R. HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC class I chain-related molecule A and B expression in human breast cancer cell lines. J. Immunol. 2012, 188, 2136–2145. [Google Scholar] [CrossRef] [Green Version]
- Vantourout, P.; Willcox, C.; Turner, A.; Swanson, C.M.; Haque, Y.; Sobolev, O.; Grigoriadis, A.; Tutt, A.; Hayday, A. Immunological visibility: Posttranscriptional regulation of human NKG2D ligands by the EGF receptor pathway. Sci. Transl. Med. 2014, 6, 231ra249. [Google Scholar] [CrossRef]
- Andresen, L.; Skovbakke, S.L.; Persson, G.; Hagemann-Jensen, M.; Hansen, K.A.; Jensen, H.; Skov, S. 2-deoxy D-glucose prevents cell surface expression of NKG2D ligands through inhibition of N-linked glycosylation. J. Immunol. 2012, 188, 1847–1855. [Google Scholar] [CrossRef] [Green Version]
- Moller, S.H.; Mellergaard, M.; Madsen, M.; Bermejo, A.V.; Jepsen, S.D.; Hansen, M.H.; Hogh, R.I.; Aldana, B.I.; Desler, C.; Rasmussen, L.J.; et al. Cytoplasmic Citrate Flux Modulates the Immune Stimulatory NKG2D Ligand MICA in Cancer Cells. Front. Immunol. 2020, 11, 1968. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, B.J.; Guerrero-Gimenez, M.E.; Prince, T.L.; Ackerman, A.; Bonorino, C.; Calderwood, S.K. Heat Shock Proteins Are Essential Components in Transformation and Tumor Progression: Cancer Cell Intrinsic Pathways and Beyond. Int. J. Mol. Sci. 2019, 20, 4507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Williams, R.M.; Zhang, X. Roles of ATM and ATR in DNA double strand breaks and replication stress. Prog. Biophys. Mol. Biol. 2021, 163, 109–119. [Google Scholar] [CrossRef]
- Textor, S.; Fiegler, N.; Arnold, A.; Porgador, A.; Hofmann, T.G.; Cerwenka, A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011, 71, 5998–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nice, T.J.; Coscoy, L.; Raulet, D.H. Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J. Exp. Med. 2009, 206, 287–298. [Google Scholar] [CrossRef]
- Schuster, C.; Berger, A.; Hoelzl, M.A.; Putz, E.M.; Frenzel, A.; Simma, O.; Moritz, N.; Hoelbl, A.; Kovacic, B.; Freissmuth, M.; et al. The cooperating mutation or “second hit” determines the immunologic visibility toward MYC-induced murine lymphomas. Blood 2011, 118, 4635–4645. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhao, Z.; Xue, M.; Wang, D.; Su, G.; Ju, X.; Yang, Q.; Zhang, S.; Fan, D.; Zhu, H.; et al. Ciclopirox olamine sensitizes leukemia cells to natural killer cell-mediated cytolysis by upregulating NKG2DLs via the Akt signaling pathway. Biochem. Biophys. Res. Commun. 2023, 659, 10–19. [Google Scholar] [CrossRef]
- Sagiv, A.; Burton, D.G.; Moshayev, Z.; Vadai, E.; Wensveen, F.; Ben-Dor, S.; Golani, O.; Polic, B.; Krizhanovsky, V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 2016, 8, 328–344. [Google Scholar] [CrossRef] [Green Version]
- Domen, A.; Deben, C.; Verswyvel, J.; Flieswasser, T.; Prenen, H.; Peeters, M.; Lardon, F.; Wouters, A. Cellular senescence in cancer: Clinical detection and prognostic implications. J. Exp. Clin. Cancer Res. 2022, 41, 360. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, R.; Xi, B.; Nie, D.; Xu, H.; Liu, A. Mechanisms of Senescence-Related NKG2D Ligands Release and Immune Escape Induced by Chemotherapy in Neuroblastoma Cells. Front. Cell Dev. Biol. 2022, 10, 829404. [Google Scholar] [CrossRef] [PubMed]
- Obiedat, A.; Seidel, E.; Mahameed, M.; Berhani, O.; Tsukerman, P.; Voutetakis, K.; Chatziioannou, A.; McMahon, M.; Avril, T.; Chevet, E.; et al. Transcription of the NKG2D ligand MICA is suppressed by the IRE1/XBP1 pathway of the unfolded protein response through the regulation of E2F1. FASEB J. 2019, 33, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, K.E.; Hur, D.; Lim, J.S.; Yang, Y.; Cho, B.J.; Kim, C.H.; Kim, T.; Bang, S.; Lee, W.J.; et al. IL-18 enhances ULBP2 expression through the MAPK pathway in leukemia cells. Immunol. Lett. 2008, 120, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, J.A.; Ogasawara, K.; Lanier, L.L. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J. Immunol. 2004, 172, 2001–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwinn, N.; Vokhminova, D.; Sucker, A.; Textor, S.; Striegel, S.; Moll, I.; Nausch, N.; Tuettenberg, J.; Steinle, A.; Cerwenka, A.; et al. Interferon-gamma down-regulates NKG2D ligand expression and impairs the NKG2D-mediated cytolysis of MHC class I-deficient melanoma by natural killer cells. Int. J. Cancer 2009, 124, 1594–1604. [Google Scholar] [CrossRef]
