Regular Sport Activity Is Able to Reduce the Level of Genomic Damage
Abstract
:Simple Summary
Abstract
1. Introduction
- (a)
- to evaluate whether physical activity affects background levels of MNi, NBUDs and binucleated cells;
- (b)
- to test whether the different sports disciplines differentially affect these levels;
- (c)
- to evaluate the possible influence of some gene polymorphisms on the frequencies of the analyzed genomic markers.
2. Materials and Methods
2.1. Population Sample
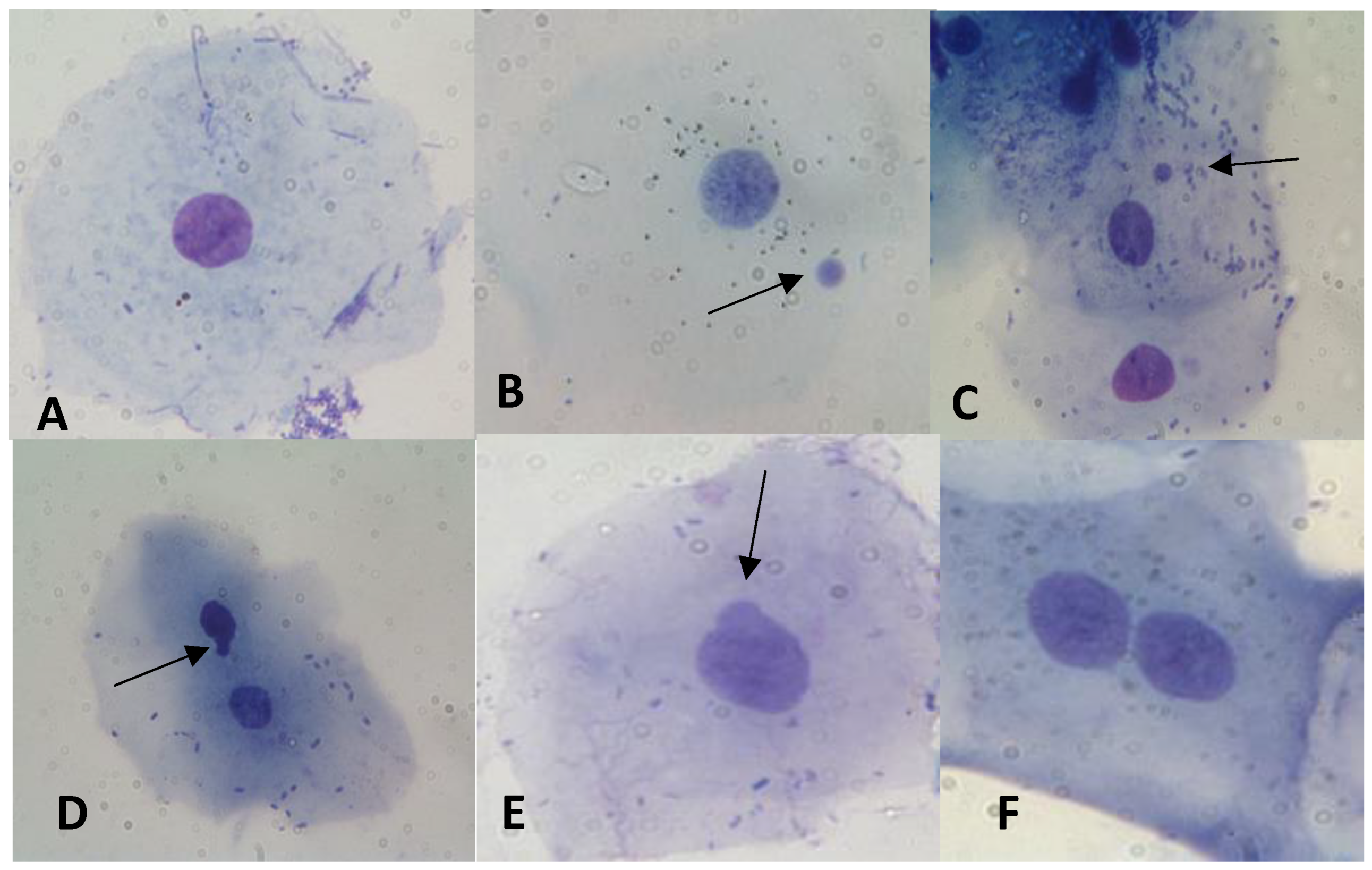
2.2. Buccal MNi Assay
2.3. DNA Extraction and Genotyping
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grazioli, E.; Dimauro, I.; Mercatelli, N.; Wang, G.; Pitsiladis, Y.; Di Luigi, L.; Caporossi, D. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genom. 2017, 18, 802. [Google Scholar] [CrossRef] [Green Version]
- Sellami, M.; Bragazzi, N.; Prince, M.S.; Denham, J.; Elrayess, M. Regular, Intense Exercise Training as a Healthy Aging Lifestyle Strategy: Preventing DNA Damage, Telomere Shortening and Adverse DNA Methylation Changes Over a Lifetime. Front. Genet. 2021, 12, 652497. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.D.; Smith, C.; Kaiser, M.; Levinger, I.; Duque, G.; Gruber, H.J.; Herrmann, M. Physical activity, a modulator of aging through effects on telomere biology. Aging 2020, 12, 13803–13823. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Rebelo-Marques, A.; De Sousa Lages, A.; Andrade, R.; Ribeiro, C.F.; Mota-Pinto, A.; Carrilho, F.; Espregueira-Mendes, J. The Benefits of Physical Exercise. Front. Endocrinol. Aging Hallm. 2018, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Rezapour, S.; Shiravand, M.; Mardani, M. Epigenetic changes due to physical activity. Biotechnol. Appl. Biochem. 2018, 65, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Cardarelli, R.; Caroll, J.; Zhang, S.; Fulda, K.G.; Gonzalez, K.; Vishwanatha, J.K.; Morabia, A.; Santella, R.M. