The Relevance of Time in Biological Scaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Major Ways That Time May Be Relevant in Biological Scaling
2.1. Time and Scaling of Biological Rates and Durations
2.2. Is Time a Legitimate Fourth Dimension in Biological Scaling?
2.2.1. The Scaling of Biological Rates and Durations Are Diverse and Do Not Necessarily Follow Quarter-Power Scaling
2.2.2. Biological Time Is Not an Independent Fourth Dimension Commensurate with Spatial Dimensions
2.2.3. Biological Time Does Not Follow a “Universal Clock” but Varies with Body Size, Age, Temperature, and Type of Process, Organ, or Tissue
- (1)
- Avian incubation and fledgling periods scale differently with body mass [141], as do the durations of gestation and lactation in primates [142], gestation time, weaning time, age at first reproduction, and life span in marsupials [92], gestation time and life span in mammals [32], and life span and age at maturity in birds, mammals, and orthopteran insects [33,118].
- (2)
- Genetic and hormonal influences and various environmental factors can dissociate the rates and timing of metabolism, growth, maturation and (or) life expectancy [28,140,145]. For example, changes in temperature can dissociate the rates of growth and maturation, thus causing the well-known temperature-size rule in ectotherms [150,151,155].
- (3)
- Differences in age-specific mortality, as caused by artificial selection, can produce changes in various life-history traits, such as growth rate and developmental time, without associated changes in metabolic rate (e.g., [161]).
- (4)
- The developmental growth rates of different organs or structures are often unequal (e.g., [1,136,162]). These differences appear to be the result of multiple local regulatory mechanisms [28,163,164,165], and they can be accentuated by experimental manipulation or artificial selection experiments (e.g., [166,167,168]). Disproportionate or discordant variation in the rates and timing of the growth and development of various parts of an organism is so common that it has been recognized by widely used specific biological terms, such as “allometry”, “relative growth”, and “heterochrony”, and has been reviewed in major synthetic books [1,136,144].
- (5)
- Cell replication rates or frequencies vary greatly among tissue types, from relatively high in tissues of the skin, blood, lymph, and gastrointestinal tract, which exhibit high levels of “cell renewal”, to relatively low in nervous, sensory and cardiac muscle tissues where no cell renewal occurs (e.g., [169,170,171,172,173,174]). Where cell renewal occurs, cell turnover times vary greatly from hours to days to months [170,174].
- (6)
- Given their various levels of cellular activity, it is not surprising that the metabolic rate of various tissue types also varies greatly from relatively high in brain, liver, heart, and kidney tissues to relatively low in adipose and musculoskeletal tissues [4,15,28,66,175,176]. The metabolic rate of skeletal muscle may also change dramatically between resting and active states [177], thereby substantially altering how whole-body metabolic rate scales with body mass [7,11,20,178].
- (7)
- The turnover times of various cellular metabolites can vary by over three orders of magnitude in the same organism (e.g., 0.01 to 40 s in Arabidopsis plants: [174]).
2.3. Scaling Biological Traits in Relation to Biological Time (Allochrony) Rather Than Physical Size (Allometry)
| Taxon | AM/AFR (Units) | L/ML (Units) | Slope (±95%CI) | Intercept (±95%CI) | r | N | p | Source |
|---|---|---|---|---|---|---|---|---|
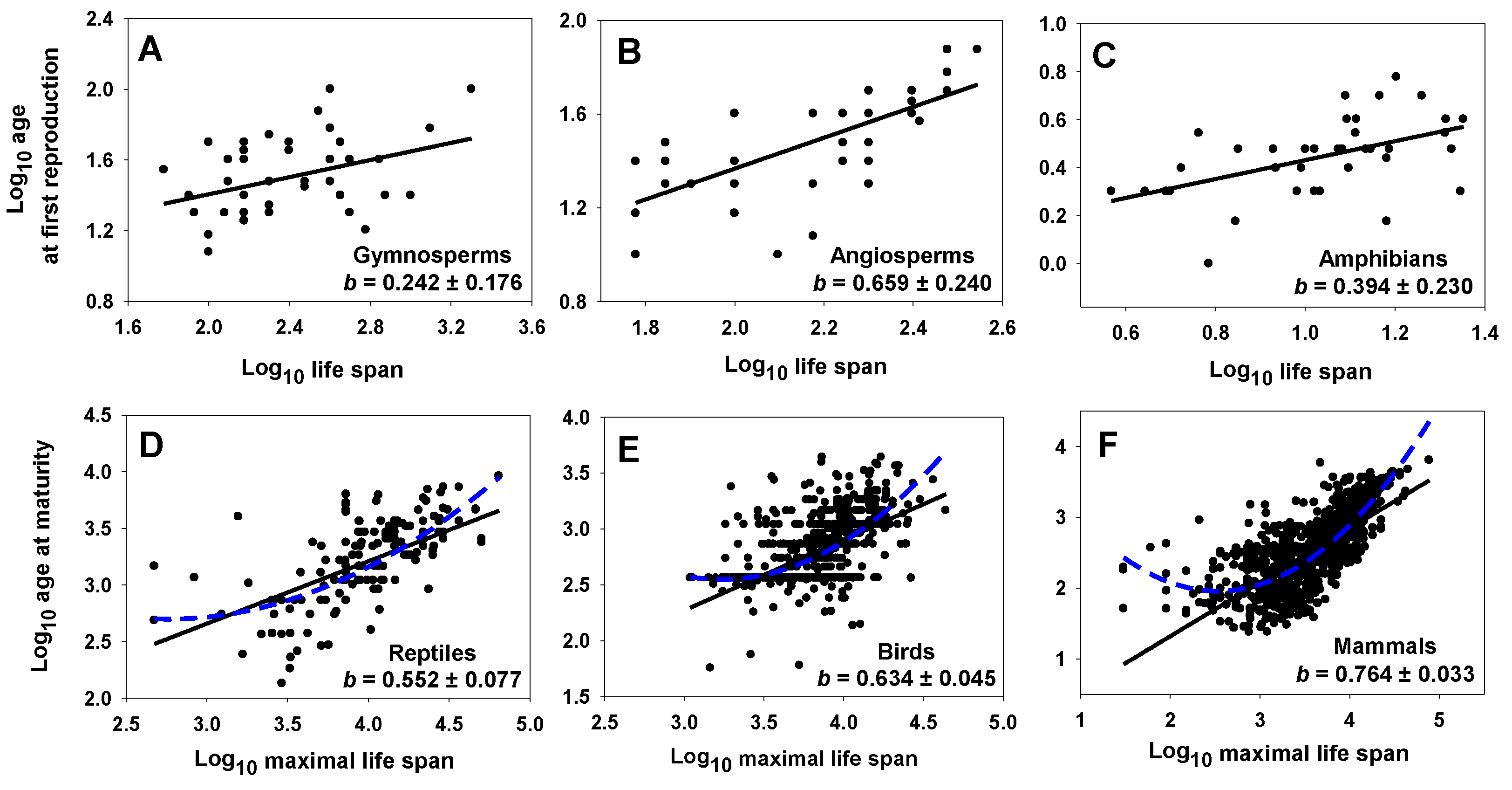
| Gymnosperms | AFR (years) | L (years) | 0.242 0.622 (±0.176) | 0.921 (±0.422) | 0.389 | 46 | 0.008 | [183] |
| Angiosperms | AFR (years) | L (years) | 0.659 0.992 (±0.240) | 0.050 (±0.527) | 0.664 | 41 | <0.0001 | [183] |
| Cladocerans | AFR (days) | L (days) | 0.430 0.517 (±0.187) | 0.179 (±0.285) | 0.832 | 13 | <0.0001 | [119] |
| Amphibians | AFR (years) | L (years) | 0.394 0.779 (±0.230) | 0.037 (±0.243) | 0.506 | 37 | 0.001 | [191] |
| Reptiles | AFR (years) | L (years) | 0.763 0.894 (±0.160) | −0.331 (±0.224) | 0.853 | 37 | <0.0001 | [191] |
| Reptiles | AM (days) | ML (days) | 0.552 0.805 (±0.077) | 1.002 (±0.307) | 0.686 | 223 | <0.0001 | [122] |
| Birds | AM (days) | ML (days) | 0.634 1.003 (±0.045) | 0.370 (±0.175) | 0.632 | 1095 | <0.0001 | [122] |
| Mammals | AM (days) | ML (days) | 0.764 1.063 (±0.033) | −0.209 (±0.124) | 0.719 | 1793 | <0.0001 | [122] |
2.4. Mortality-Imposed Time Limits on the Rates and Durations of Various Biological Processes and Their Scaling with Body Size
- (1)
- At the population level, the maximal intrinsic rate of increase of various kinds of unicellular and multicellular organisms scales with body mass with a slope near –1/4 [211] that is similar to the scaling of mortality rate (b ~ –1/4: [34]) but not that of mass-specific metabolic rate (b ~ 0) [212,213] or whole-body metabolic rate (b ~ 1) [34,213]. As expected from the principle of “ecological compensation” [202], the mortality rate of a stable (persistent) population should be balanced by its reproductive rate [56,125,199,200,201,214]. Of course, populations may temporarily increase/decrease in size, but these trends cannot continue indefinitely because of resource limitation or inevitable population extirpation.