- Yadav, D.; Ngolab, J.; Lim, R.S.; Krishnamurthy, S.; Bui, J.D. Cutting edge: Down-regulation of MHC class I-related chain A on tumor cells by IFN-gamma-induced microRNA. J. Immunol. 2009, 182, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Niu, J.; Zhang, J.; Wang, Y.; Zhou, Z.; Zhang, J.; Tian, Z. Opposing effects of interferon-alpha and interferon-gamma on the expression of major histocompatibility complex class I chain-related A in tumors. Cancer Sci. 2008, 99, 1279–1286. [Google Scholar] [CrossRef]
- Bui, J.D.; Carayannopoulos, L.N.; Lanier, L.L.; Yokoyama, W.M.; Schreiber, R.D. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J. Immunol. 2006, 176, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Bedel, R.; Thiery-Vuillemin, A.; Grandclement, C.; Balland, J.; Remy-Martin, J.P.; Kantelip, B.; Pallandre, J.R.; Pivot, X.; Ferrand, C.; Tiberghien, P.; et al. Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res. 2011, 71, 1615–1626. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lu, X.; Jia, Z.; Zhang, X.; Han, W.; Rong, X.; Ma, L.; Zhou, M.; Chen, B. STAT3 contributes to NK cell recognition by modulating expression of NKG2D ligands in adriamycin-resistant K562/AO2 cells. Int. J. Hematol. 2015, 102, 536–543. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Shen, M.; Yang, D.R.; Fang, L.; Weng, G.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Inhibition of IL-6-JAK/Stat3 signaling in castration-resistant prostate cancer cells enhances the NK cell-mediated cytotoxicity via alteration of PD-L1/NKG2D ligand levels. Mol. Oncol. 2018, 12, 269–286. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.E.; Menares-Castillo, E.; Garrido-Tapia, M.; Ribeiro, C.H.; Hernandez, C.J.; Mendoza-Naranjo, A.; Gatica-Andrades, M.; Valenzuela-Diaz, R.; Zuniga, R.; Lopez, M.N.; et al. Interleukin 10 decreases MICA expression on melanoma cell surface. Immunol. Cell Biol. 2011, 89, 447–457. [Google Scholar] [CrossRef]
- Schulz, U.; Kreutz, M.; Multhoff, G.; Stoelcker, B.; Kohler, M.; Andreesen, R.; Holler, E. Interleukin-10 promotes NK cell killing of autologous macrophages by stimulating expression of NKG2D ligands. Scand. J. Immunol. 2010, 72, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Eisele, G.; Wischhusen, J.; Mittelbronn, M.; Meyermann, R.; Waldhauer, I.; Steinle, A.; Weller, M.; Friese, M.A. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain 2006, 129, 2416–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, T.L.; Kandell, W.M.; Donatelli, S.S.; Tu, N.; Tejera, M.M.; Gilvary, D.L.; Eksioglu, E.A.; Burnette, A.; Adams, W.A.; Liu, J.; et al. Immune evasion by TGFbeta-induced miR-183 repression of MICA/B expression in human lung tumor cells. Oncoimmunology 2019, 8, e1557372. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Soto, A.; Huergo-Zapico, L.; Galvan, J.A.; Rodrigo, L.; de Herreros, A.G.; Astudillo, A.; Gonzalez, S. Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J. Immunol. 2013, 190, 4408–4419. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Tian, X.; Li, Y.; Liu, Y.; Yang, T.; Han, Z.; An, J.; Kong, L.; Li, Y. Epithelial-mesenchymal transition may be involved in the immune evasion of circulating gastric tumor cells via downregulation of ULBP1. Cancer Med. 2020, 9, 2686–2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, C.A.; Austgen, K.; Haberthur, K.; Hofmann, C.; Moyes, K.W.; Avanesyan, L.; Fong, L.; Campbell, M.J.; Cooper, S.; Oakes, S.A.; et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc. Natl. Acad. Sci. USA 2014, 111, 12823–12828. [Google Scholar] [CrossRef]
- Agaugue, S.; Hargreaves, A.; Gilham, D.E. The high expression of NKG2D ligands on tumor and non-tumor cells and a lack of surface expression on healthy tissues provide a strong rationale to support NKG2D-based therapeutic approaches for cancer. Ann. Oncol. 2019, 29. Abstract 1179P (Poster presentation). [Google Scholar]
- Wang, B.; Wang, Q.; Wang, Z.; Jiang, J.; Yu, S.C.; Ping, Y.F.; Yang, J.; Xu, S.L.; Ye, X.Z.; Xu, C.; et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014, 74, 5746–5757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paczulla, A.M.; Rothfelder, K.; Raffel, S.; Konantz, M.; Steinbacher, J.; Wang, H.; Tandler, C.; Mbarga, M.; Schaefer, T.; Falcone, M.; et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 2019, 572, 254–259. [Google Scholar] [CrossRef]