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics 2011, 6, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Larsen, F.J.; Schiffer, T.A.; Ørtenblad, N.; Zinner, C.; Morales-Alamo, D.; Willis, S.J.; Calbet, J.A.; Holmberg, H.C.; Boushel, R. High-intensity sprint training inhibits mitochondrial respiration through aconitase inactivation. FASEB J. 2016, 30, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Steinbacger, M.B.P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Margaritelis, N.V.; Matsakas, A. Quantitative Redox Biology of Exercise. Int. J. Sports Med. 2020, 41, 633–645. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul, E.B.H.; Duchnik, E. Exercise-induced oxidative stress and melatonin supplementation: Current evidence. J. Physiol. Sci. 2021, 71, 27. [Google Scholar] [CrossRef]
- Meneghini, R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef]
- Renaudin, X. Reactive oxygen species and DNA damage response in cancer. Int. Rev. Cell Mol. Biol. 2021, 364, 139–161. [Google Scholar]
- Brancaccio, M.; Menniti, C.; Cesaro, A.; Fimiani, F.; Moscarella, E.; Caiazza, M.; Gragnano, F.; Ranieri, A.; D’Alicandro, G.; Tinto, N.; et al. Dietary Thiols: A Potential Supporting Strategy against Oxidative Stress in Heart Failure and Muscular Damage during Sports Activity. Int. J. Environ. Res. Pub. Health 2020, 17, 9424. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Fiebig, R.; Gore, M.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Eur. J. Physiol. 2001, 442, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Harvey, S.; Gruner, T.; Fenech, M. The buccal cytome and micronucleus frequency is substantially altered in Down’s syndrome and normal ageing compared to young healthy controls. Mutat. Res. 2008, 638, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagen. 