- (2)
- At the organismal level, larval growth and developmental rates relate positively to the intensity of adult mortality rate in the fruit fly Drosophila melanogaster [161], whereas the post-maturational growth rate (and supporting metabolic rate) of the freshwater amphipod Gammarus minus relates negatively to the intensity of adult mortality, as caused by size-selective fish predation [12,215]. Growth rates of the plant Arabidopsis thaliana are also inversely related to life span (and thus positively with rate of mortality) [216]. These examples are important because they show that although higher rates or risks of mortality often favor increases in the rates of specific biological processes, the opposite may also occur if increased mortality threats involve size-specific predation, thus favoring reduced rates of foraging and growth that decrease the visibility of adults to visually hunting predators. In short, age- and size-related patterns of mortality may have variable effects on age- and size-specific rates of various biological processes. Comparative studies have also shown that organisms with characteristics that reduce mortality (e.g., flight and hibernation) have slower paces of life (e.g., [217,218]).
- (3)
- At the organ level, Sibly and Calow [219] developed a theoretical model showing that the risk of mortality may influence the differential allocation of resources to organs and, thus, their varying growth rates during ontogeny. In fact, empirical data show that rates of growth and photosynthesis of plant leaves are inversely related to their life span (or positively with their rate of mortality) both across [220,221] and within species [216].
- (4)
- At the tissue level, it is well known that the replication rate of cells (and the need for “cell renewal”) correlates strongly with their mortality (turnover) rate in different tissue types, which is in turn related to their frequency of injury and exposure to environmental hazards [169,170,171,172,173]. As the reproductive and mortality rates of organisms are matched in stable (non-growing or non-declining) ecological populations, so are the reproductive and mortality rates of cells in stable organismal tissues [219].
- (5)
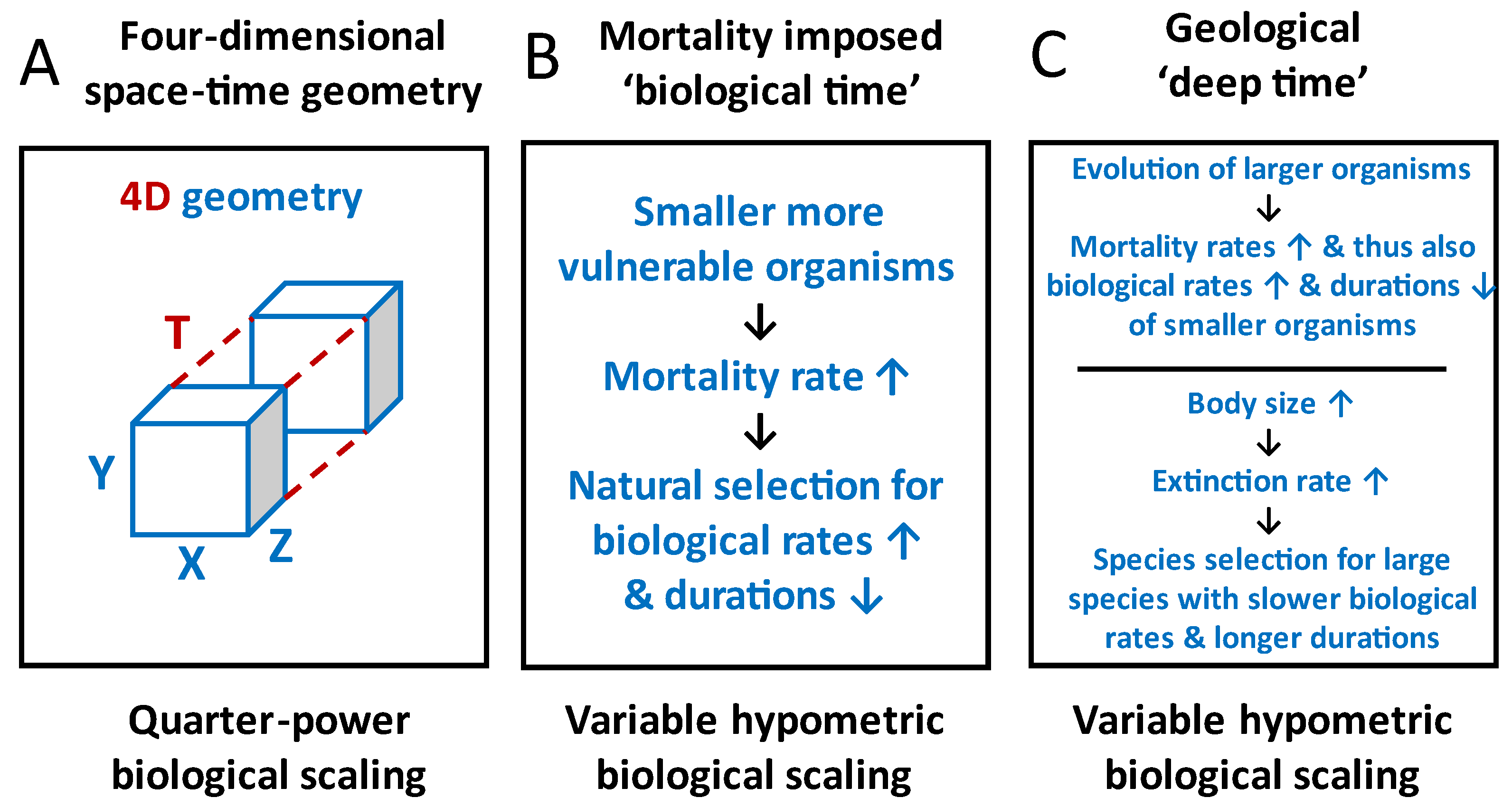
2.5. Biological Scaling Viewed in Geological “Deep Time”
3. Conclusions and Prospects
4. Appendix: What Is “Allochrony”?
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huxley, J.S. Problems of Relative Growth; Dover Publications: New York, NY, USA, 1932. [Google Scholar]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Calder, W.A. Size, Function and Life History; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Schmidt-Nielsen, K. Scaling: Why Is Animal Size So Important? Cambridge University Press: New York, NY, USA, 1984. [Google Scholar]
- Brown, J.H.; West, G.B. (Eds.) Scaling in Biology; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Glazier, D.S. Metabolic scaling in complex living systems. Systems 2014, 2, 451–540. [Google Scholar] [CrossRef]
- Glazier, D.S. Beyond the “3/4-power law”: Variation in the intra-and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005, 80, 611–662. [Google Scholar] [CrossRef]
- Glazier, D.S. Rediscovering and reviving old observations and explanations of metabolic scaling in living systems. Systems 2018, 6, 4. [Google Scholar] [CrossRef]
- Glazier, D.S. Variable metabolic scaling breaks the law: From ‘Newtonian’ to ‘Darwinian’ approaches. Proc. R. Soc. B 2022, 289, 20221605. [Google Scholar] [CrossRef]
- Glazier, D.S. The 3/4-power law is not universal: Evolution of isometric, ontogenetic metabolic scaling in pelagic animals. BioScience 2006, 56, 325–332. [Google Scholar] [CrossRef]
- Glazier, D.S. Scaling of metabolic scaling within physical limits. Systems 2014, 2, 425–450. [Google Scholar] [CrossRef]
- Glazier, D.S.; Butler, E.M.; Lombardi, S.A.; Deptola, T.J.; Reese, A.J.; Satterthwaite, E.V. Ecological effects on metabolic scaling: Amphipod responses to fish predators in freshwater springs. Ecol. Monogr. 2011, 81, 599–618. [Google Scholar] [CrossRef]
- Uyeda, J.C.; Pennell, M.W.; Miller, E.T.; Maia, R.; McClain, C.R. The evolution of energetic scaling across the vertebrate tree of life. Am. Nat. 2017, 190, 185–199. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Marshall, D.J.; Alton, L.A.; Arnold, P.A.; Beaman, J.E.; Bywater, C.L.; Condon, C.; Crispin, T.S.; Janetzki, A.; Pirtle, E.; et al. The origin and maintenance of metabolic allometry in animals. Nat. Ecol. Evol. 2019, 3, 598–603. [Google Scholar] [CrossRef]
- Kozłowski, J.; Konarzewski, M.; Czarnoleski, M. Coevolution of body size and metabolic rate in vertebrates: A life-history perspective. Biol. Rev. 2020, 95, 1393–1417. [Google Scholar] [CrossRef]
- Gavrilov, V.M.; Golubeva, T.B.; Bushuev, A.V. Evolution of metabolic scaling among the tetrapod: Effect of phylogeny, the geologic time of class formation, and uniformity of species within a class. Integr. Zool. 2022, 17, 904–917. [Google Scholar] [CrossRef]
- White, C.R.; Alton, L.A.; Bywater, C.L.; Lombardi, E.J.; Marshall, D.J. Metabolic scaling is the product of life-history optimization. Science 2022, 377, 834–839. [Google Scholar] [CrossRef]
- Harrison, J.F.; Biewener, A.; Bernhardt, J.R.; Burger, J.R.; Brown, J.H.; Coto, Z.N.; Duell, M.E.; Lynch, M.; Moffett, E.R.; Norin, T.; et al. White paper: An integrated perspective on the causes of hypometric metabolic scaling in animals. Integr. Comp. Biol. 2022, 62, 1395–1418. [Google Scholar] [CrossRef]
- Giancarli, S.M.; Dunham, A.E.; O’Connor, M.P. Clade-specific allometries in avian basal metabolic rate demand a broader theory of allometry. Physiol. Biochem. Zool. 2023, 96. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 2010, 85, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T. Intrinsic rate of natural increase: The relationship with body size. Oecologia 1974, 14, 317–326. Available online: https://www.jstor.org/stable/4214933 (accessed on 30 July 2023). [CrossRef] [PubMed]
- McNab, B.K. Food habits, energetics, and the population biology of mammals. Am. Nat. 1980, 116, 106–124. [Google Scholar] [CrossRef]
- Western, D.; Ssemakula, J. Life history patterns in birds and mammals and their evolutionary interpretation. Oecologia 1982, 54, 281–290. [Google Scholar] [CrossRef]
- Glazier, D.S. Relationship between metabolic rate and energy expenditure for lactation in Peromyscus. Comp. Biochem. Physiol. Part A Physiol. 1985, 80, 587–590. [Google Scholar] [CrossRef]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Cornell University Press: Ithaca, NY, USA, 2002. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Pontzer, H.; Raichlen, D.A.; Gordon, A.D.; Schroepfer-Walker, K.K.; Hare, B.; O’Neill, M.C.; Muldoon, K.M.; Dunsworth, H.M.; Wood, B.M.; Isler, K.; et al. Primate energy expenditure and life history. Proc. Natl. Acad. Sci. USA 2014, 111, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Is metabolic rate a universal ‘pacemaker’ for biological processes? Biol. Rev. 2015, 90, 377–407. [Google Scholar] [CrossRef]
- Schuster, L.; Cameron, H.; White, C.R.; Marshall, D.J. Metabolism drives demography in an experimental field test. Proc. Natl. Acad. Sci. USA 2021, 118, e2104942118. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Forsyth, D.M.; Hone, J. Testing the metabolic theory of ecology: Allometric scaling exponents in mammals. Ecology 2007, 88, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, B.G. Age at first reproduction and growth rate are independent of basal metabolic rate in mammals. J. Comp. Physiol. B 2009, 179, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, J.F.; Müller, D.W.; Clauss, M. A test of the metabolic theory of ecology with two longevity data sets reveals no common cause of scaling in biological times. Mamm. Rev. 2014, 44, 204–214. [Google Scholar] [CrossRef]
- Hallmann, K.; Griebeler, E.M. An exploration of differences in the scaling of life history traits with body mass within reptiles and between amniotes. Ecol. Evol. 2018, 8, 5480–5494. [Google Scholar] [CrossRef]
- Hatton, I.A.; Dobson, A.P.; Storch, D.; Galbraith, E.D.; Loreau, M. Linking scaling laws across eukaryotes. Proc. Natl. Acad. Sci. USA 2019, 116, 21616–21622. [Google Scholar] [CrossRef]
- Glazier, D.S. Does death drive the scaling of life? In Scaling in Biology; Enquist, B.J., Kempes, C.P., O’Connor, M.I., Eds.; Santa Fe Institute Press: Santa Fe, NM, USA, in review.
