Hydrogen Sulfide Prevents LPS-Induced Depression-like Behavior through the Suppression of NLRP3 Inflammasome and Pyroptosis and the Improvement of Mitochondrial Function in the Hippocampus of Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Behavior Analysis
2.2.1. Sucrose Preference Test (SPT)
2.2.2. Forced Swimming Test (FST)
2.2.3. Open Field Test (OFT)
2.2.4. Elevate Zero Maze Test (EZM)
2.2.5. Integrated Behavioral Z-Normalization
2.3. Mitochondria Isolation and Measurement of Mitochondrial Function
2.4. Mitochondrial Respirometry
2.5. Electron Microscopy
2.6. Western Blotting Analysis
2.7. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
2.8. Statistical Analysis
3. Results
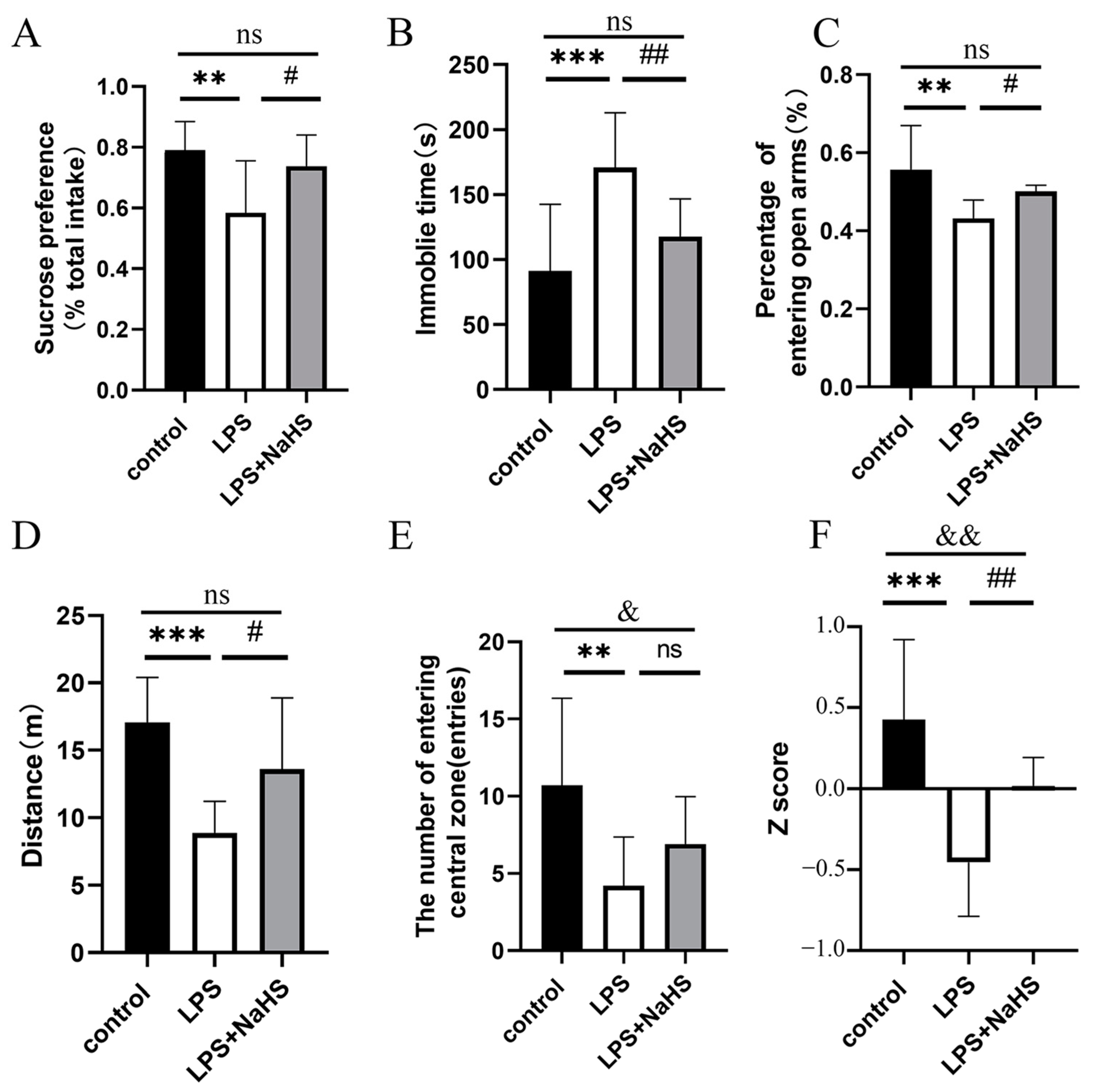
3.1. NaHS Pretreatment Prevents LPS-Induced Depression- and Anxiety-like Behavior
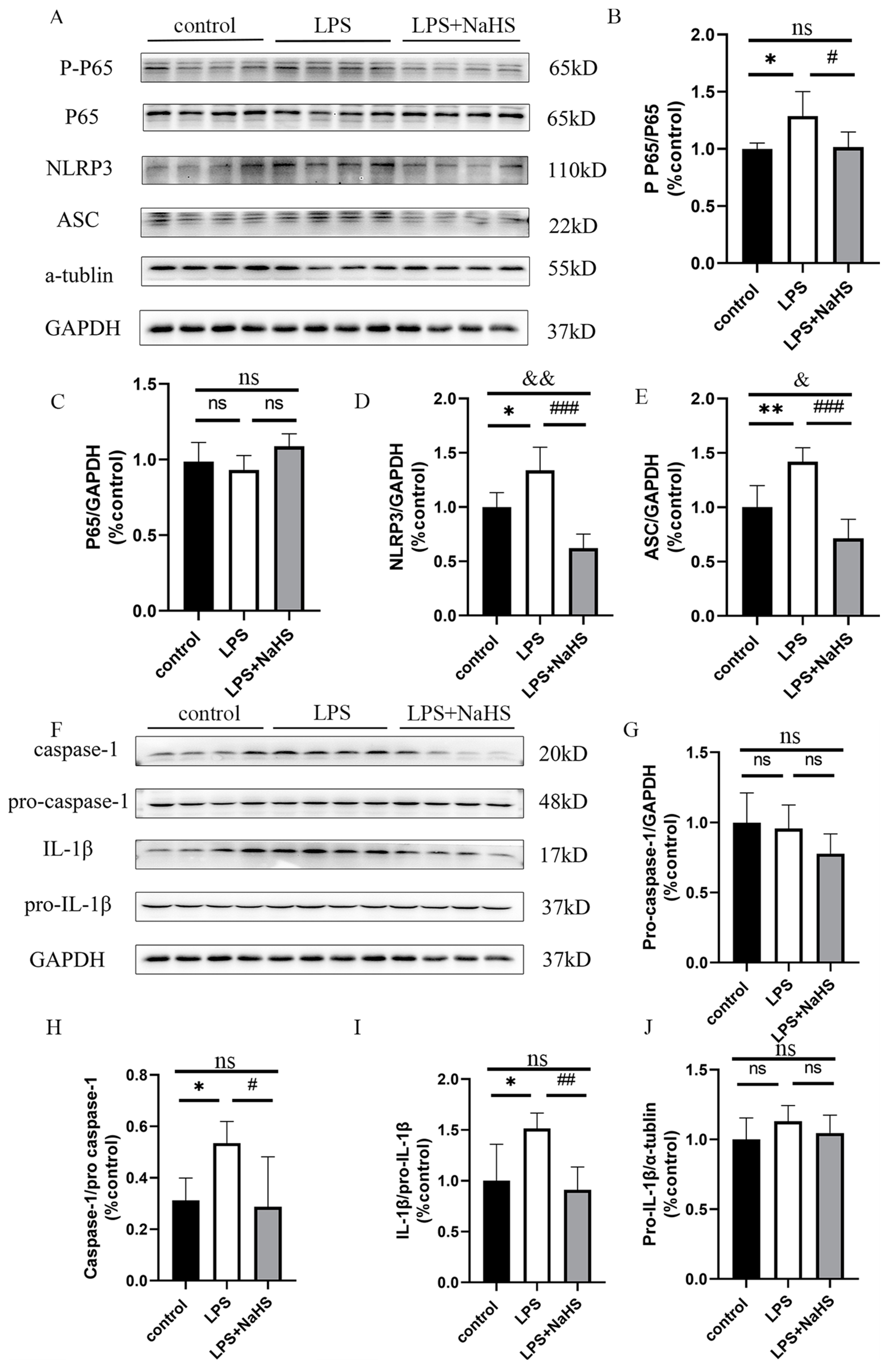
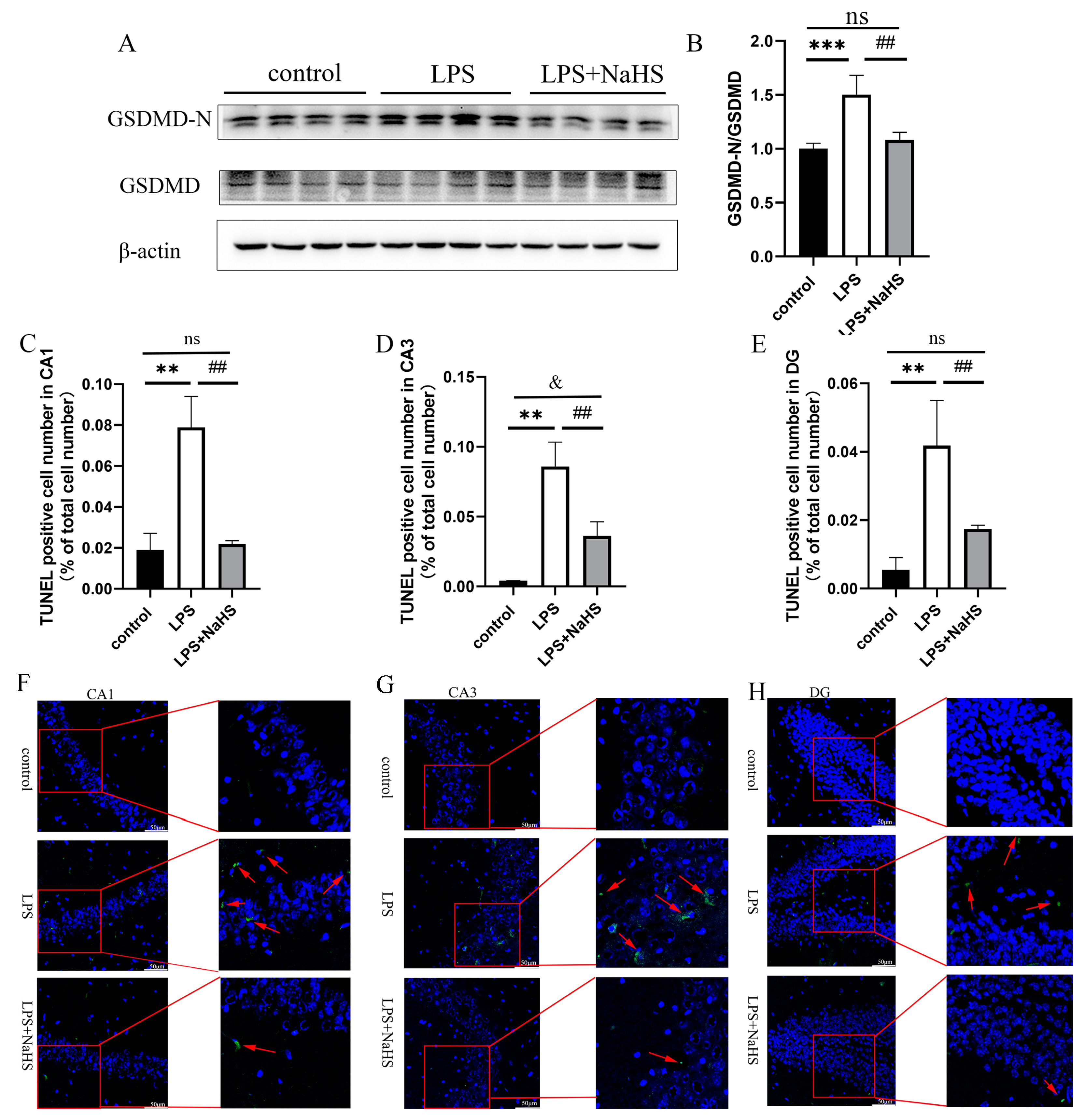
3.2. H2S Pretreatment Alleviates NF-κB and NLRP3 Inflammasome Activation and Pyroptosis in the Hippocampus of LPS-Treated Mice
3.3. H2S Prevents Mitochondrial Damage in the Hippocampus of LPS-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [PubMed]
- Gardner, A.; Boles, R.G. Beyond the serotonin hypothesis: Mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 730–743. [Google Scholar] [CrossRef]
- Peng, G.J.; Tian, J.S.; Gao, X.X.; Zhou, Y.Z.; Qin, X.M. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, H.; Baune, B.T. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology 2012, 37, 1397–1416. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, X.; Jiang, T.; Jin, L.; Zhao, X.D.; Cheng, J.H.; Jin, X.J.; Ma, J.; Piao, H.N.; Piao, L.X. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Int. Immunopharmacol. 2019, 67, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Zhuang, K.; Liu, Z.; Huang, C.; Gao, Y.; Chen, G.; Lin, H.; Hu, Y.; Wu, D.; Shi, M.; et al. Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation. Neuron 2018, 100, 551–563.e557. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.; Liu, Z.; Ali Shah, F.; Murtaza, I.; Zhang, Z.; Yang, X.; Liu, G.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667. [Google Scholar] [CrossRef]