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay–An update and expanded photogallery. Mutat. Res. Rev. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal micronucleus cytome assay. Nat. Prot. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Pittaluga, M.; Parisi, P.; Sabatini, S.; Ceci, R.; Caporossi, D.; Catani, M.V.; Savini, I.; Avigliano, L. Cellular and biochemical parameters of exercise-induced oxidative stress: Relationship with training levels. Free. Radic. Res. 2006, 40, 607–614. [Google Scholar] [CrossRef]
- Sharma, R.; Shailey; Gandhi, G. Pre-cancerous (DNA and chromosomal) lesions in professional sports. J. Cancer Res. Ther. 2012, 8, 578–585. [Google Scholar] [CrossRef]
- Franzke, B.; Schober-Halper, B.; Hofmann, M.; Oesen, S.; Tosevska, A.; Nersesyan, A.; Knasmüller, S.; Strasser, E.M.; Wallner, M.; Wessner, B.; et al. Chromosomal stability in buccal cells was linked to age but not affected by exercise and nutrients–Vienna Active Ageing Study (VAAS), a randomized controlled trial. Redox Biol. 2020, 28, 101362. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, F.; Cocchi, E.; Buglisi, M.; Squillacioti, G.; Bellisario, V.; Bono, R.; Santovito, A. The role of phase I, phase II, and DNA-repair gene polymorphisms in the damage induced by formaldehyde in pathologists. Sci. Rep. 2021, 11, 10507. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Delsoglio, M.; Manitta, E.; Picco, G.; Meschiati, G.; Chiarizio, M.; Gendusa, C.; Cervella, P. Association of GSTT1 null, XPD 751 CC and XPC 939 CC genotypes with increased levels of genomic damage among hospital pathologists. Biomarkers 2017, 22, 557–565. [Google Scholar] [CrossRef]
- Santovito, A.; Cervella, P.; Delpero, M. Chromosomal damage in peripheral blood lymphocytes from nurses occupationally exposed to chemicals. Hum. Exp. Toxicol. 2014, 33, 897–903. [Google Scholar] [CrossRef]