- Wong, S.; Bigman, J.S.; Dulvy, N.K. The metabolic pace of life histories across fishes. Proc. R. Soc. B 2021, 288, 20210910. [Google Scholar] [CrossRef]
- Norin, T. Growth and mortality as causes of variation in metabolic scaling among taxa and taxonomic levels. Integr. Comp. Biol. 2022, 62, 1448–1459. [Google Scholar] [CrossRef]
- Minkowski, H. Raum und zeit. Phys. Z. 1909, 10, 104–111. [Google Scholar]
- Archibald, R.C. Time as a fourth dimension. Bull. Am. Math. Soc. 1914, 20, 409–412. [Google Scholar] [CrossRef]
- Einstein, A. Relativity: The Special and General Theory, A Popular Exposition, 3rd ed.; Methuen: London, UK, 1920. [Google Scholar]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- Savage, V.M.; Gillooly, J.F.; Woodruff, W.H.; West, G.B.; Allen, A.P.; Enquist, B.J.; Brown, J.H. The predominance of quarter-power scaling in biology. Funct. Ecol. 2004, 18, 257–282. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Savage, V.M.; Allen, A.P.; Gillooly, J.F. Allometry and metabolic scaling in ecology. In Encyclopedia of Life Sciences; Jansson, R., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–10. [Google Scholar] [CrossRef]
- Sibly, R.M.; Brown, J.H.; Kodric-Brown, A. Metabolic Ecology: A Scaling Approach; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Schramski, J.R.; Dell, A.I.; Grady, J.M.; Sibly, R.M.; Brown, J.H. Metabolic theory predicts whole-ecosystem properties. Proc. Natl. Acad. Sci. USA 2015, 112, 2617–2622. [Google Scholar] [CrossRef]
- Carrel, A. Physiological time. Science 1931, 74, 618–621. [Google Scholar] [CrossRef]
- Sider, T. Four-Dimensionalism: An Ontology of Persistence and Time; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Morgan, W. Are organisms substances or processes? Australas. J. Philos. 2022, 100, 605–619. [Google Scholar] [CrossRef]
- Günther, B. Dimensional analysis and theory of biological similarity. Physiol. Rev. 1975, 55, 659–699. [Google Scholar] [CrossRef]
- Hainsworth, F.R. Animal Physiology: Adaptations in Function; Addison-Wesley: Reading, MA, USA, 1981. [Google Scholar]
- Lindstedt, S.L.; Calder, W.A. Body size, physiological time, and longevity of homeothermic animals. Q. Rev. Biol. 1981, 56, 1–16. [Google Scholar] [CrossRef]
- Günther, B.; Morgado, E. Allometric scaling of biological rhythms in mammals. Biol. Res. 2005, 38, 207–212. [Google Scholar] [CrossRef]
- da Silva, J.K.L.; Garcia, G.J.; Barbosa, L.A. Allometric scaling laws of metabolism. Phys. Life Rev. 2006, 3, 229–261. [Google Scholar] [CrossRef]
- Ginzburg, L.; Damuth, J. The space-lifetime hypothesis: Viewing organisms in four dimensions, literally. Am. Nat. 2008, 171, 125–131. [Google Scholar] [CrossRef]
- Burger, J.R.; Hou, C.; Hall, C.A.S.; Brown, J.H. Universal rules of life: Metabolic rates, biological times and the equal fitness paradigm. Ecol. Lett. 2021, 24, 1262–1281. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Burger, J.R.; Hou, C.; Hall, C.A.S. The pace of life: Metabolic energy, biological time, and life history. Integr. Comp. Biol. 2022, 62, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H. The origin of allometric scaling laws in biology from genomes to ecosystems: Towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005, 208, 1575–1592. [Google Scholar] [CrossRef] [PubMed]
- Prinzinger, R. Life span in birds and the ageing theory of absolute metabolic scope. Comp. Biochem. Physiol. Part A Physiol. 1993, 105, 609–615. [Google Scholar] [CrossRef]
- Mortola, J.P. The mouse-to-elephant metabolic curve: Historical overview. Compr. Physiol. 2023, 13, 4513–4558. [Google Scholar] [CrossRef]
- Bokma, F. Evidence against universal metabolic allometry. Funct. Ecol. 2004, 18, 184–187. [Google Scholar] [CrossRef]
- White, C.R.; Cassey, P.; Blackburn, T.M. Allometric exponents do not support a universal metabolic allometry. Ecology 2007, 88, 315–323. [Google Scholar] [CrossRef]
- DeLong, J.P.; Okie, J.G.; Moses, M.E.; Sibly, R.M.; Brown, J.H. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc. Natl. Acad. Sci. USA 2010, 107, 12941–12945. [Google Scholar] [CrossRef]
- Killen, S.S.; Atkinson, D.; Glazier, D.S. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 2010, 13, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Complications with body-size correction in comparative biology: Possible solutions and an appeal for new approaches. J. Exp. Biol. 2022, 225 (Suppl. S1), jeb243313. [Google Scholar] [CrossRef] [PubMed]
- Zeuthen, E. Oxygen uptake as related to body size in organisms. Q. Rev. Biol. 1953, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. How metabolic rate relates to cell size. Biology 2022, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Costa, I.; Brown, J.A.; Gamperl, A.K. Little left in the tank: Metabolic scaling in marine teleosts and its implications for aerobic scope. Proc. R. Soc. B Biol. Sci. 2007, 274, 431–438. [Google Scholar] [CrossRef]
- Moran, D.; Wells, R.M. Ontogenetic scaling of fish metabolism in the mouse-to-elephant mass magnitude range. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 611–620. [Google Scholar] [CrossRef]
- Streicher, J.W.; Cox, C.L.; Birchard, G.F. Non-linear scaling of oxygen consumption and heart rate in a very large cockroach species (Gromphadorhina portentosa): Correlated changes with body size and temperature. J. Exp. Biol. 2012, 215, 1137–1143. [Google Scholar] [CrossRef]
- Glazier, D.S.; Hirst, A.G.; Atkinson, D. Shape shifting predicts ontogenetic changes in metabolic scaling in diverse aquatic invertebrates. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142302. [Google Scholar] [CrossRef]
- Forlenza, A.E.; Galbraith, H.S.; Blakeslee, C.J.; Glazier, D.S. Ontogenetic changes in body shape and the scaling of metabolic rate in the American Eel (Anguilla rostrata). Physiol. Biochem. Zool. 2022, 95, 430–437. [Google Scholar] [CrossRef]
- Sánchez-González, J.R.; Nicieza, A.G. Declining metabolic scaling parallels an ontogenetic change from elongate to deep-bodied shapes in juvenile Brown trout. Curr. Zool. 2022, 69, 294–303. [Google Scholar] [CrossRef]
- Hayssen, V.; Lacy, R.C. Basal metabolic rates in mammals: Taxonomic differences in the allometry of BMR and body mass. Comp. Biochem. Physiol. Part A Physiol. 1985, 81, 741–754. [Google Scholar] [CrossRef]
- Kozłowski, J.; Konarzewski, M. West, Brown and Enquist’s model of allometric scaling again: The same questions remain. Funct. Ecol. 2005, 19, 739–743. [Google Scholar] [CrossRef]
- Clarke, A.; Rothery, P.; Isaac, N.J. Scaling of basal metabolic rate with body mass and temperature in mammals. J. Anim. Ecol. 2010, 79, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Kolokotrones, T.; Savage, V.; Deeds, E.J.; Fontana, W. Curvature in metabolic scaling. Nature 2010, 464, 753–756. [Google Scholar] [CrossRef]
- Müller, D.W.; Codron, D.; Werner, J.; Fritz, J.; Hummel, J.; Griebeler, E.M.; Clauss, M. Dichotomy of eutherian reproduction and metabolism. Oikos 2012, 121, 102–115. [Google Scholar] [CrossRef]
- Ehnes, R.B.; Rall, B.C.; Brose, U. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 2011, 14, 993–1000. [Google Scholar] [CrossRef]
- Cheng, D.L.; Li, T.; Zhong, Q.L.; Wang, G.X. Scaling relationship between tree respiration rates and biomass. Biol. Lett. 2010, 6, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Yamaji, K.; Ishida, A.; Prokushkin, S.G.; Masyagina, O.V.; Hagihara, A.; Hoque, A.R.; Suwa, R.; Osawa, A.; Nishizono, T.; et al. Mixed-power scaling of whole-plant respiration from seedlings to giant trees. Proc. Natl. Acad. Sci. USA 2010, 107, 1447–1451. [Google Scholar] [CrossRef]