- Frenois, F.; Moreau, M.; O’Connor, J.; Lawson, M.; Micon, C.; Lestage, J.; Kelley, K.W.; Dantzer, R.; Castanon, N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 2007, 32, 516–531. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ali, T.; He, K.; Liu, Z.; Shah, F.A.; Ren, Q.; Liu, Y.; Jiang, A.; Li, S. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav. Immun. 2021, 92, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, H.; Bao, Q.; Wang, Y.; Lu, J.; Ni, X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav. Immun. 2016, 56, 175–186. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Brkic, Z.; Milosavljevic, M.; Glavonic, E.; Adzic, M. Mitochondrial signaling in inflammation-induced depressive behavior in female and male rats: The role of glucocorticoid receptor. Brain Res. Bull. 2019, 150, 317–327. [Google Scholar] [CrossRef]
- Xia, C.Y.; Guo, Y.X.; Lian, W.W.; Yan, Y.; Ma, B.Z.; Cheng, Y.C.; Xu, J.K.; He, J.; Zhang, W.K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, Y.; Gong, Y.; Xu, Y.; Lu, J.; Sheng, H.; Ni, X. Treadmill Exercise Ameliorates Depression-Like Behavior in the Rats With Prenatal Dexamethasone Exposure: The Role of Hippocampal Mitochondria. Front. Neurosci. 2019, 13, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol. Psychiatry 2021, 27, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Gebara, E.; Zanoletti, O.; Ghosal, S.; Grosse, J.; Schneider, B.L.; Knott, G.; Astori, S.; Sandi, C. Mitofusin-2 in the Nucleus Accumbens Regulates Anxiety and Depression-like Behaviors Through Mitochondrial and Neuronal Actions. Biol. Psychiatry 2021, 89, 1033–1044. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Gao, C.; Xie, L.; Zhai, L.; Cui, G.; Yin, X. Mitochondrial transplantation attenuates lipopolysaccharide- induced depression-like behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 240–249. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, L.; Lu, S.; Li, C.; Bai, M.; Xu, E.; Shen, J.; Li, Y. Baicalin ameliorates CUMS-induced depression-like behaviors through activating AMPK/PGC-1α pathway and enhancing NIX-mediated mitophagy in mice. Eur. J. Pharmacol. 2023, 938, 175435. [Google Scholar] [CrossRef]
- Głombik, K.; Stachowicz, A.; Olszanecki, R.; Ślusarczyk, J.; Trojan, E.; Lasoń, W.; Kubera, M.; Budziszewska, B.; Spedding, M.; Basta-Kaim, A. The effect of chronic tianeptine administration on the brain mitochondria: Direct links with an animal model of depression. Mol. Neurobiol. 2016, 53, 7351–7362. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, H.; Xue, X.; Xia, J.; Liu, J.; Qi, Z.; Ji, L. OGT-related mitochondrial motility is associated with sex differences and exercise effects in depression induced by prenatal exposure to glucocorticoids. J. Affect. Disord. 2018, 226, 203–215. [Google Scholar] [CrossRef]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid. Med. Cell Longev. 2020, 2020, 2972968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.Y.; Li, D.; Zeng, H.Y.; Kan, L.Y.; Zou, W.; Zhang, P.; Gu, H.F.; Tang, X.Q. Hydrogen Sulfide Inhibits Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior by Upregulation of Sirt-1: Involvement in Suppression of Hippocampal Endoplasmic Reticulum Stress. Int. J. Neuropsychopharmacol. 2017, 20, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Arora, P.; Sandhir, R. Hydrogen Sulfide Reverses LPS-Induced Behavioral Deficits by Suppressing Microglial Activation and Promoting M2 Polarization. J. Neuroimmune Pharmacol. 2021, 16, 483–499. [Google Scholar] [CrossRef]
- Ruilian, L.; Honglin, Q.; Jun, X.; Jianxin, L.; Qingyun, B.; Yilin, C.; Haifeng, M. H(2)S-mediated aerobic exercise antagonizes the hippocampal inflammatory response in CUMS-depressed mice. J. Affect. Disord. 2021, 283, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Kan, L.Y.; Zeng, H.Y.; Tang, Y.Y.; Huang, H.L.; Xie, M.; Zou, W.; Wang, C.Y.; Zhang, P.; Tang, X.Q. BDNF/TrkB Pathway Mediates the Antidepressant-Like Role of H(2)S in CUMS-Exposed Rats by Inhibition of Hippocampal ER Stress. Neuromolecular Med. 2018, 20, 252–261. [Google Scholar] [CrossRef]