- Santovito, A.; Cervella, P.; Delpero, M. Increased frequency of chromosomal aberrations and sister chromatid exchanges in peripheral lymphocytes of radiology technicians chronically exposed to low levels of ionizing radiations. Environ. Toxicol. Pharmacol. 2014, 37, 396–403. [Google Scholar] [CrossRef]
- Santovito, A.; Caravella, P.; Delpero, M. Evaluation of Genomic Damage in Peripheral Lymphocytes from Occupationally Exposed Anesthetists: Assessment of the Effects of Age, Sex, and GSTT1 Gene Polymorphism. J. Biochem. Mol. Toxicol. 2015, 29, 234–239. [Google Scholar] [CrossRef]
- Santovito, A.; Cervella, P.; Delpero, M. Evidence of genotoxicity in lymphocytes of non-smoking alcoholics. Mol. Biol. Rep. 2015, 42, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Bouzid, M.A.; Filaire, E.; McCall, A.; Fabre, C. Radical Oxygen Species, Exercise and Aging: An Update. Sports Med. 2015, 45, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Phillips, S.; Cassaro Vechin, F.; Ugrinowitsch, C. A Review of Resistance Training-Induced Changes in Skeletal Muscle Protein Synthesis and Their Contribution to Hypertrophy. Sports Med. 2015, 45, 801–807. [Google Scholar] [CrossRef]
- Vecchio, M.; Currò, M.; Trimarchi, F.; Naccari, S.; Caccamo, D.; Riccardo, L.; Barreca, D.; Di Mauro, D. The Oxidative Stress Response in Elite Water Polo Players: Effects of Genetic Background. BioMed Res. Int. 2017, 2017, 7019694. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J. Effect of exercise on oxidative stress biomarkers. Adv. Clin. Chem. 2008, 46, 1–50. [Google Scholar] [PubMed]
- Bloomer, R.J.; Goldfarb, A.H. Anaerobic exercise and oxidative stress: A review. Can. J. Appl. Physiol. 2004, 29, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Suzuki, K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc. Immunol. Rev. 2004, 10, 129–141. [Google Scholar] [PubMed]