- Zhang, X.; Rao, A.; Sette, P.; Deibert, C.; Pomerantz, A.; Kim, W.J.; Kohanbash, G.; Chang, Y.; Park, Y.; Engh, J.; et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro-Oncology 2016, 18, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Fluh, C.; Chitadze, G.; Adamski, V.; Hattermann, K.; Synowitz, M.; Kabelitz, D.; Held-Feindt, J. NKG2D ligands in glioma stem-like cells: Expression in situ and in vitro. Histochem. Cell Biol. 2018, 149, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Chen, M.; Smith, R.C.; Hagino, T.; Perez-Cunningham, J.; Sckisel, G.D.; Urayama, S.; et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J. Immunol. 2015, 195, 4010–4019. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell. Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Yang, J.I.; Kim, O.; Ahn, E.J.; Kang, W.D.; Lee, J.H.; Moon, K.S.; Lee, K.H.; Cho, D. Human U87 glioblastoma cells with stemness features display enhanced sensitivity to natural killer cell cytotoxicity through altered expression of NKG2D ligand. Cancer Cell Int. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Basher, F.; Wu, J.D. NKG2D Ligands in Tumor Immunity: Two Sides of a Coin. Front. Immunol. 2015, 6, 97. [Google Scholar] [CrossRef]
- Morimoto, Y.; Yamashita, N.; Daimon, T.; Hirose, H.; Yamano, S.; Haratake, N.; Ishikawa, S.; Bhattacharya, A.; Fushimi, A.; Ahmad, R.; et al. MUC1-C is a master regulator of MICA/B NKG2D ligand and exosome secretion in human cancer cells. J. Immunother. Cancer 2023, 11, e006238. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Ferrari de Andrade, L. NKG2D and MICA/B shedding: A ‘tag game’ between NK cells and malignant cells. Clin. Transl. Immunol. 2020, 9, e1230. [Google Scholar] [CrossRef]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef]
- Chitadze, G.; Kabelitz, D. Immune surveillance in glioblastoma: Role of the NKG2D system and novel cell-based therapeutic approaches. Scand. J. Immunol. 2022, 96, e13201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, F.; Dong, K. Soluble NKG2D ligands impair CD8(+) T cell antitumor function dependent of NKG2D downregulation in neuroblastoma. Oncol. Lett. 2023, 26, 297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Pan, K.; Gu, M.F.; Chen, M.S.; Zhao, J.J.; Wang, H.; Liang, X.T.; Sun, J.C.; Xia, J.C. Prognostic value of soluble MICA levels in the serum of patients with advanced hepatocellular carcinoma. Chin. J. Cancer 2013, 32, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kegasawa, T.; Tatsumi, T.; Yoshioka, T.; Suda, T.; Ikezawa, K.; Nakabori, T.; Yamada, R.; Kodama, T.; Shigekawa, M.; Hikita, H.; et al. Soluble UL16-binding protein 2 is associated with a poor prognosis in pancreatic cancer patients. Biochem. Biophys. Res. Commun. 2019, 517, 84–88. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Zhu, X.X. Abnormal expression levels of sMICA and NKG2D are correlated with poor prognosis in pancreatic cancer. Ther. Clin. Risk Manag. 2016, 12, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Vyas, M.; Reinartz, S.; Hoffmann, N.; Reiners, K.S.; Lieber, S.; Jansen, J.M.; Wagner, U.; Muller, R.; von Strandmann, E.P. Soluble NKG2D ligands in the ovarian cancer microenvironment are associated with an adverse clinical outcome and decreased memory effector T cells independent of NKG2D downregulation. Oncoimmunology 2017, 6, e1339854. [Google Scholar] [CrossRef] [Green Version]
- Jinushi, M.; Hodi, F.S.; Dranoff, G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2006, 103, 9190–9195. [Google Scholar] [CrossRef]
- Salih, H.R.; Rammensee, H.G.; Steinle, A. Cutting edge: Down-regulation of MICA on human tumors by proteolytic shedding. J. Immunol. 2002, 169, 4098–4102. [Google Scholar] [CrossRef] [Green Version]
- Chitadze, G.; Lettau, M.; Bhat, J.; Wesch, D.; Steinle, A.; Furst, D.; Mytilineos, J.; Kalthoff, H.; Janssen, O.; Oberg, H.H.; et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: Heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int. J. Cancer 2013, 133, 1557–1566. [Google Scholar] [CrossRef]
- Fernandez-Messina, L.; Ashiru, O.; Boutet, P.; Aguera-Gonzalez, S.; Skepper, J.N.; Reyburn, H.T.; Vales-Gomez, M. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J. Biol. Chem. 2010, 285, 8543–8551. [Google Scholar] [CrossRef] [Green Version]
- Bernareggi, D.; Xie, Q.; Prager, B.C.; Yun, J.; Cruz, L.S.; Pham, T.V.; Kim, W.; Lee, X.; Coffey, M.; Zalfa, C.; et al. CHMP2A regulates tumor sensitivity to natural killer cell-mediated cytotoxicity. Nat. Commun. 2022, 13, 1899. [Google Scholar] [CrossRef]