- Tan, H.; Zou, W.; Jiang, J.; Tian, Y.; Xiao, Z.; Bi, L.; Zeng, H.; Tang, X. Disturbance of hippocampal H2S generation contributes to CUMS-induced depression-like behavior: Involvement in endoplasmic reticulum stress of hippocampus. Acta Biochim. Biophys. Sin. 2015, 47, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.J.; Du, J.K.; Hu, X.; Yu, Q.; Li, D.X.; Wang, C.N.; Zhu, X.Y.; Liu, Y.J. Protective effects of resveratrol on mitochondrial function in the hippocampus improves inflammation-induced depressive-like behavior. Physiol. Behav. 2017, 182, 54–61. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar]
- Tucker, L.B.; McCabe, J.T. Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze. Front. Behav. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Guilloux, J.P.; Seney, M.; Edgar, N.; Sibille, E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. J. Neurosci. Methods 2011, 197, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Burtscher, J.; Zangrandi, L.; Schwarzer, C.; Gnaiger, E. Differences in mitochondrial function in homogenated samples from healthy and epileptic specific brain tissues revealed by high-resolution respirometry. Mitochondrion 2015, 25, 104–112. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Sun, Y.; Song, M.; Song, Y.; Fang, Y.; Zhang, Q.; Li, X.; Song, N.; Ding, J.; Lu, M.; et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 2021, 6, e146852. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Wang, J.; Wang, H. The Role of the Effects of Autophagy on NLRP3 Inflammasome in Inflammatory Nervous System Diseases. Front. Cell Dev. Biol. 2021, 9, 657478. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Pitharouli, M.C.; Hagenaars, S.P.; Glanville, K.P.; Coleman, J.R.I.; Hotopf, M.; Lewis, C.M.; Pariante, C.M. Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am. J. Psychiatry 2021, 178, 522–529. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef] [PubMed]