- Koyama, K. Exercise-induced oxidative stress: A tool for “hormesis” and “adaptive response”. J. Physic. Fit. Sports Med. 2014, 3, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Santovito, A.; Gendusa, C. Micronuclei frequency in peripheral blood lymphocytes of healthy subjects living in Turin (North-Italy): Contribution of body mass index, age and sex. Ann. Hum. Biol. 2020, 47, 48–54. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Oreščanin, V.; Garaj-Vrhovac, V. Cytokinesis-block micronucleus cytome assay parameters in peripheral blood lymphocytes of the general population: Contribution of age, sex, seasonal variations and lifestyle factors. Ecotoxicol. Environ. Saf. 2018, 148, 561–570. [Google Scholar] [CrossRef]
- Cotton, S.C.; Sharp, L.; Little, J.; Brockton, N. Glutathione S-transferase polymorphisms and colorectal cancer: A HuGE review. Am. J. Epidemiol. 2000, 151, 7–32. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Lee, S.Y.; Ki, C.S.; Jong-Won, K. GSTM1, GSTT1 and GSTP1 polymorphisms in the Korean population. J. Korean Med. Sci. 2005, 20, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xue, K.; Xu, L.; Ma, G.; Wu, J. Polymorphisms of the CYP1A1 and GSTM1 genes in relation to individual suscepti-bility to lung carcinoma in Chinese population. Mutat. Res. 2001, 458, 41–47. [Google Scholar]
- Hu, Z.; Wang, Y.; Wang XLiang, G.; Miao, X.; Xu, Y.; Tan, W.; Wei, Q.; Lin, D.; Shen, H. DNA repair gene XPC geno-types/haplotypes and risk of lung cancer in a Chinese population. Int. J. Cancer 2005, 11, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Pemble, S.; Schroeder, K.R.; Spencer, S.R.; Meyer, D.J.; Hallier, E.; Bolt, H.M.; Ketterer, B.; Taylo, J.B. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J. 1994, 300, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ji, F.; Sun, Y.; Qui, Y.L.; Wang, W.; Wu, F.; Miao, W.; Li, Y.; Brandt-Rauf, P.W.; Xia, Z. Genetic polymorphisms of XRCC1, HOGG1 and MGMT and micronucleus occurrence in Chinese vinyl chloride-exposed workers. Carcinogenesis 2010, 31, 1068–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Wyllie, A.H.; Barnes, D.; Wolf, C.R.; Spurr, N.K. Relationship between GSTM1 genetic polymorphism and suscep-tibility to bladder, breast and colon cancer. Carcinogenesis 1993, 14, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
| Subjects | N (Frequency) | Age ( ± σ) | Weight ( ± σ) |
|---|---|---|---|
| Total Subjects | 206 | 21.631 ± 3.494 | 70.560 ± 11.770 |
| Males | 148 | 21.858 ± 3.720 | 74.418 ± 10.081 A |
| Females | 58 | 21.052 ± 2.781 | 60.716 ± 9.942 |
| Athlete | 125 | 21.528 ± 4.063 | 72.654 ± 12.103 |
| Males | 89 | 21.697 ± 4.342 | 76.526 ± 10.496 |
| Females | 36 | 21.111 ± 3.293 | 63.083 ± 10.470 |
| Martial Arts | 34 | 22.500 ± 4.460 | 70.941 ± 9.739 |
| Basketball | 29 | 20.207 ± 3.668 | 81.652 ± 9.992 B,C |
| Volleyball | 40 | 21.300 ± 4.201 | 72.223 ± 12.722 |
| Sprinters | 22 | 22.182 ± 3.319 | 64.227 ± 9.532 |
| Controls | 81 | 21.790 ± 2.375 | 67.327 ± 10.512 |
| Males | 59 | 22.102 ± 2.524 | 71.237 ± 8.563 |
| Females | 22 | 20.955 ± 1.704 | 56.841 ± 7.763 |
| Subjects | N | MNi Nab ( ± σ) | NBUDs Nab ( ± σ) | Binucleated Cells Nab ( ± σ) |
|---|---|---|---|---|
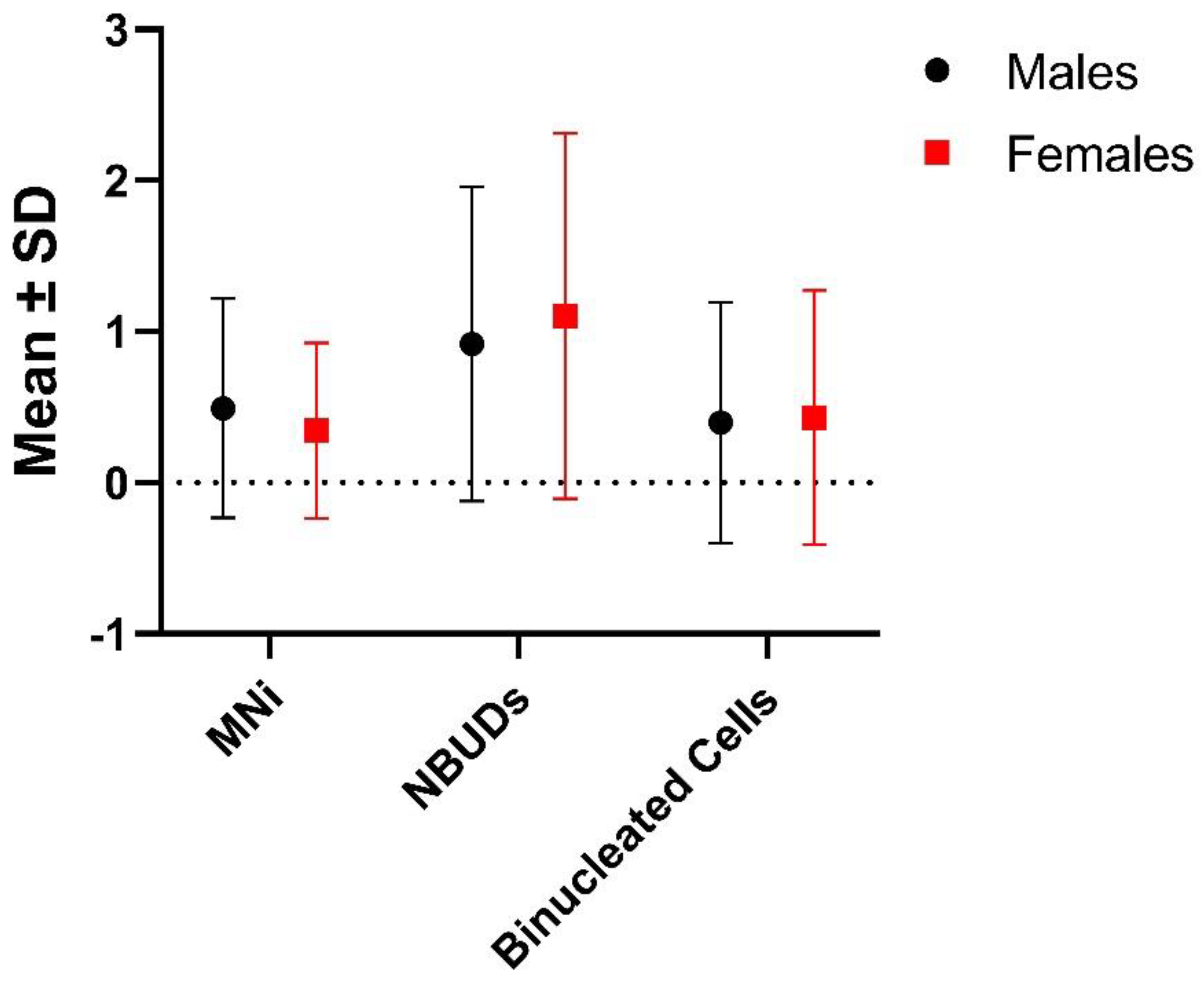
| Males | 148 | 73 (0.493 ± 0.724) | 136 (0.919 ± 1.040) | 59 (0.399 ± 0.797) |
| Females | 58 | 20 (0.345 ± 0.579) | 64 (1.103 ± 1.209) | 25 (0.431 ± 0.840) |
| Subjects | N | MNi Nab ( ± σ) | NBUDs Nab ( ± σ) | Binucleated Cells Nab ( ± σ) |
|---|---|---|---|---|
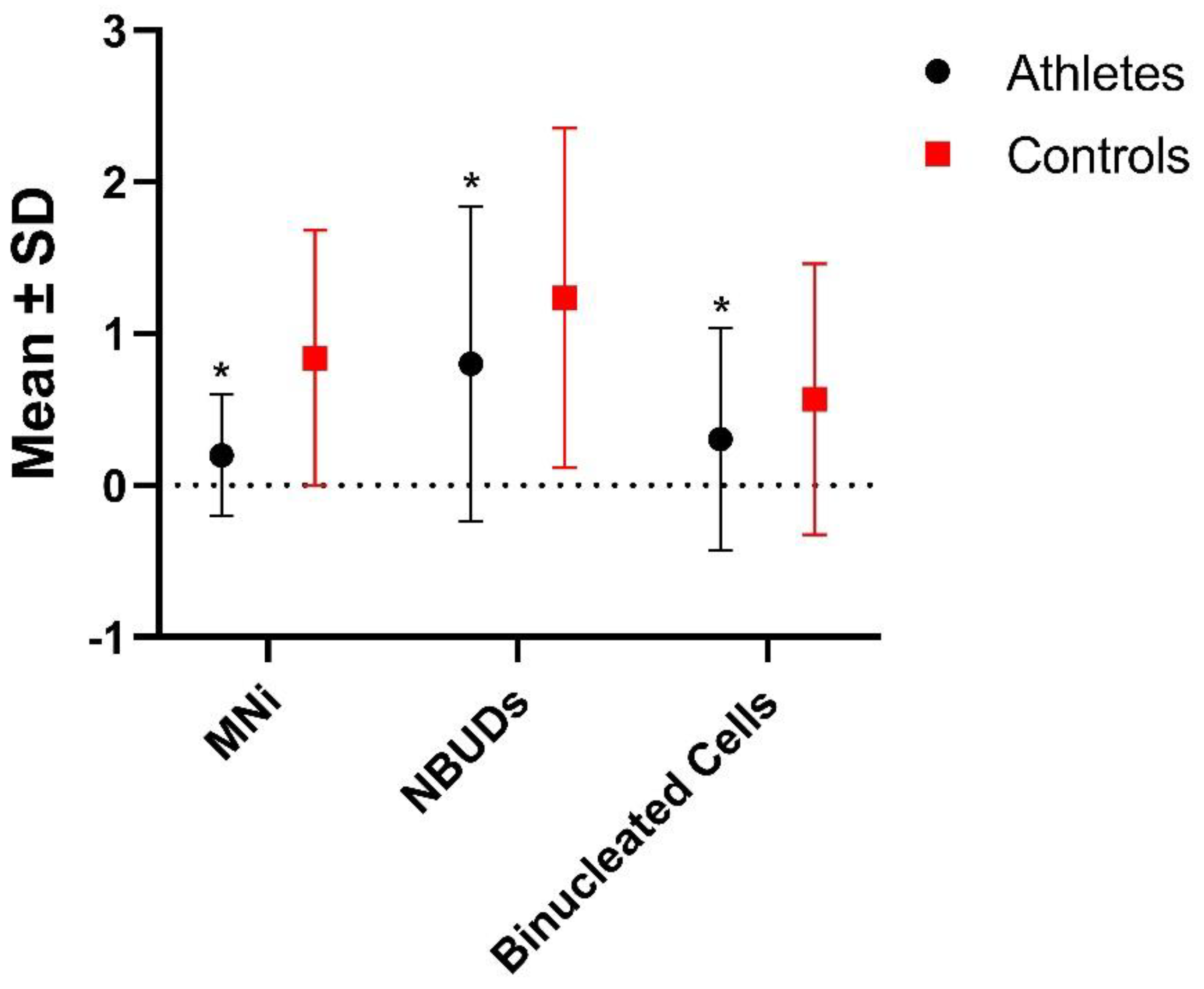
| Athletes | 125 | 25 (0.200 ± 0.402) A | 100 (0.800 ± 1.040) B | 38 (0.304 ± 0.732) C |
| Controls | 81 | 68 (0.840 ± 0.843) | 100 (1.235 ± 1.121) | 46 (0.568 ± 0.894) |