- Campos-Silva, C.; Lopez-Borrego, S.; Felgueres, M.J.; Esteso, G.; Vales-Gomez, M. NKG2D Ligands in Liquid Biopsy: The Importance of Soluble and Vesicle-Bound Proteins for Immune Modulation. Crit. Rev. Immunol. 2022, 42, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Girart, M.V.; Molinero, L.L.; Domaica, C.I.; Rossi, L.E.; Barrio, M.M.; Mordoh, J.; Rabinovich, G.A.; Zwirner, N.W. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J. Immunol. 2008, 180, 4606–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champsaur, M.; Lanier, L.L. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 2010, 235, 267–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chen, L.; Baker, K.; Olszak, T.; Zeissig, S.; Huang, Y.H.; Kuo, T.T.; Mandelboim, O.; Beauchemin, N.; Lanier, L.L.; et al. CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J. Exp. Med. 2011, 208, 2633–2640. [Google Scholar] [CrossRef]
- Petillo, S.; Capuano, C.; Molfetta, R.; Fionda, C.; Mekhloufi, A.; Pighi, C.; Antonangeli, F.; Zingoni, A.; Soriani, A.; Petrucci, M.T.; et al. Immunomodulatory effect of NEDD8-activating enzyme inhibition in Multiple Myeloma: Upregulation of NKG2D ligands and sensitization to Natural Killer cell recognition. Cell Death Dis. 2021, 12, 836. [Google Scholar] [CrossRef]
- Domaica, C.I.; Fuertes, M.B.; Rossi, L.E.; Girart, M.V.; Avila, D.E.; Rabinovich, G.A.; Zwirner, N.W. Tumour-experienced T cells promote NK cell activity through trogocytosis of NKG2D and NKp46 ligands. EMBO Rep. 2009, 10, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Nakayama, M.; Kawano, M.; Amagai, R.; Ishii, T.; Harigae, H.; Ogasawara, K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl. Acad. Sci. USA 2013, 110, 9421–9426. [Google Scholar] [CrossRef]
- Sheppard, S.; Ferry, A.; Guedes, J.; Guerra, N. The Paradoxical Role of NKG2D in Cancer Immunity. Front. Immunol. 2018, 9, 1808. [Google Scholar] [CrossRef]
- Cerwenka, A.; Baron, J.L.; Lanier, L.L. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 11521–11526. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, A.; Jensen, E.R.; Jamieson, A.M.; Raulet, D.H. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 2001, 413, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, N.; Tan, Y.X.; Joncker, N.T.; Choy, A.; Gallardo, F.; Xiong, N.; Knoblaugh, S.; Cado, D.; Greenberg, N.M.; Raulet, D.H. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, M.J.; Swann, J.; Cretney, E.; Zerafa, N.; Yokoyama, W.M.; Hayakawa, Y. NKG2D function protects the host from tumor initiation. J. Exp. Med. 2005, 202, 583–588. [Google Scholar] [CrossRef]
- Liu, G.; Lu, S.; Wang, X.; Page, S.T.; Higano, C.S.; Plymate, S.R.; Greenberg, N.M.; Sun, S.; Li, Z.; Wu, J.D. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J. Clin. Investig. 2013, 123, 4410–4422. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, N.; Yu, Y.; Zhou, L.; Niu, C.; Liu, Y.; Tian, H.; Lv, Z.; Han, F.; Cui, J. Prognostic value of MICA/B in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 96384–96395. [Google Scholar] [CrossRef] [Green Version]
- Babic, M.; Romagnani, C. The Role of Natural Killer Group 2, Member D in Chronic Inflammation and Autoimmunity. Front. Immunol. 2018, 9, 1219. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, D.E.; Roberts, S.J.; Clarke, S.L.; Filler, R.; Lewis, J.M.; Tigelaar, R.E.; Girardi, M.; Hayday, A.C. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005, 6, 928–937. [Google Scholar] [CrossRef]
- Coudert, J.D.; Zimmer, J.; Tomasello, E.; Cebecauer, M.; Colonna, M.; Vivier, E.; Held, W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood 2005, 106, 1711–1717. [Google Scholar] [CrossRef] [Green Version]
- Coudert, J.D.; Scarpellino, L.; Gros, F.; Vivier, E.; Held, W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 2008, 111, 3571–3578. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, S.; Guedes, J.; Mroz, A.; Zavitsanou, A.M.; Kudo, H.; Rothery, S.M.; Angelopoulos, P.; Goldin, R.; Guerra, N. The immunoreceptor NKG2D promotes tumour growth in a model of hepatocellular carcinoma. Nat. Commun. 2017, 8, 13930. [Google Scholar] [CrossRef]
- Curio, S.; Lin, W.; Bromley, C.; McGovern, J.; Triulzi, C.; Jonsson, G.; Ghislat, G.; Zelenay, S.; Guerra, N. NKG2D Fine-Tunes the Local Inflammatory Response in Colorectal Cancer. Cancers 2023, 15, 1792. [Google Scholar] [CrossRef] [PubMed]
- Rohner, A.; Langenkamp, U.; Siegler, U.; Kalberer, C.P.; Wodnar-Filipowicz, A. Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk. Res. 2007, 31, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Armeanu, S.; Bitzer, M.; Lauer, U.M.; Venturelli, S.; Pathil, A.; Krusch, M.; Kaiser, S.; Jobst, J.; Smirnow, I.; Wagner, A.; et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005, 65, 6321–6329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, N.; Tanaka, J.; Sugita, J.; Toubai, T.; Miura, Y.; Ibata, M.; Syono, Y.; Ota, S.; Kondo, T.; Asaka, M.; et al. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia 2007, 21, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Vales-Gomez, M.; Chisholm, S.E.; Cassady-Cain, R.L.; Roda-Navarro, P.; Reyburn, H.T. Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res. 2008, 68, 1546–1554. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, X.; Jiang, L.; Sun, X.; Zhou, H.; Jia, Z.; Zhang, X.; Ma, L. STAT3 signaling pathway is involved in decitabine induced biological phenotype regulation of acute myeloid leukemia cells. Am. J. Transl. Res. 2015, 7, 1896–1907. [Google Scholar]