- Yue, N.; Huang, H.; Zhu, X.; Han, Q.; Wang, Y.; Li, B.; Liu, Q.; Wu, G.; Zhang, Y.; Yu, J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J. Neuroinflammation 2017, 14, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jin, H.; Shi, Y.; Guo, Y.; Zhang, H. Pyroptosis, a novel mechanism implicated in cataracts. Mol. Med. Rep. 2018, 18, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflammation 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, Z.; Huang, Y.; Wang, G.; Tang, Z.; Lu, J.; Wang, C.; Ni, X. Reduced hydrogen sulfide production contributes to adrenal insufficiency induced by hypoxia via modulation of NLRP3 inflammasome activation. Redox Rep. 2023, 28, 2163354. [Google Scholar] [CrossRef]
- Pei, L.; Wallace, D.C. Mitochondrial Etiology of Neuropsychiatric Disorders. Biol. Psychiatry 2018, 83, 722–730. [Google Scholar] [CrossRef]
- Zhao, F.L.; Fang, F.; Qiao, P.F.; Yan, N.; Gao, D.; Yan, Y. AP39, a Mitochondria-Targeted Hydrogen Sulfide Donor, Supports Cellular Bioenergetics and Protects against Alzheimer’s Disease by Preserving Mitochondrial Function in APP/PS1 Mice and Neurons. Oxid. Med. Cell Longev. 2016, 2016, 8360738. [Google Scholar] [CrossRef] [Green Version]
- Lohakul, J.; Jeayeng, S.; Chaiprasongsuk, A.; Torregrossa, R.; Wood, M.E.; Saelim, M.; Thangboonjit, W.; Whiteman, M.; Panich, U. Mitochondria-Targeted Hydrogen Sulfide Delivery Molecules Protect Against UVA-Induced Photoaging in Human Dermal Fibroblasts, and in Mouse Skin In Vivo. Antioxid. Redox Signal 2021, 36, 1268–1288. [Google Scholar] [CrossRef] [PubMed]