| Subjects | N | MNi Nab ( ± σ) | NBUDs Nab ( ± σ) | Binucleated Cells Nab ( ± σ) |
|---|---|---|---|---|
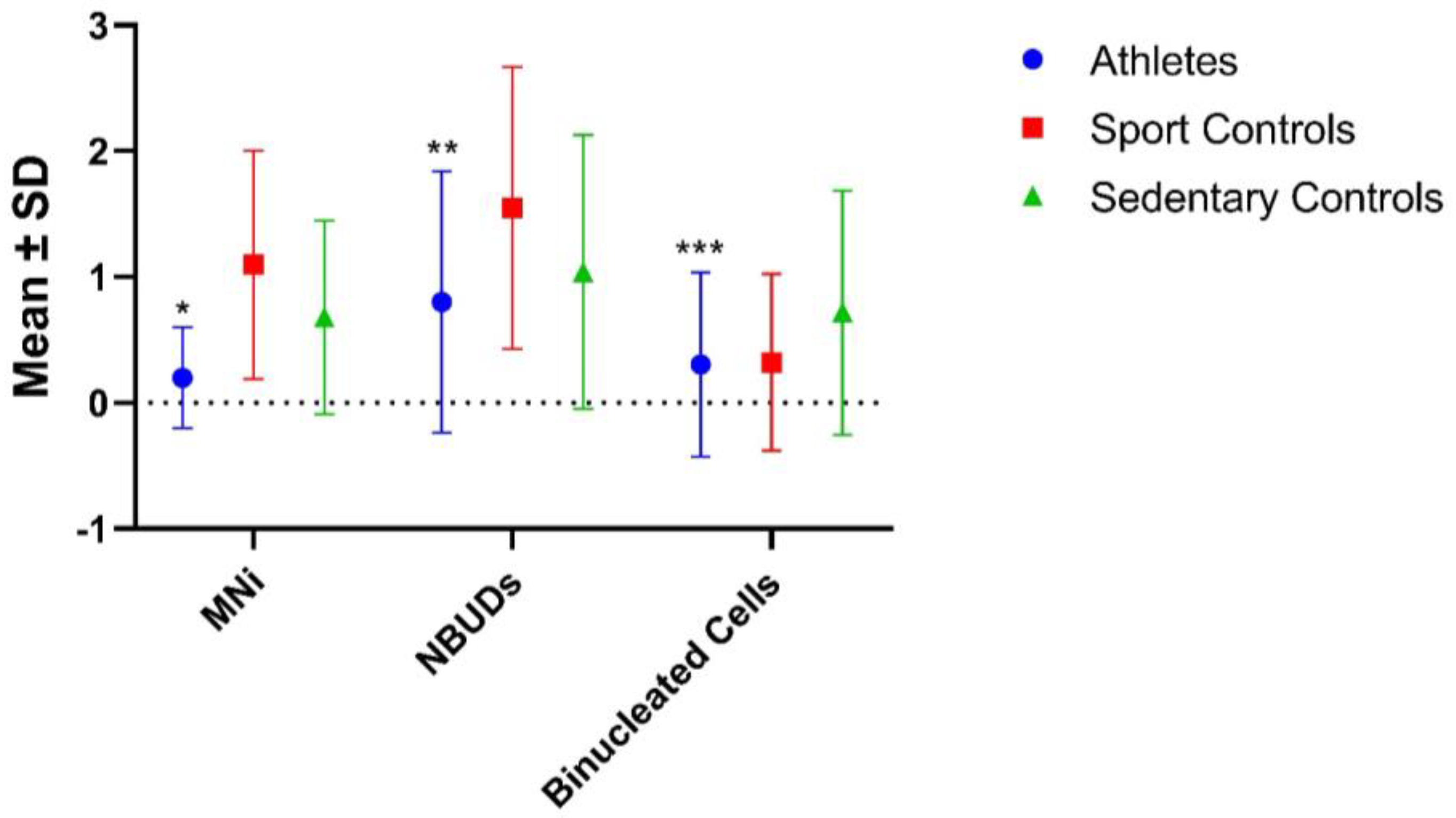
| Athletes | 125 | 25 (0.200 ± 0.402) A | 100 (0.800 ± 1.040) B | 38 (0.304 ± 0.732) C |
| Sport Controls | 31 | 34 (1.097 ± 0.908) D | 48 (1.548 ± 1.121) E | 10 (0.323 ± 0.702) |
| Sedentary Controls | 50 | 34 (0.680 ± 0.768) | 52 (1.040 ± 1.087) | 36 (0.720 ± 0.970) |
| Subjects | N | MNi Nab ( ± σ) | NBUDs Nab ( ± σ) | Binucleated Cells Nab ( ± σ) |
|---|---|---|---|---|
| Martial Arts | 34 | 5 (0.147 ± 0.359) | 21 (0.618 ±0.779) | 18 (0.529 ± 0.961) D |
| Basketball | 29 | 4 (0.138 ± 0.351) | 16 (0.552 ± 0.736) | 5 (0.172 ± 0.468) |
| Volleyball | 40 | 6 (0.150 ± 0.707) | 50 (1.250 ± 3.536) B | 15 (0.375 ± 0.000) |
| Sprinters | 22 | 10 (0.455 ± 0.510) A | 13 (0.591 ± 0.666) | 0 (0.000 ± 0.000) C |
| Gene Polymorphisms | Allele | N | Frequency | Genotype | N | Frequency | HWE p-Value | χ2-Value |
|---|---|---|---|---|---|---|---|---|
| GSTT | Positive | -- | -- | Positive | 152 | 0.738 | n.a. | n.a. |
| Null | -- | -- | Null | 54 | 0.262 | |||
| GSTM | Positive | -- | -- | Positive | 142 | 0.689 | n.a. | n.a. |
| Null | -- | -- | Null | 64 | 0.310 | |||
| CYP1A1 | A | 299 | 0.726 | AA | 103 | 0.500 | p > 0.05 | 3.701 |
| G | 113 | 0.274 | AG | 93 | 0.451 | |||
| GG | 10 | 0.049 | ||||||
| XRCC1 | C | 295 | 0.716 | CC | 100 | 0.485 | p > 0.05 | 3.699 |
| T | 117 | 0.284 | CT | 95 | 0.461 | |||
| TT | 11 | 0.054 | ||||||
| XPC | A | 282 | 0.684 | AA | 98 | 0.476 | p > 0.05 | 0.231 |
| C | 130 | 0.316 | AC | 86 | 0.417 | |||
| CC | 22 | 0.107 |
| Gene | Genotype | N | Total MNi | ± SE | Total NBUDs | ± SE | Total BIN | ± SE |
|---|---|---|---|---|---|---|---|---|
| GSTT1 | Positive | 152 | 66 | 0.434 ± 0.055 | 152 | 1.000 ± 0.080 | 72 | 0.474 ± 0.071 |