- Okita, R.; Yukawa, T.; Nojima, Y.; Maeda, A.; Saisho, S.; Shimizu, K.; Nakata, M. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2016, 65, 499–509. [Google Scholar] [CrossRef]
- Abruzzese, M.P.; Bilotta, M.T.; Fionda, C.; Zingoni, A.; Soriani, A.; Vulpis, E.; Borrelli, C.; Zitti, B.; Petrucci, M.T.; Ricciardi, M.R.; et al. Inhibition of bromodomain and extra-terminal (BET) proteins increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: Role of cMYC-IRF4-miR-125b interplay. J. Hematol. Oncol. 2016, 9, 134. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, X.T.; Guo, K.Y.; Li, Y.H.; Wu, B.Y.; Song, C.Y.; He, Y.J. Sunitinib Induces NK-kappaB-dependent NKG2D Ligand Expression in Nasopharyngeal Carcinoma and Hepatoma Cells. J. Immunother. 2017, 40, 164–174. [Google Scholar] [CrossRef]
- McCarthy, M.T.; Lin, D.; Soga, T.; Adam, J.; O’Callaghan, C.A. Inosine pranobex enhances human NK cell cytotoxicity by inducing metabolic activation and NKG2D ligand expression. Eur. J. Immunol. 2020, 50, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veneziani, I.; Infante, P.; Ferretti, E.; Melaiu, O.; Battistelli, C.; Lucarini, V.; Compagnone, M.; Nicoletti, C.; Castellano, A.; Petrini, S.; et al. Nutlin-3a Enhances Natural Killer Cell-Mediated Killing of Neuroblastoma by Restoring p53-Dependent Expression of Ligands for NKG2D and DNAM-1 Receptors. Cancer Immunol. Res. 2021, 9, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Catellani, S.; Garuti, A.; Pierri, I.; Gobbi, M.; Zocchi, M.R. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia 2009, 23, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Tajima, H.; Shoji, M.; Nakanuma, S.; Sakai, S.; Makino, I.; Hayashi, H.; Nakagawara, H.; Takamura, H.; Kitagawa, H.; et al. Gemcitabine augments major histocompatibility complex class I-related chain A expression in pancreatic cancer. Gan Kagaku Ryoho Cancer Chemother. 2013, 40, 1600–1602. [Google Scholar]
- Lin, X.; Huang, M.; Xie, F.; Zhou, H.; Yang, J.; Huang, Q. Gemcitabine inhibits immune escape of pancreatic cancer by down regulating the soluble ULBP2 protein. Oncotarget 2016, 7, 70092–70099. [Google Scholar] [CrossRef] [Green Version]
- Ferrari de Andrade, L.; Tay, R.E.; Pan, D.; Luoma, A.M.; Ito, Y.; Badrinath, S.; Tsoucas, D.; Franz, B.; May, K.F., Jr.; Harvey, C.J.; et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 2018, 359, 1537–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, H.; Yamagiwa, S.; Tsuchiya, A.; Takamura, M.; Matsuda, Y.; Ohkoshi, S.; Inoue, M.; Wakai, T.; Shirai, Y.; Nomoto, M.; et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J. Hepatol. 2012, 56, 381–388. [Google Scholar] [CrossRef]
- Sroijak, N.; Ponglikitmongkol, M. 17beta-Estradiol suppresses MHC class I chain-related B gene expression via an intact GC box. Biochem. Cell Biol. 2013, 91, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Pioli, P.A.; Conejo-Garcia, J.; Wira, C.R.; Sentman, C.L. Estradiol regulates MICA expression in human endometrial cells. Clin. Immunol. 2008, 129, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Nie, Y.; Lv, M.; Shen, S.; Tang, R.; Xu, Y.; Hou, Y.; Zhao, S.; Wang, T. Estrogen upregulates MICA/B expression in human non-small cell lung cancer through the regulation of ADAM17. Cell. Mol. Immunol. 2015, 12, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Gong, J.; Wang, Y.; Liu, R.; Li, Z.; Wang, Z.; Zhang, Y.; Zhang, C.; Song, C.; Yang, A.; et al. MICA/B expression is inhibited by unfolded protein response and associated with poor prognosis in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2014, 33, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Li, W.; Zhelev, D.V.; Mellors, J.W.; Dimitrov, D.S.; Baek, D.S. Chimeric antigen receptor-T cells are effective against CEACAM5 expressing non-small cell lung cancer cells resistant to antibody-drug conjugates. Front. Oncol. 2023, 13, 1124039. [Google Scholar] [CrossRef] [PubMed]