- Lindstedt, S.L.; Calder, W.A. Body size and longevity in birds. Condor 1976, 78, 91–94. [Google Scholar] [CrossRef]
- Millar, J.S. Adaptive features of mammalian reproduction. Evolution 1977, 31, 370–386. [Google Scholar] [CrossRef]
- Martin, R.D.; Genoud, M.; Hemelrijk, C.K. Problems of allometric scaling analysis: Examples from mammalian reproductive biology. J. Exp. Biol. 2005, 208, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, J.P.; Costa, J.; Church, G.M. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Frazier, M.R. Thermodynamics constrains allometric scaling of optimal development time in insects. PLoS ONE 2013, 8, e84308. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Dittmann, M.T.; Müller, D.W.; Zerbe, P.; Codron, D. Low scaling of a life history variable: Analysing eutherian gestation periods with and without phylogeny-informed statistics. Mamm. Biol. 2014, 79, 9–16. [Google Scholar] [CrossRef]
- Healy, K.; Guillerme, T.; Finlay, S.; Kane, A.; Kelly, S.B.; McClean, D.; Kelly, D.J.; Donohue, I.; Jackson, A.L.; Cooper, N. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140298. [Google Scholar] [CrossRef]
- Jackson, G.; Mooers, A.Ø.; Dubman, E.; Hutchen, J.; Collard, M. Basal metabolic rate and maternal energetic investment durations in mammals. BMC Evol. Biol. 2014, 14, 194. [Google Scholar] [CrossRef]
- Scharf, I.; Feldman, A.; Novosolov, M.; Pincheira-Donoso, D.; Das, I.; Böhm, M.; Uetz, P.; Torres-Carvajal, O.; Bauer, A.; Roll, U.; et al. Late bloomers and baby boomers: Ecological drivers of longevity in squamates and the tuatara. Glob. Ecol. Biogeogr. 2015, 24, 396–405. [Google Scholar] [CrossRef]
- Szekely, P.; Korem, Y.; Moran, U.; Mayo, A.; Alon, U. The mass-longevity triangle: Pareto optimality and the geometry of life-history trait space. PLoS Comp. Biol. 2015, 11, e1004524. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, S.; Watanabe, Y.Y.; Kawano, M.; Kawabata, Y. Factors affecting gestation periods in elasmobranch fishes. Biol. Open 2022, 11, bio059270. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.J.; Davidson, A.D.; Sibly, R.M.; Brown, J.H. Universal scaling of production rates across mammalian lineages. Proc. R. Soc. B Biol. Sci. 2011, 278, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; López-Urrutia, Á. Scaling up the curvature of mammalian metabolism. Front. Ecol. Evol. 2014, 2, 61. [Google Scholar] [CrossRef]
- Douhard, F.; Lemaître, J.F.; Rauw, W.M.; Friggens, N.C. Allometric scaling of the elevation of maternal energy intake during lactation. Front. Zool. 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Banavar, J.R.; Cooke, T.J.; Rinaldo, A.; Maritan, A. Form, function, and evolution of living organisms. Proc. Natl. Acad. Sci. USA 2014, 111, 3332–3337. [Google Scholar] [CrossRef] [PubMed]
- Economos, A.C. On the origin of biological similarity. J. Theor. Biol. 1982, 94, 25–60. [Google Scholar] [CrossRef]
- Hirst, A.G.; Glazier, D.S.; Atkinson, D. Body shape shifting during growth permits tests that distinguish between competing geometric theories of metabolic scaling. Ecol. Lett. 2014, 17, 1274–1281. [Google Scholar] [CrossRef]
- Tan, H.; Hirst, A.G.; Glazier, D.S.; Atkinson, D. Ecological pressures and the contrasting scaling of metabolism and body shape in coexisting taxa: Cephalopods versus teleost fish. Philos. Trans. R. Soc. B 2019, 374, 20180543. [Google Scholar] [CrossRef]
- Blum, J.J. On the geometry of four-dimensions and the relationship between metabolism and body mass. J. Theor. Biol. 1977, 6, 599–601. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. The fourth dimension of life: Fractal geometry and allometric scaling of organisms. Science 1999, 284, 1677–1679. [Google Scholar] [CrossRef]
- West, D.; West, B.J. Physiologic time: A hypothesis. Phys. Life Rev. 2013, 10, 210–224. [Google Scholar] [CrossRef]
- Lambert, R.; Teissier, G. Théorie de la similitude biologique. Ann. Physiol. Physicochim. Biol. 1927, 13, 212–246. [Google Scholar]
- Hill, A.V. The dimensions of animals and their muscular dynamics. Sci. Progr. 1950, 38, 209–230. [Google Scholar]
- Morin, L.P. A concept of physiological time: Rhythms in behavior and reproductive physiology. Ann. N. Y. Acad. Sci. 1986, 474, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Caughley, G. Mortality patterns in mammals. Ecology 1966, 47, 906–918. [Google Scholar] [CrossRef]
- Millar, J.S.; Zammuto, R.M. Life histories of mammals: An analysis of life tables. Ecology 1983, 64, 631–635. [Google Scholar] [CrossRef]
- Pacifici, M.; Santini, L.; Di Marco, M.; Baisero, D.; Francucci, L.; Marasini, G.G.; Visconti, P.; Rondinini, C. Generation length for mammals. Nat. Conserv. 2013, 5, 87–95. [Google Scholar] [CrossRef]
- Bird, J.P.; Martin, R.; Akçakaya, H.R.; Gilroy, J.; Burfield, I.J.; Garnett, S.T.; Symes, A.; Taylor, J.; Şekercioğlu, Ç.H.; Butchart, S.H. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 2020, 34, 1252–1261. [Google Scholar] [CrossRef]
- Pearl, R. The Biology of Death; Lippincott: Philadelphia, PA, USA, 1922. [Google Scholar]
- Běhrádek, J. Temperature coefficients in biology. Biol. Rev. 1930, 5, 30–58. [Google Scholar] [CrossRef]
- Brody, S. Bioenergetics and Growth; Hafner: New York, NY, USA, 1945. [Google Scholar]
- Reiss, J.O. The meaning of developmental time: A metric for comparative embryology. Am. Nat. 1989, 134, 170–189. [Google Scholar] [CrossRef]
- Donner, K. The living time—On the relativity of biological time. Duodecim 2002, 118, 2411–2417. [Google Scholar]
- Gillooly, J.F.; Charnov, E.L.; West, G.B.; Savage, V.M.; Brown, J.H. Effects of size and temperature on developmental time. Nature 2002, 417, 70–73. [Google Scholar] [CrossRef]
- Szasz, O.; Szigeti, P.G.; Szasz, A. The intrinsic self-time of biosystems. Open J. Biophys. 2019, 9, 131–145. [Google Scholar] [CrossRef]
- Bonner, J.T. Size and Cycle: An Essay on the Structure of Biology; Princeton University Press: Princeton, NJ, USA, 1965. [Google Scholar]
- Glazier, D.S. Biological scaling analyses are more than statistical line fitting. J. Exp. Biol. 2021, 224, jeb241059. [Google Scholar] [CrossRef]
- Bakewell, A.T.; Davis, K.E.; Freckleton, R.P.; Isaac, N.J.; Mayhew, P.J. Comparing life histories across taxonomic groups in multiple dimensions: How mammal-like are insects? Am. Nat. 2020, 195, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M. The evolution of cladoceran life histories. Q. Rev. Biol. 1980, 55, 23–42. [Google Scholar] [CrossRef]
- Denney, N.H.; Jennings, S.; Reynolds, J.D. Life-history correlates of maximum population growth rates in marine fishes. Proc. R. Soc. B Biol. Sci. 2002, 269, 2229–2237. [Google Scholar] [CrossRef]
- Dunham, A.E.; Miles, D.B.; Reznick, D.N. Life history patterns in squamate reptiles. In Biology of the Reptilia. Ecology B, Defense and Life History; Gans, C., Huey, R.B., Alan, R., Eds.; Liss: New York, NY, USA, 1988; Volume 16, pp. 441–522. [Google Scholar]
- Myhrvold, N.P.; Baldridge, E.; Chan, B.; Sivam, D.; Freeman, D.L.; Ernest, S.M. An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles. Ecol. Soc. Am. 2015, 96, 3109. [Google Scholar] [CrossRef]
- Eisenberg, J.F. The Mammalian Radiations: An Analysis of Trends in Evolution, Adaptation, and Behavior; University of Chicago Press: Chicago, IL, USA, 1981. [Google Scholar]
- MammalBase Community 2022. MammalBase—Database of Traits, Measurements and Diets of the Species in Class Mammalia. Available online: https://www.mammalbase.net (accessed on 8 November 2022).