- Latorre, E.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Harries, L.W. Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2. Aging (Albany N. Y.) 2018, 10, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Genovese, I.; Perrone, M.; Vezzani, B.; Vitto, V.A.M.; Giorgi, C. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. J. Clin. Med. 2020, 9, 740. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Yao, H. Mitochondrial dysfunction in inflammatory responses and cellular senescence: Pathogenesis and pharmacological targets for chronic lung diseases. Br. J. Pharmacol. 2016, 173, 2305–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal cortex and depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Grogans, S.E.; Fox, A.S.; Shackman, A.J. The Amygdala and Depression: A Sober Reconsideration. Am. J. Psychiatry 2022, 179, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, P.; Gong, Y.; Wang, Y.; Xu, M.; Qian, Z.; Ni, X.; Lu, J. Hydrogen Sulfide Prevents LPS-Induced Depression-like Behavior through the Suppression of NLRP3 Inflammasome and Pyroptosis and the Improvement of Mitochondrial Function in the Hippocampus of Mice. Biology 2023, 12, 1092. https://doi.org/10.3390/biology12081092
Bao P, Gong Y, Wang Y, Xu M, Qian Z, Ni X, Lu J. Hydrogen Sulfide Prevents LPS-Induced Depression-like Behavior through the Suppression of NLRP3 Inflammasome and Pyroptosis and the Improvement of Mitochondrial Function in the Hippocampus of Mice. Biology. 2023; 12(8):1092. https://doi.org/10.3390/biology12081092
Chicago/Turabian StyleBao, Peng, Yuxiang Gong, Yanjie Wang, Miaomiao Xu, Zhenyu Qian, Xin Ni, and Jianqiang Lu. 2023. "Hydrogen Sulfide Prevents LPS-Induced Depression-like Behavior through the Suppression of NLRP3 Inflammasome and Pyroptosis and the Improvement of Mitochondrial Function in the Hippocampus of Mice" Biology 12, no. 8: 1092. https://doi.org/10.3390/biology12081092
APA StyleBao, P., Gong, Y., Wang, Y., Xu, M., Qian, Z., Ni, X., & Lu, J. (2023). Hydrogen Sulfide Prevents LPS-Induced Depression-like Behavior through the Suppression of NLRP3 Inflammasome and Pyroptosis and the Improvement of Mitochondrial Function in the Hippocampus of Mice. Biology, 12(8), 1092. https://doi.org/10.3390/biology12081092