- Frazao, A.; Colombo, M.; Fourmentraux-Neves, E.; Messaoudene, M.; Rusakiewicz, S.; Zitvogel, L.; Vivier, E.; Vely, F.; Faure, F.; Dreno, B.; et al. Shifting the Balance of Activating and Inhibitory Natural Killer Receptor Ligands on BRAF(V600E) Melanoma Lines with Vemurafenib. Cancer Immunol. Res. 2017, 5, 582–593. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Bai, Y.; Lu, X.; Ding, J.; Qi, C. Rapamycin downregulates NKG2D ligands in acute myeloid leukemia cells via an activation of the STAT3 pathway: A potential mechanism for rapamycin-induced immune escape in leukemia. Transl. Cancer Res. 2019, 8, 473–482. [Google Scholar] [CrossRef]
- Xu, X.; Rao, G.; Li, Y. Xanthine oxidoreductase is required for genotoxic stress-induced NKG2D ligand expression and gemcitabine-mediated antitumor activity. Oncotarget 2016, 7, 59220–59235. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, K.; Xiao, T.; Liu, P.; Prinz, R.A.; Xu, X. Uric acid accumulation in DNA-damaged tumor cells induces NKG2D ligand expression and antitumor immunity by activating TGF-beta-activated kinase 1. Oncoimmunology 2022, 11, 2016159. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Hatanaka, Y.; Sutoh, Y.; Suzuki, Y.; Oba, K.; Hatanaka, K.C.; Mitsuhashi, T.; Otsuka, N.; Fugo, K.; Kasahara, M.; et al. Immunohistochemical validation and expression profiling of NKG2D ligands in a wide spectrum of human epithelial neoplasms. J. Histochem. Cytochem. 2015, 63, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Watson, N.F.; Spendlove, I.; Madjd, Z.; McGilvray, R.; Green, A.R.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int. J. Cancer 2006, 118, 1445–1452. [Google Scholar] [CrossRef]
- McGilvray, R.W.; Eagle, R.A.; Watson, N.F.; Al-Attar, A.; Ball, G.; Jafferji, I.; Trowsdale, J.; Durrant, L.G. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin. Cancer Res. 2009, 15, 6993–7002. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, I.; Agarwal, S.; Sakiyama, M.; Shenoy, V.; Orr, W.S.; Diffalha, S.A.; Prizment, A.; Varambally, S.; Manne, U.; Gomez, C.R. Expression of MHC class I polypeptide-related sequence A (MICA) in colorectal cancer. Front. Biosci. 2021, 26, 765–776. [Google Scholar] [CrossRef]
- Ruan, G.T.; Xie, H.L.; Zhu, L.C.; Ge, Y.Z.; Yan, L.; Liao, C.; Gong, Y.Z.; Shi, H.P. Immune ULBP1 is Elevated in Colon Adenocarcinoma and Predicts Prognosis. Front. Genet. 2022, 13, 762514. [Google Scholar] [CrossRef] [PubMed]
- Madjd, Z.; Spendlove, I.; Moss, R.; Bevin, S.; Pinder, S.E.; Watson, N.F.; Ellis, I.; Durrant, L.G. Upregulation of MICA on high-grade invasive operable breast carcinoma. Cancer Immun. 2007, 7, 17. [Google Scholar] [PubMed]
- de Kruijf, E.M.; Sajet, A.; van Nes, J.G.; Putter, H.; Smit, V.T.; Eagle, R.A.; Jafferji, I.; Trowsdale, J.; Liefers, G.J.; van de Velde, C.J.; et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: An observational study. BMC Cancer 2012, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lin, G.; Guo, Z.Q.; Zhou, Z.F.; He, Z.Y.; Ye, Y.B. Effects of MICA expression on the prognosis of advanced non-small cell lung cancer and the efficacy of CIK therapy. PLoS ONE 2013, 8, e69044. [Google Scholar] [CrossRef] [PubMed]
- McGilvray, R.W.; Eagle, R.A.; Rolland, P.; Jafferji, I.; Trowsdale, J.; Durrant, L.G. ULBP2 and RAET1E NKG2D ligands are independent predictors of poor prognosis in ovarian cancer patients. Int. J. Cancer 2010, 127, 1412–1420. [Google Scholar] [CrossRef]
- Li, K.; Mandai, M.; Hamanishi, J.; Matsumura, N.; Suzuki, A.; Yagi, H.; Yamaguchi, K.; Baba, T.; Fujii, S.; Konishi, I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: High expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol. Immunother. 2009, 58, 641–652. [Google Scholar] [CrossRef]
- Paschen, A.; Sucker, A.; Hill, B.; Moll, I.; Zapatka, M.; Nguyen, X.D.; Sim, G.C.; Gutmann, I.; Hassel, J.; Becker, J.C.; et al. Differential clinical significance of individual NKG2D ligands in melanoma: Soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin. Cancer Res. 2009, 15, 5208–5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuckel, H.; Switala, M.; Sellmann, L.; Horn, P.A.; Durig, J.; Duhrsen, U.; Kuppers, R.; Grosse-Wilde, H.; Rebmann, V. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia 2010, 24, 1152–1159. [Google Scholar] [CrossRef]
- Kumar, P.; Ranmale, S.; Mehta, S.; Tongaonkar, H.; Patel, V.; Singh, A.K.; Mania-Pramanik, J. Immune profile of primary and recurrent epithelial ovarian cancer cases indicates immune suppression, a major cause of progression and relapse of ovarian cancer. J. Ovarian Res. 2023, 16, 114. [Google Scholar] [CrossRef]