- Harvey, P.H.; Pagel, M.D. The Comparative Method in Evolutionary Biology; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Alencar, L.R.; Hodge, J.R.; Friedman, S.T.; Wainwright, P.C.; Price, S.A. Size as a complex trait and the scaling relationships of its components across teleosts. Evol. Ecol. 2022, 36, 471–487. [Google Scholar] [CrossRef]
- Hirst, A.G. Intraspecific scaling of mass to length in pelagic animals: Ontogenetic shape change and its implications. Limnol. Oceanogr. 2012, 57, 1579–1590. [Google Scholar] [CrossRef]
- Okie, J.G. General models for the spectra of surface area scaling strategies of cells and organisms: Fractality, geometric dissimilitude, and internalization. Am. Nat. 2013, 181, 421–439. [Google Scholar] [CrossRef]
- Adolph, E.F. Quantitative relations in the physiological constitutions of mammals. Science 1949, 109, 579–585. [Google Scholar] [CrossRef]
- Stahl, W.R. Similarity and dimensional methods in biology: They promise to show quantitative similarities between biological organisms and models of biological systems. Science 1962, 137, 205–212. [Google Scholar] [CrossRef]
- Günther, B. Physiological time and its evolution. In Biogenesis Evolution Homeostasis; Locker, A., Ed.; Springer: Berlin, Germany, 1973; pp. 127–135. [Google Scholar]
- Prinzinger, R. Programmed ageing: The theory of maximal metabolic scope: How does the biological clock tick? EMBO Rep. 2005, 6, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Bromage, T.G.; Hogg, R.T.; Lacruz, R.S.; Hou, C. Primate enamel evinces long period biological timing and regulation of life history. J. Theor. Biol. 2012, 305, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Neill, D. Individual cell longevity, ‘life’s timekeeper’, and metazoan evolution. Curr. Aging Sci. 2016, 9, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, S.L. Body size, time and dimensions of oxygen diffusion. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 252, 110847. [Google Scholar] [CrossRef]
- Gould, S.J. Ontogeny and Phylogeny; Harvard University Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Smith, B.H. Life history and the evolution of human maturation. Evol. Anthropol. 1992, 1, 134–142. [Google Scholar] [CrossRef]
- Glazier, D.S.; Newcomer, S.D. Allochrony: A new way of analysing life histories, as illustrated with mammals. Evol. Ecol. Res. 1999, 1, 333–346. [Google Scholar]
- Kooijman, S.A.L.M. Dynamic Energy and Mass Budgets in Biological Systems; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Needham, J. On the dissociability of the fundamental processes in ontogenesis. Biol. Rev. 1933, 8, 180–223. [Google Scholar] [CrossRef]
- Sæther, B.E. The influence of body weight on the covariation between reproductive traits in European birds. Oikos 1987, 48, 79–88. [Google Scholar] [CrossRef]
- Dubman, E.; Collard, M.; Mooers, A.Ø. Evidence that gestation duration and lactation duration are coupled traits in primates. Biol. Lett. 2012, 8, 998–1001. [Google Scholar] [CrossRef]
- Crozier, W.J. On biological oxidations as function of temperature. J. Gen. Physiol. 1924, 7, 189–216. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.J. Shapes of Time: The Evolution of Growth and Development; Johns Hopkins University Press: Baltimore, MD, USA, 1997. [Google Scholar]
- Hoeflich, A.; Reyer, A.; Ohde, D.; Schindler, N.; Brenmoehl, J.; Spitschak, M.; Langhammer, M.; Tuchscherer, A.; Wirthgen, E.; Renner-Müller, I.; et al. Dissociation of somatic growth, time of sexual maturity, and life expectancy by overexpression of an RGD-deficient IGFBP-2 variant in female transgenic mice. Aging Cell 2016, 15, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.P.; Bullock, T.H. Q10 as a function of size and habitat temperature in poikilotherms. Am. Nat. 1954, 88, 33–44. [Google Scholar] [CrossRef]
- Prosser, C.L. Temperature. In Comparative Animal Physiology, 2nd ed.; Prosser, C.L., Brown, F.A., Eds.; Saunders: Philadelphia, PA, USA, 1961; pp. 238–284. [Google Scholar]
- Taylor, F. Ecology and evolution of physiological time in insects. Am. Nat. 1981, 117, 1–23. [Google Scholar] [CrossRef]
- Cossins, A.R.; Bowler, K. Temperature Biology of Animals; Chapman and Hall: London, UK, 1987. [Google Scholar]
- Van der Have, T.M.; De Jong, G. Adult size in ectotherms: Temperature effects on growth and differentiation. J. Theor. Biol. 1996, 183, 329–340. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Steury, T.D.; Sears, M.W. Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integr. Comp. Biol. 2004, 44, 498–509. [Google Scholar] [CrossRef]
- Walters, R.J.; Hassall, M. The temperature-size rule in ectotherms: May a general explanation exist after all? Am. Nat. 2006, 167, 510–523. [Google Scholar] [CrossRef]
- Irlich, U.M.; Terblanche, J.S.; Blackburn, T.M.; Chown, S.L. Insect rate-temperature relationships: Environmental variation and the metabolic theory of ecology. Am. Nat. 2009, 174, 819–835. [Google Scholar] [CrossRef]
- Dell, A.I.; Pawar, S.; Savage, V.M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 10591–10596. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G. The temperature-size rule emerges from ontogenetic differences between growth and development rates. Funct. Ecol. 2012, 26, 483–492. [Google Scholar] [CrossRef]
- Lemoine, N.P.; Burkepile, D.E. Temperature-induced mismatches between consumption and metabolism reduce consumer fitness. Ecology 2012, 93, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Hille, S.M.; Cooper, C.B. Elevational trends in life histories: Revising the pace-of-life framework. Biol. Rev. 2015, 90, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J., Jr.; Dunham, A.E. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Activity alters how temperature influences intraspecific metabolic scaling: Testing the metabolic-level boundaries hypothesis. J. Comp. Physiol. B 2020, 190, 445–454. [Google Scholar] [CrossRef]
- Carraco, G.; Martins-Jesus, A.P.; Andrade, R.P. The vertebrate Embryo Clock: Common players dancing to a different beat. Front. Cell Dev. Biol. 2022, 10, 944016. [Google Scholar] [CrossRef]
- Gasser, M.; Kaiser, M.; Berrigan, D.; Stearns, S.C. Life-history correlates of evolution under high and low adult mortality. Evolution 2000, 54, 1260–1272. [Google Scholar] [CrossRef]
- Mehnert, E. Kainogenese eine gesetzmässige Abänderung der embryonalen Entfaltung in Folge von erblicher Uebertragung in der Phylogenese erworbener Eigentümlichkeiten. Morph. Arb. 1897, 7, 1–156. [Google Scholar]
- Lui, J.C.; Baron, J. Mechanisms limiting body growth in mammals. Endocr. Rev. 2011, 32, 422–440. [Google Scholar] [CrossRef]
- Emlen, D.J.; Warren, I.A.; Johns, A.; Dworkin, I.; Lavine, L.C. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 2012, 337, 860–864. [Google Scholar] [CrossRef]
- Penzo-Méndez, A.I.; Stanger, B.Z. Organ-size regulation in mammals. Cold Spring Harb. Perspect. Biol. 2015, 7, a019240. [Google Scholar] [CrossRef]
- Lilja, C.; Sperber, I.; Marks, H.L. Postnatal growth and organ development in Japanese quail selected for high growth rate. Growth 1985, 49, 51–62. [Google Scholar] [PubMed]
- Konarzewski, M.; Gavin, A.; McDevitt, R.; Wallis, I.R. Metabolic and organ mass responses to selection for high growth rates in the domestic chicken (Gallus domesticus). Physiol. Biochem. Zool. 2000, 73, 237–248. [Google Scholar] [CrossRef]