| Null | 54 | 27 | 0.500 ± 0.098 | 48 | 0.889 ± 0.142 | 12 | 0.222 ± 0.073 | |
| GSTM | Positive | 142 | 66 | 0.465 ± 0.059 | 141 | 0.993 ± 0.089 | 60 | 0.423 ± 0.068 |
| Null | 64 | 27 | 0.422 ± 0.083 | 59 | 0.922 ± 0.147 | 24 | 0.375 ± 0.101 | |
| CYP1A1 | AA | 103 | 53 | 0.515 ± 0.070 | 109 | 1.058 ± 0.119 | 35 | 0.340 ± 0.075 |
| AG | 93 | 36 | 0.387 ± 0.066 | 79 | 0.849 ± 0.093 | 47 | 0.505 ± 0.092 | |
| GG | 10 | 4 | 0.400 ± 0.221 | 12 | 1.200 ± 0.389 | 2 | 0.200 ± 0.133 | |
| XRCC1 | CC | 100 | 45 | 0.450 ± 0.067 | 103 | 1.030 ± 0.110 | 39 | 0.390 ± 0.075 |
| CT | 95 | 44 | 0.463 ± 0.073 | 89 | 0.937 ± 0.113 | 44 | 0.463 ± 0.092 | |
| TT | 11 | 5 | 0.455 ± 0.027 | 10 | 0.909 ± 0.315 | 3 | 0.273 ± 0.195 | |
| XPC | AA | 98 | 41 | 0.418 ± 0.066 | 83 | 0.847 ± 0.100 | 35 | 0.357 ± 0.077 |
| AC | 86 | 41 | 0.477 ± 0.073 | 91 | 1.058 ± 0.123 | 44 | 0.512 ± 0.097 | |
| CC | 22 | 11 | 0.500 ± 0.183 | 26 | 1.182 ± 0.276 | 5 | 0.227 ± 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santovito, A.; Agostinovna Nigretti, A.; Sellitri, A.; Scarfò, M.; Nota, A. Regular Sport Activity Is Able to Reduce the Level of Genomic Damage. Biology 2023, 12, 1110. https://doi.org/10.3390/biology12081110
Santovito A, Agostinovna Nigretti A, Sellitri A, Scarfò M, Nota A. Regular Sport Activity Is Able to Reduce the Level of Genomic Damage. Biology. 2023; 12(8):1110. https://doi.org/10.3390/biology12081110
Chicago/Turabian StyleSantovito, Alfredo, Angiolina Agostinovna Nigretti, Amedeo Sellitri, Manuel Scarfò, and Alessandro Nota. 2023. "Regular Sport Activity Is Able to Reduce the Level of Genomic Damage" Biology 12, no. 8: 1110. https://doi.org/10.3390/biology12081110
APA StyleSantovito, A., Agostinovna Nigretti, A., Sellitri, A., Scarfò, M., & Nota, A. (2023). Regular Sport Activity Is Able to Reduce the Level of Genomic Damage. Biology, 12(8), 1110. https://doi.org/10.3390/biology12081110