- Cho, H.; Chung, J.Y.; Kim, S.; Braunschweig, T.; Kang, T.H.; Kim, J.; Chung, E.J.; Hewitt, S.M.; Kim, J.H. MICA/B and ULBP1 NKG2D ligands are independent predictors of good prognosis in cervical cancer. BMC Cancer 2014, 14, 957. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhou, L.; Zong, J.; Ye, Y.; Chen, G.; Chen, Y.; Liao, X.; Guo, Q.; Qiu, S.; Lin, S.; et al. Decreased expression of the NKG2D ligand ULBP4 may be an indicator of poor prognosis in patients with nasopharyngeal carcinoma. Oncotarget 2017, 8, 42007–42019. [Google Scholar] [CrossRef]
- Cadoux, M.; Caruso, S.; Pham, S.; Gougelet, A.; Pophillat, C.; Riou, R.; Loesch, R.; Colnot, S.; Nguyen, C.T.; Calderaro, J.; et al. Expression of NKG2D ligands is downregulated by beta-catenin signalling and associates with HCC aggressiveness. J. Hepatol. 2021, 74, 1386–1397. [Google Scholar] [CrossRef]
- Vetter, C.S.; Groh, V.; thor Straten, P.; Spies, T.; Brocker, E.B.; Becker, J.C. Expression of stress-induced MHC class I related chain molecules on human melanoma. J. Investig. Dermatol. 2002, 118, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Su, T.; He, L.; Wang, H.; Ji, G.; Liu, X.; Zhang, Y.; Dong, G. Identification and functional analysis of ligands for natural killer cell activating receptors in colon carcinoma. Tohoku J. Exp. Med. 2012, 226, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, J.; Grosse-Hovest, L.; Grunebach, F.; Buechele, C.; Nuebling, T.; Raum, T.; Steinle, A.; Salih, H.R. Comprehensive analysis of NKG2D ligand expression and release in leukemia: Implications for NKG2D-mediated NK cell responses. J. Immunol. 2012, 189, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Correa, B.; Morgado, S.; Gayoso, I.; Bergua, J.M.; Casado, J.G.; Arcos, M.J.; Bengochea, M.L.; Duran, E.; Solana, R.; Tarazona, R. Human NK cells in acute myeloid leukaemia patients: Analysis of NK cell-activating receptors and their ligands. Cancer Immunol. Immunother. 2011, 60, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Spaggiari, G.M.; Marcenaro, S.; Martini, S.; Rivera, P.; Capobianco, A.; Falco, M.; Lanino, E.; Pierri, I.; Zambello, R.; et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 2005, 105, 2066–2073. [Google Scholar] [CrossRef]
- Diermayr, S.; Himmelreich, H.; Durovic, B.; Mathys-Schneeberger, A.; Siegler, U.; Langenkamp, U.; Hofsteenge, J.; Gratwohl, A.; Tichelli, A.; Paluszewska, M.; et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood 2008, 111, 1428–1436. [Google Scholar] [CrossRef]
- Schlegel, P.; Ditthard, K.; Lang, P.; Mezger, M.; Michaelis, S.; Handgretinger, R.; Pfeiffer, M. NKG2D Signaling Leads to NK Cell Mediated Lysis of Childhood AML. J. Immunol. Res. 2015, 2015, 473175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salih, H.R.; Antropius, H.; Gieseke, F.; Lutz, S.Z.; Kanz, L.; Rammensee, H.G.; Steinle, A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 2003, 102, 1389–1396. [Google Scholar] [CrossRef] [Green Version]
- Driouk, L.; Gicobi, J.K.; Kamihara, Y.; Rutherford, K.; Dranoff, G.; Ritz, J.; Baumeister, S.H.C. Chimeric Antigen Receptor T Cells Targeting NKG2D-Ligands Show Robust Efficacy Against Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Front. Immunol. 2020, 11, 580328. [Google Scholar] [CrossRef]
- Crane, C.A.; Han, S.J.; Barry, J.J.; Ahn, B.J.; Lanier, L.L.; Parsa, A.T. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-Oncology 2010, 12, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberthur, K.; Brennan, K.; Hoglund, V.; Balcaitis, S.; Chinn, H.; Davis, A.; Kreuser, S.; Winter, C.; Leary, S.E.; Deutsch, G.H.; et al. NKG2D ligand expression in pediatric brain tumors. Cancer Biol. Ther. 2016, 17, 1253–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffaghello, L.; Prigione, I.; Airoldi, I.; Camoriano, M.; Levreri, I.; Gambini, C.; Pende, D.; Steinle, A.; Ferrone, S.; Pistoia, V. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004, 6, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Tsukagoshi, M.; Wada, S.; Yokobori, T.; Altan, B.; Ishii, N.; Watanabe, A.; Kubo, N.; Saito, F.; Araki, K.; Suzuki, H.; et al. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci. 2016, 107, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; An, H.J.; Kim, T.H.; Kim, G.; Park, K.S.; Park, H.; Lee, T.H.; Kwon, A.Y. Clinical Impact of Natural Killer Group 2D Receptor Expression and That of Its Ligand in Ovarian Carcinomas: A Retrospective Study. Yonsei Med. J. 2021, 62, 288–297. [Google Scholar] [CrossRef]
- Okita, R.; Maeda, A.; Shimizu, K.; Nojima, Y.; Saisho, S.; Nakata, M. Clinicopathological relevance of tumor expression of NK group 2 member D ligands in resected non-small cell lung cancer. Oncotarget 2019, 10, 6805–6815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busche, A.; Goldmann, T.; Naumann, U.; Steinle, A.; Brandau, S. Natural killer cell-mediated rejection of experimental human lung cancer by genetic overexpression of major histocompatibility complex class I chain-related gene A. Hum. Gene Ther. 2006, 17, 135–146. [Google Scholar] [CrossRef]