- Gagnat, M.R.; Wold, P.A.; Bardal, T.; Øie, G.; Kjørsvik, E. Allometric growth and development of organs in ballan wrasse (Labrus bergylta Ascanius, 1767) larvae in relation to different live prey diets and growth rates. Biol. Open 2016, 5, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.G. The Size and Growth of Tissue Cells; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1953. [Google Scholar]
- Leblond, C.P.; Walker, B.E. Renewal of cell populations. Physiol. Rev. 1956, 36, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Klein, R.E. Cell renewal in adult mouse tissues. Am. J. Pathol. 1961, 38, 437–453. [Google Scholar]
- Bertalanffy, F.D.; Lau, C. Cell renewal. Int. Rev. Cytol. 1962, 13, 357–366. [Google Scholar] [CrossRef]
- Lipkin, M. Proliferation and differentiation of gastrointestinal cells. Physiol. Rev. 1973, 53, 891–915. [Google Scholar] [CrossRef]
- Milo, R.; Phillips, R. Cell Biology by the Numbers; Garland Science: New York, NY, USA, 2015. [Google Scholar]
- Elia, M. Organ and tissue contribution to metabolic rate. In Energy Metabolism: Tissue Determinants and Cellular Corollaries; Kinney, J.M., Tucker, H.N., Eds.; Raven Press: New York, NY, USA, 1992; pp. 61–79. [Google Scholar]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Weibel, E.R.; Hoppeler, H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J. Exp. Biol. 2005, 208, 1635–1644. [Google Scholar] [CrossRef]
- Glazier, D.S. Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. J. Comp. Physiol. B 2009, 179, 821–828. [Google Scholar] [CrossRef]
- Hull, D.L. Individuality and selection. Annu. Rev. Ecol. Syst. 1980, 11, 311–332. [Google Scholar] [CrossRef]
- Strassmann, J.E.; Queller, D.C. The social organism: Congresses, parties, and committees. Evolution 2010, 64, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Harvey, P.H.; Zammuto, R.M. Patterns of mortality and age at first reproduction in natural populations of mammals. Nature 1985, 315, 319–320. [Google Scholar] [CrossRef]
- Loehle, C. Tree life history strategies: The role of defenses. Can. J. For. Res. 1988, 18, 209–222. [Google Scholar] [CrossRef]
- Beverton, R.J.H. Patterns of reproductive strategy parameters in some marine teleost fishes. J. Fish Biol. 1992, 41, 137–160. [Google Scholar] [CrossRef]
- Prothero, J. Adult life span as a function of age at maturity. Exp. Gerontol. 1993, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Charnov, E.L. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Charnov, E.L. A note on dimensionless life histories for birds versus mammals. Evol. Ecol. 1995, 9, 288–291. [Google Scholar] [CrossRef]
- Blanck, A.; Lamouroux, N. Large-scale intraspecific variation in life-history traits of European freshwater fish. J. Biogeogr. 2007, 34, 862–875. [Google Scholar] [CrossRef]
- Ridgway, I.D.; Richardson, C.A.; Austad, S.N. Maximum shell size, growth rate, and maturation age correlate with longevity in bivalve molluscs. J. Gerontol. Part A Biomed. Sci. Med. Sci. 2011, 66, 183–190. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. Longevity and sexual maturity vary across species with number of cortical neurons, and humans are no exception. J. Comp. Neurol. 2019, 527, 1689–1705. [Google Scholar] [CrossRef]
- Reinke, B.A.; Cayuela, H.; Janzen, F.J.; Lemaître, J.F.; Gaillard, J.M.; Lawing, A.M.; Iverson, J.B.; Christiansen, D.G.; Martínez-Solano, I.; Sánchez-Montes, G.; et al. Diverse aging rates in ectothermic tetrapods provide insights for the evolution of aging and longevity. Science 2022, 376, 1459–1466. [Google Scholar] [CrossRef]
- Cayuela, H.; Gaillard, J.M.; Vieira, C.; Ronget, V.; Gippet, J.M.; García, T.C.; Marais, G.A.; Lemaître, J.F. Sex differences in adult lifespan and aging rate across mammals: A test of the ‘Mother Curse hypothesis’. Mech. Ageing Dev. 2023, 212, 111799. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman: New York, NY, USA, 1995. [Google Scholar]
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. 2006, 81, 259–291. [Google Scholar] [CrossRef]
- Smith, R.J. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 2009, 140, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Al-Wathiqui, N.; Rodríguez, R.L. Allometric slopes not underestimated by ordinary least squares regression: A case study with Enchenopa treehoppers (Hemiptera: Membracidae). Ann. Ent. Soc. Am. 2011, 104, 562–566. [Google Scholar] [CrossRef]
- Kilmer, J.T.; Rodríguez, R.L. Ordinary least squares regression is indicated for studies of allometry. J. Evol. Biol. 2017, 30, 4–12. [Google Scholar] [CrossRef]
- Charnov, E.L.; Berrigan, D. Dimensionless numbers and life history evolution: Age of maturity versus the adult lifespan. Evol. Ecol. 1990, 4, 273–275. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species by Means of Natural Selection; Murray: London, UK, 1859. [Google Scholar]
- Schmalhausen, I.I. Factors of Evolution: The Theory of Stabilizing Selection; Blakiston: Philadelphia, PA, USA, 1949. [Google Scholar]
- Cole, L.C. The population consequences of life history phenomena. Q. Rev. Biol. 1954, 29, 103–137. [Google Scholar] [CrossRef]
- Sibly, R.; Calow, P. Ecological compensation—A complication for testing life-history theory. J. Theor. Biol. 1987, 125, 177–186. [Google Scholar] [CrossRef]
- Rubner, M. Das Problem der Lebensdauer und seine Beziehungen zum Wachstum und Ernährung; Oldenbourg: Munich, Germany, 1908. [Google Scholar]
- Pearl, R. The Rate of Living; University of London Press: London, UK, 1928. [Google Scholar]
- McCoy, M.W.; Gillooly, J.F. Predicting natural mortality rates of plants and animals. Ecol. Lett. 2008, 11, 710–716. [Google Scholar] [CrossRef]
- Ng, J.C.; Schooling, C.M. Effect of basal metabolic rate on lifespan: A sex-specific Mendelian randomization study. Sci. Rep. 2023, 13, 7761. [Google Scholar] [CrossRef]
- Stark, G.; Pincheira-Donoso, D.; Meiri, S. No evidence for the ‘rate-of-living’ theory across the tetrapod tree of life. Glob. Ecol. Biogeogr. 2020, 29, 857–884. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Zhu, W.L. Evidence for the ‘rate-of-living’ hypothesis between mammals and lizards, but not in birds, with field metabolic rate. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 253, 110867. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.C. Pleiotropy, natural selection, and the evolution of senescence: Evolution 11, 398–411 (1957). Sci. Aging Knowl. Environ. 2001, 2001, cp13. [Google Scholar] [CrossRef]
- Daan, S.; Masman, D.; Groenewold, A. Avian basal metabolic rates: Their association with body composition and energy expenditure in nature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990, 259, R333–R340. [Google Scholar] [CrossRef] [PubMed]
- Blueweiss, L.; Fox, H.; Kudzma, V.; Nakashima, D.; Peters, R.; Sams, S. Relationships between body size and some life history parameters. Oecologia 1978, 37, 257–272. [Google Scholar] [CrossRef]
- Makarieva, A.M.; Gorshkov, V.G.; Li, B.L.; Chown, S.L.; Reich, P.B.; Gavrilov, V.M. Mean mass-specific metabolic rates are strikingly similar across life’s major domains: Evidence for life’s metabolic optimum. Proc. Natl. Acad. Sci. USA 2008, 105, 16994–16999. [Google Scholar] [CrossRef]
- Makarieva, A.M.; Nefiodov, A.V.; Li, B.L. Life’s energy and information: Contrasting evolution of volume-versus surface-specific rates of energy consumption. Entropy 2020, 22, 1025. [Google Scholar] [CrossRef]
- Bronson, F.H. Mammalian Reproductive Biology; University of Chicago Press: Chicago, IL, USA, 1989. [Google Scholar]
- Glazier, D.S.; Borrelli, J.J.; Hoffman, C.L. Effects of fish predators on the mass-related energetics of a keystone freshwater crustacean. Biology 2020, 9, 40. [Google Scholar] [CrossRef]
- Sartori, K.; Vasseur, F.; Violle, C.; Baron, E.; Gerard, M.; Rowe, N.; Ayala-Garay, O.; Christophe, A.; Jalón, L.G.D.; Masclef, D.; et al. Leaf economics and slow-fast adaptation across the geographic range of Arabidopsis thaliana. Sci. Rep. 2019, 9, 10758. [Google Scholar] [CrossRef]
- Barclay, R.M.; Harder, L.D. Life histories of bats: Life in the slow lane. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 209–253. [Google Scholar]
- Turbill, C.; Bieber, C.; Ruf, T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B Biol. Sci. 2011, 278, 3355–3363. [Google Scholar] [CrossRef]
- Sibly, R.; Calow, P. Physiological Ecology of Animals: An Evolutionary Approach; Blackwell: Oxford, UK, 1986. [Google Scholar]
- Chabot, B.F.; Hicks, D.J. The ecology of leaf life spans. Ann. Rev. Ecol. Syst. 1982, 13, 229–259. Available online: https://www.jstor.org/stable/2097068 (accessed on 30 July 2023). [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- O’Brien, L.E. Tissue homeostasis and non-homeostasis: From cell life cycles to organ states. Annu. Rev. Cell Dev. Biol. 2022, 38, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.; Tweto, J. Measurement of protein turnover in animal cells. Meth. Cell Biol. 1975, 10, 235–260. [Google Scholar] [CrossRef]
- Nelson, C.J.; Alexova, R.; Jacoby, R.P.; Millar, A.H. Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol. 2014, 166, 91–108. [Google Scholar] [CrossRef]
- Nelson, C.J.; Millar, A.H. Protein turnover in plant biology. Nat. Plants 2015, 1, 15017. [Google Scholar] [CrossRef]
- Basisty, N.; Meyer, J.G.; Schilling, B. Protein turnover in aging and longevity. Proteomics 2018, 18, 1700108. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Bionomic strategies and population parameters. In Theoretical Ecology: Principles and Applications; May, R.M., Ed.; Sinauer Associates: Sunderland, MA, USA, 1981; pp. 30–52. [Google Scholar]
- Sinclair, A.R.; Mduma, S.; Brashares, J.S. Patterns of predation in a diverse predator-prey system. Nature 2003, 425, 288–290. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. Body size and mortality rates in coral reef fishes: A three-phase relationship. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161858. [Google Scholar] [CrossRef]
- Mihalitsis, M.; Hemingson, C.R.; Goatley, C.H.; Bellwood, D.R. The role of fishes as food: A functional perspective on predator–prey interactions. Funct. Ecol. 2021, 35, 1109–1119. [Google Scholar] [CrossRef]
- Otto, S.B.; Rall, B.C.; Brose, U. Allometric degree distributions facilitate food-web stability. Nature 2007, 450, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Yvon-Durocher, G.; Reiss, J.; Blanchard, J.; Ebenman, B.; Perkins, D.M.; Reuman, D.C.; Thierry, A.; Woodward, G.; Petchey, O.L. Across ecosystem comparisons of size structure: Methods, approaches and prospects. Oikos 2011, 120, 550–563. [Google Scholar] [CrossRef]
- Reichard, M.; Polačik, M. Nothobranchius furzeri, an ‘instant’ fish from an ephemeral habitat. eLife 2019, 8, e41548. [Google Scholar] [CrossRef]
- Cichoń, M. Evolution of longevity through optimal resource allocation. Proc. R. Soc. B Biol. Sci. 1997, 264, 1383–1388. [Google Scholar] [CrossRef]
- Smith, F.A.; Payne, J.L.; Heim, N.A.; Balk, M.A.; Finnegan, S.; Kowalewski, M.; Lyons, S.K.; McClain, C.R.; McShea, D.W.; Novack-Gottshall, P.M.; et al. Body size evolution across the Geozoic. Annu. Rev. Earth Planet. Sci. 2016, 44, 523–553. [Google Scholar] [CrossRef]
- Garland, T., Jr.; Bennett, A.F.; Rezende, E.L. Phylogenetic approaches in comparative physiology. J. Exp. Biol. 2005, 208, 3015–3035. [Google Scholar] [CrossRef] [PubMed]
- Sibly, R.M.; Brown, J.H. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl. Acad. Sci. USA 2007, 104, 17707–17712. [Google Scholar] [CrossRef]
- Shattuck, M.R.; Williams, S.A. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl. Acad. Sci. USA 2010, 107, 4635–4639. [Google Scholar] [CrossRef]
- Damuth, J. Interspecific allometry of population density in mammals and other animals: The independence of body mass and population energy-use. Biol. J. Linn. Soc. 1987, 31, 193–246. [Google Scholar] [CrossRef]
- White, E.P.; Ernest, S.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.; Mace, G.M.; Jones, K.E.; Bielby, J.; Bininda-Emonds, O.R.; Sechrest, W.; Orme, C.D.L.; Purvis, A. Multiple causes of high extinction risk in large mammal species. Science 2005, 309, 1239–1241. [Google Scholar] [CrossRef]
- Smith, F.A.; Elliott Smith, R.E.; Lyons, S.K.; Payne, J.L. Body size downgrading of mammals over the late Quaternary. Science 2018, 360, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Boyer, A.G.; Jetz, W. Conservation biology. In Metabolic Ecology: A Scaling Approach; Sibly, R.M., Brown, J.H., Kodric-Brown, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 271–279. [Google Scholar]
- Newsome, T.M.; Wolf, C.; Nimmo, D.G.; Kopf, R.K.; Ritchie, E.G.; Smith, F.A.; Ripple, W.J. Constraints on vertebrate range size predict extinction risk. Glob. Ecol. Biogeogr. 2020, 29, 76–86. [Google Scholar] [CrossRef]
- Brown, J.H. Two decades of homage to Santa Rosalia: Toward a general theory of diversity. Am. Zool. 1981, 21, 877–888. [Google Scholar] [CrossRef]
- Brown, J.H.; Maurer, B.A. Evolution of species assemblages: Effects of energetic constraints and species dynamics on the diversification of the North American avifauna. Am. Nat. 1987, 130, 1–17. [Google Scholar] [CrossRef]
- Chichorro, F.; Juslén, A.; Cardoso, P. A review of the relation between species traits and extinction risk. Biol. Conserv. 2019, 237, 220–229. [Google Scholar] [CrossRef]
- Agosta, S.J.; Bernardo, J.; Ceballos, G.; Steele, M.A. A macrophysiological analysis of energetic constraints on geographic range size in mammals. PLoS ONE 2013, 8, e72731. [Google Scholar] [CrossRef]
- Gould, S.J. The Structure of Evolutionary Theory; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Jablonski, D. Species selection: Theory and data. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 501–524. [Google Scholar] [CrossRef]
- Cagan, A.; Baez-Ortega, A.; Brzozowska, N.; Abascal, F.; Coorens, T.H.; Sanders, M.A.; Lawson, A.R.; Harvey, L.M.; Bhosle, S.; Jones, D.; et al. Somatic mutation rates scale with lifespan across mammals. Nature 2022, 604, 517–524. [Google Scholar] [CrossRef]
- Weibel, E.R. Symmorphosis: On Form and Function in Shaping Life; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Ricklefs, R.E.; Wikelski, M. The physiology/life-history nexus. Trends Ecol. Evol. 2002, 17, 462–468. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl. Acad. Sci. USA 2010, 107, 10314–10319. [Google Scholar] [CrossRef] [PubMed]
- Smaers, J.B.; Rothman, R.S.; Hudson, D.R.; Balanoff, A.M.; Beatty, B.; Dechmann, D.K.; de Vries, D.; Dunn, J.C.; Fleagle, J.G.; Gilbert, C.C.; et al. The evolution of mammalian brain size. Sci. Adv. 2021, 7, eabe2101. [Google Scholar] [CrossRef] [PubMed]