- Ribeiro, C.H.; Kramm, K.; Galvez-Jiron, F.; Pola, V.; Bustamante, M.; Contreras, H.R.; Sabag, A.; Garrido-Tapia, M.; Hernandez, C.J.; Zuniga, R.; et al. Clinical significance of tumor expression of major histocompatibility complex class I-related chains A and B (MICA/B) in gastric cancer patients. Oncol. Rep. 2016, 35, 1309–1317. [Google Scholar] [CrossRef] [Green Version]
- Kamei, R.; Yoshimura, K.; Yoshino, S.; Inoue, M.; Asao, T.; Fuse, M.; Wada, S.; Kuramasu, A.; Furuya-Kondo, T.; Oga, A.; et al. Expression levels of UL16 binding protein 1 and natural killer group 2 member D affect overall survival in patients with gastric cancer following gastrectomy. Oncol. Lett. 2018, 15, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.D.; Higgins, L.M.; Steinle, A.; Cosman, D.; Haugk, K.; Plymate, S.R. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J. Clin. Investig. 2004, 114, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yan, L.; Jiao, W.; Ren, J.; Xing, N.; Zhang, Y.; Zang, Y.; Wang, J.; Xu, Z. The clinical and biological significance of MICA in clear cell renal cell carcinoma patients. Tumour Biol. 2016, 37, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.Y.; Zhang, J.F.; Hu, G.M.; Li, Q.R.; Liu, P.P.; Du, Y. Upregulated expression of NKG2D and its ligands give potential therapeutic targets for patients with thymoma. Cancer Gene Ther. 2015, 22, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Takehara, T.; Tatsumi, T.; Kanto, T.; Groh, V.; Spies, T.; Kimura, R.; Miyagi, T.; Mochizuki, K.; Sasaki, Y.; et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int. J. Cancer 2003, 104, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Z.; Zhou, X.; Zhang, H.; Yang, N.; Wu, Y.; Chen, Y.; Yang, G.; Ren, T. Loss of expression of MHC class I-related chain A (MICA) is a frequent event and predicts poor survival in patients with hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 3123–3131. [Google Scholar]
- Duan, X.; Deng, L.; Chen, X.; Lu, Y.; Zhang, Q.; Zhang, K.; Hu, Y.; Zeng, J.; Sun, W. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med. Oncol. 2011, 28, 466–474. [Google Scholar] [CrossRef]
- Dambrauskas, Z.; Svensson, H.; Joshi, M.; Hyltander, A.; Naredi, P.; Iresjo, B.M. Expression of major histocompatibility complex class I-related chain A/B (MICA/B) in pancreatic carcinoma. Int. J. Oncol. 2014, 44, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Caballero-Benitez, A.; Gewe, M.M.; Jenkins, I.C.; Drescher, C.W.; Strong, R.K.; Spies, T.; Groh, V. Control of Tumor Initiation by NKG2D Naturally Expressed on Ovarian Cancer Cells. Neoplasia 2017, 19, 471–482. [Google Scholar] [CrossRef]
- Benitez, A.C.; Dai, Z.; Mann, H.H.; Reeves, R.S.; Margineantu, D.H.; Gooley, T.A.; Groh, V.; Spies, T. Expression, signaling proficiency, and stimulatory function of the NKG2D lymphocyte receptor in human cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4081–4086. [Google Scholar] [CrossRef]
- Cai, X.; Dai, Z.; Reeves, R.S.; Caballero-Benitez, A.; Duran, K.L.; Delrow, J.J.; Porter, P.L.; Spies, T.; Groh, V. Autonomous stimulation of cancer cell plasticity by the human NKG2D lymphocyte receptor coexpressed with its ligands on cancer cells. PLoS ONE 2014, 9, e108942. [Google Scholar] [CrossRef]
- Zuo, J.; Mohammed, F.; Moss, P. The Biological Influence and Clinical Relevance of Polymorphism Within the NKG2D Ligands. Front. Immunol. 2018, 9, 1820. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Imai, K.; Morishita, Y.; Hayashi, I.; Kusunoki, Y.; Nakachi, K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 2006, 66, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, G.; Spillane, K.M.; Maher, J. The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer. Biology 2023, 12, 1079. https://doi.org/10.3390/biology12081079
Tan G, Spillane KM, Maher J. The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer. Biology. 2023; 12(8):1079. https://doi.org/10.3390/biology12081079
Chicago/Turabian StyleTan, Ge, Katelyn M. Spillane, and John Maher. 2023. "The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer" Biology 12, no. 8: 1079. https://doi.org/10.3390/biology12081079
APA StyleTan, G., Spillane, K. M., & Maher, J. (2023). The Role and Regulation of the NKG2D/NKG2D Ligand System in Cancer. Biology, 12(8), 1079. https://doi.org/10.3390/biology12081079