- De Jong, G. Is invariance across animal species just an illusion? Science 2005, 309, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Nee, S.; Colegrave, N.; West, S.A.; Grafen, A. The illusion of invariant quantities in life histories. Science 2005, 309, 1236–1239. [Google Scholar] [CrossRef]
- Nespolo, R.F. New invariants and dimensionless numbers: Futile renaissance of old fallacies? Biol. Res. 2005, 38, 27–29. [Google Scholar] [CrossRef]
- Price, C.A.; Wright, I.J.; Ackerly, D.D.; Niinemets, Ü.; Reich, P.B.; Veneklaas, E.J. Are leaf functional traits ‘invariant’ with plant size and what is ‘invariance’ anyway? Funct. Ecol. 2014, 28, 1330–1343. [Google Scholar] [CrossRef]
- Hallmann, K.; Griebeler, E.M. An identification of invariants in life history traits of amphibians and reptiles. Ecol. Evol. 2020, 10, 1233–1251. [Google Scholar] [CrossRef]
- Price, C.A.; Weitz, J.S.; Savage, V.M.; Stegen, J.; Clarke, A.; Coomes, D.A.; Dodds, P.S.; Etienne, R.S.; Kerkhoff, A.J.; McCulloh, K.; et al. Testing the metabolic theory of ecology. Ecol. Lett. 2012, 15, 1465–1474. [Google Scholar] [CrossRef]
- Auer, S.K.; Dick, C.A.; Metcalfe, N.B.; Reznick, D.N. Metabolic rate evolves rapidly and in parallel with the pace of life history. Nat. Commun. 2018, 9, 14. [Google Scholar] [CrossRef]
- Harvey, P.H.; Pagel, M.D.; Rees, J.A. Mammalian metabolism and life histories. Am. Nat. 1991, 137, 556–566. [Google Scholar] [CrossRef]
- Johnson, M.S.; Thomson, S.C.; Speakman, J.R. Limits to sustained energy intake: II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. J. Exp. Biol. 2001, 204, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Simmen, B.; Morino, L.; Blanc, S.; Garcia, C. The energy allocation trade-offs underlying life history traits in hypometabolic strepsirhines and other primates. Sci. Rep. 2021, 11, 14196. [Google Scholar] [CrossRef] [PubMed]
- Åsheim, E.R.; Prokkola, J.M.; Morozov, S.; Aykanat, T.; Primmer, C.R. Standard metabolic rate does not associate with age-at-maturity genotype in juvenile Atlantic salmon. Ecol. Evol. 2022, 12, e8408. [Google Scholar] [CrossRef]
- Charnov, E.L.; Schaffer, W.M. Life-history consequences of natural selection: Cole’s result revisited. Am. Nat. 1973, 107, 791–793. [Google Scholar] [CrossRef]
- Promislow, D.E.; Harvey, P.H. Living fast and dying young: A comparative analysis of life-history variation among mammals. J. Zool. 1990, 220, 417–437. [Google Scholar] [CrossRef]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis; Chapman & Hall: New York, NY, USA, 1992. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Dańko, M.J.; Burger, O.; Argasiński, K.; Kozłowski, J. Extrinsic mortality can shape life-history traits, including senescence. Evol. Biol. 2018, 45, 395–404. [Google Scholar] [CrossRef]
- Healy, K.; Ezard, T.H.; Jones, O.R.; Salguero-Gómez, R.; Buckley, Y.M. Animal life history is shaped by the pace of life and the distribution of age-specific mortality and reproduction. Nat. Ecol. Evol. 2019, 3, 1217–1224. [Google Scholar] [CrossRef]
- Kozłowski, J.; Weiner, J. Interspecific allometries are by-products of body size optimization. Am. Nat. 1997, 149, 352–380. [Google Scholar] [CrossRef]
- Glazier, D.S. Variation in offspring investment within and among populations of Gammarus minus Say (Crustacea: Amphipoda) in ten mid-Appalachian springs (USA). Arch. Hydrobiol. 1999, 146, 257–283. [Google Scholar] [CrossRef]
- Sibly, R.M.; Brown, J.H. Mammal reproductive strategies driven by offspring mortality-size relationships. Am. Nat. 2009, 173, E185–E199. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Noriega, M.; White, C.R.; Kozłowski, J.; Day, T.; Marshall, D.J. Life history optimisation drives latitudinal gradients and responses to global change in marine fishes. PLoS Biol. 2023, 21, e3002114. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.; Bezerra, G.; Edwards, B.; Brown, J.; Forrest, S. Energy and time determine scaling in biological and computer designs. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150446. [Google Scholar] [CrossRef]
- Lewontin, R.C. Selection for colonizing ability. In The Genetics of Colonizing Species; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: New York, NY, USA, 1965; pp. 77–94. [Google Scholar]
- Sibly, R.; Calow, P. Why breeding earlier is always worthwhile. J. Theor. Biol. 1986, 123, 311–319. [Google Scholar] [CrossRef]
- Bergman, C.M.; Fryxell, J.M.; Gates, C.C.; Fortin, D. Ungulate foraging strategies: Energy maximizing or time minimizing? J. Anim. Ecol. 2001, 70, 289–300. [Google Scholar] [CrossRef]
- Gerber, L.R.; Reichman, O.J.; Roughgarden, J. Food hoarding: Future value in optimal foraging decisions. Ecol. Modell. 2004, 175, 77–85. [Google Scholar] [CrossRef]
- Alexander, R.D.; Bigelow, R.S. Allochronic speciation in field crickets, and a new species, Acheta veletis. Evolution 1960, 14, 334–346. [Google Scholar] [CrossRef]
- Taylor, R.S.; Friesen, V.L. The role of allochrony in speciation. Mol. Ecol. 2017, 26, 3330–3342. [Google Scholar] [CrossRef]
- Matsuda, M.; Hayashi, H.; Garcia-Ojalvo, J.; Yoshioka-Kobayashi, K.; Kageyama, R.; Yamanaka, Y.; Ikeya, M.; Toguchida, J.; Alev, C.; Ebisuya, M. Species-specific segmentation clock periods are due to differential biochemical reaction speeds. Science 2020, 369, 1450–1455. [Google Scholar] [CrossRef]
- Rayon, T.; Briscoe, J. Cross-species comparisons and in vitro models to study tempo in development and homeostasis. Interface Focus 2021, 11, 20200069. [Google Scholar] [CrossRef]
- Rayon, T. Cell time: How cells control developmental timetables. Sci. Adv. 2023, 9, eadh1849. [Google Scholar] [CrossRef] [PubMed]
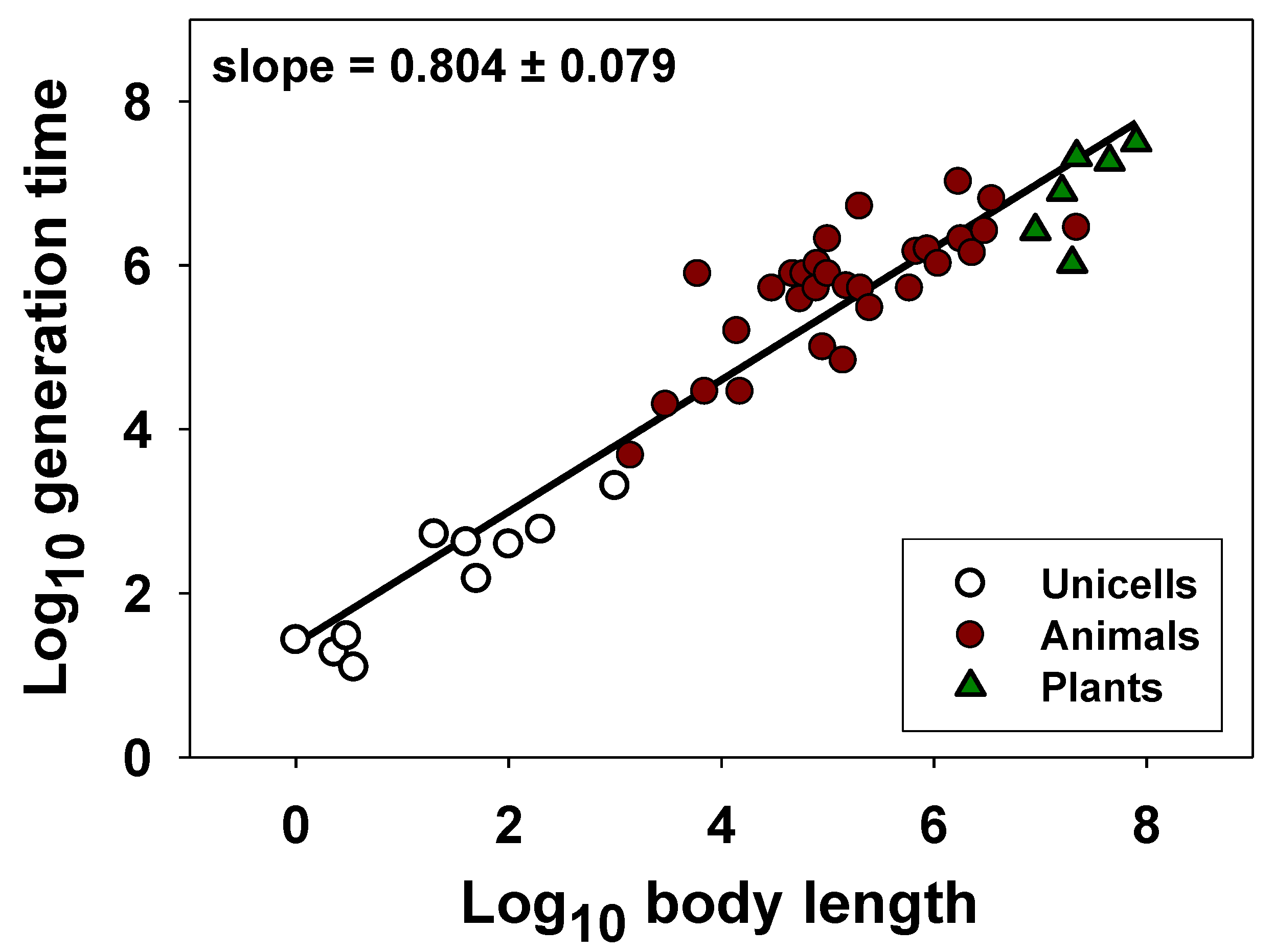
| Taxon | G/AM/AFR (Units) | Body Length (Units) | Slope (±95%CI) | Intercept (±95%CI) | r | N | p | Source |
|---|---|---|---|---|---|---|---|---|
| Unicellular and multicellular organisms | AFR (minutes) | µm | 0.804 (±0.079) | 1.384 (±0.394) | 0.953 | 46 | <0.0001 | [116] |
| Unicells | AFR (minutes) | µm | 0.731 (±0.249) | 1.178 (±0.403) | 0.920 | 10 | <0.0001 | [116] |
| Animals | AFR (minutes) | µm | 0.607 (±0.194) | 2.591 (±1.020) | 0.771 | 30 | <0.0001 | [116] |
| Cladocerans | AFR (days) | mm | 0.479 (±0.289) | 0.791 (±0.078) | 0.740 | 13 | 0.004 | [119] |
| Teleosts | AM (years) | cm | 0.799 (±0.354) | −0.925 (±0.614) | 0.791 | 16 | <0.0001 | [120] |
| Squamates | AM (months) | mm | 0.313 (±0.084) | 0.595 (±0.178) | 0.521 | 145 | <0.0001 | [121] |
| Reptiles | AM (days) | cm | 0.292 (±0.138) | 2.478 (±0.183) | 0.441 | 76 | <0.0001 | [122] |
| Birds | AM (days) | cm | 0.694 (±0.080) | 1.656 (±0.135) | 0.629 | 442 | <0.0001 | [122] |
| Mammals | G (years) | mm | 0.817 (±0.153) | −1.832 (±0.433) | 0.904 | 29 | <0.0001 | [106,122,123,124] |
| Mammals | AM (days) | cm | 0.674 (±0.035) | 1.487 (±0.055) | 0.664 | 1815 | <0.0001 | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazier, D.S. The Relevance of Time in Biological Scaling. Biology 2023, 12, 1084. https://doi.org/10.3390/biology12081084
Glazier DS. The Relevance of Time in Biological Scaling. Biology. 2023; 12(8):1084. https://doi.org/10.3390/biology12081084
Chicago/Turabian StyleGlazier, Douglas S. 2023. "The Relevance of Time in Biological Scaling" Biology 12, no. 8: 1084. https://doi.org/10.3390/biology12081084
APA StyleGlazier, D. S. (2023). The Relevance of Time in Biological Scaling. Biology, 12(8), 1084. https://doi.org/10.3390/biology12081084






