Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phenolic Extraction from Olive Leaves
2.1.1. LC–MS/MS Analysis for Single Phenols
2.1.2. Quantitative Analysis for Single Phenols
2.1.3. HPLC for Total Polar Phenols Quantification
2.2. Formulation of Treatments
2.3. In Vitro Evaluation of Antibacterial Activity
2.4. In Planta Set up and Evaluation of the Xylematic Treatments
3. Results and Discussions
3.1. Quantification of Total and Single Phenols in Olive Leaves
3.2. In Vitro Screening
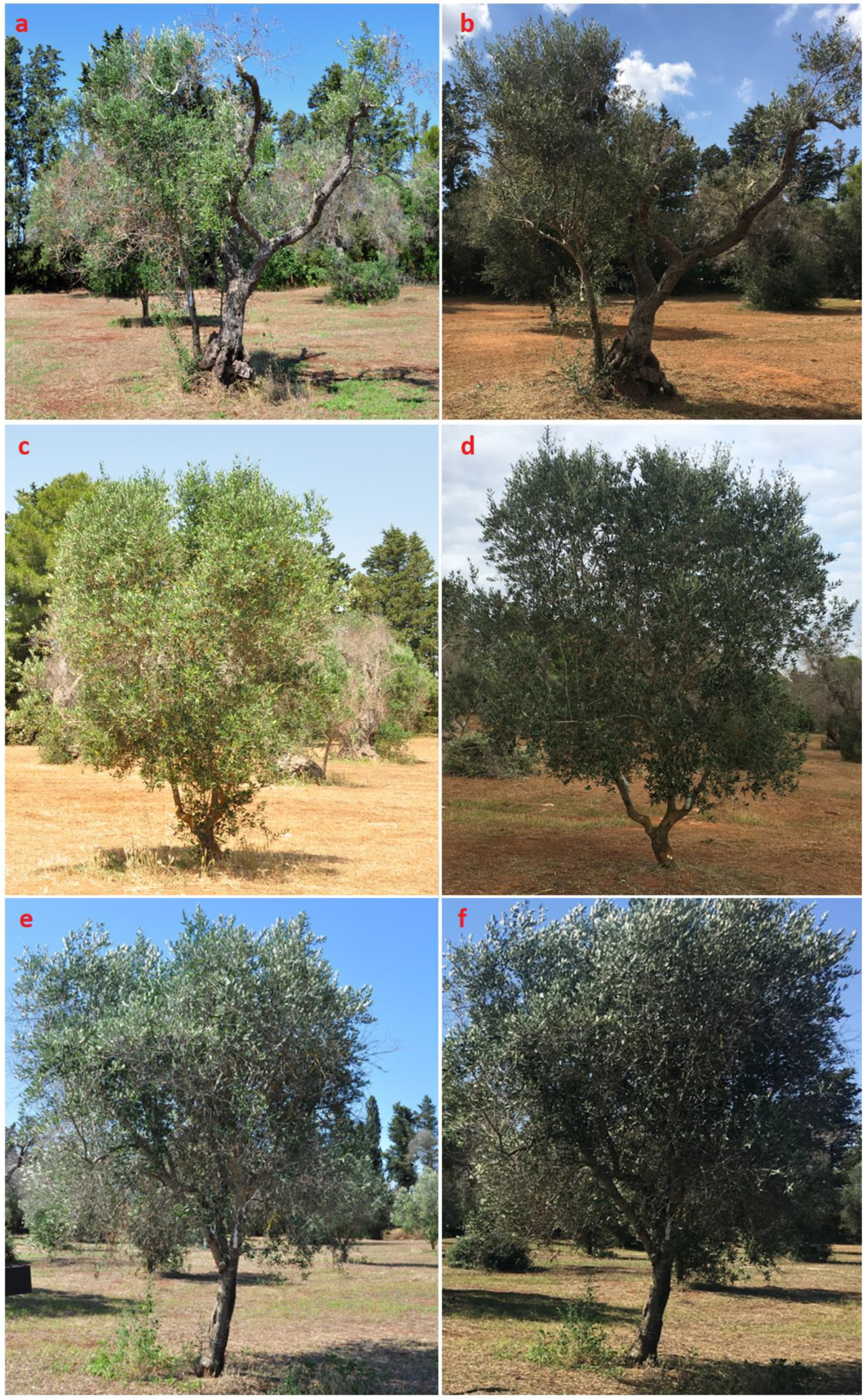
3.3. In Planta Evaluation of the Endotherapeutic Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wells, J.M.; Raju, B.C.; Hung, H.-Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa Gen. Nov., Sp. Nov: Gram-Negative, Xylem-Limited, Fastidious Plant Bacteria Related to Xanthomonas spp. Int. J. Syst. Evol. Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- De Souza, A.A.; Takita, M.A.; Pereira, E.O.; Coletta-Filho, H.D.; Machado, M.A. Expression of Pathogenicity-Related Genes of Xylella fastidiosa in vitro and in Planta. Curr. Microbiol. 2005, 50, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA Sequences Related to Xylella fastidiosa in Oleander, Almond and Olive Trees Exhibiting Leaf Scorch Symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- European Food Safety Authority; Delbianco, A.; Gibin, D.; Pasinato, L.; Morelli, M. Update of the Xylella spp. Host Plant Database–Systematic Literature Search up to 30 June 2021. EFSA J. 2022, 20, e07039. [Google Scholar] [CrossRef]
- Cariddi, C.; Saponari, M.; Boscia, D.; De Stradis, A.; Loconsole, G.; Nigro, F.; Porcelli, F.; Potere, O.; Martelli, G.P. Isolation of a Xylella fastidiosa Strain Infecting Olive and Oleander in Apulia, Italy. J. Plant Pathol. 2014, 96, 1–5. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Saponari, M.; Almeida, R.P.; Essakhi, S.; Boscia, D.; Loconsole, G.; Saldarelli, P. Complete Genome Sequence of the Olive-Infecting Strain Xylella fastidiosa subsp. pauca De Donno. Genome Announc. 2017, 5, e00569-17. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S. Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Saponari, M.; Vanhove, M.; Castillo, A.I.; Giampetruzzi, A.; Loconsole, G.; Saldarelli, P.; Boscia, D.; Neema, C.; Almeida, R.P. Introduction and Adaptation of an Emerging Pathogen to Olive Trees in Italy. Microb. Genomics 2021, 7, 000735. [Google Scholar] [CrossRef]
- Montilon, V.; De Stradis, A.; Saponari, M.; Abou Kubaa, R.; Giampetruzzi, A.; D’Attoma, G.; Saldarelli, P. Xylella fastidiosa subsp. pauca ST53 Exploits Pit Membranes of Susceptible Olive Cultivars to Spread Systemically in the Xylem. Plant Pathol. 2023, 72, 144–153. [Google Scholar] [CrossRef]
- Cardinale, M.; Luvisi, A.; Meyer, J.B.; Sabella, E.; De Bellis, L.; Cruz, A.C.; Ampatzidis, Y.; Cherubini, P. Specific Fluorescence in Situ Hybridization (FISH) Test to Highlight Colonization of Xylem Vessels by Xylella fastidiosa in Naturally Infected Olive Trees (Olea europaea L.). Front. Plant Sci. 2018, 9, 431. [Google Scholar] [CrossRef]
- De Benedictis, M.; De Caroli, M.; Baccelli, I.; Marchi, G.; Bleve, G.; Gallo, A.; Ranaldi, F.; Falco, V.; Pasquali, V.; Piro, G. Vessel Occlusion in Three Cultivars of Olea europaea Naturally Exposed to Xylella fastidiosa in Open Field. J. Phytopathol. 2017, 165, 589–594. [Google Scholar] [CrossRef]
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and Gums: A Review of Structure, Function and Occurrence of Vessel Occlusions. IAWA J. 2016, 37, 186–205. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.N.; Martelli, G.P.; Saldarelli, P. Transcriptome Profiling of Two Olive Cultivars in Response to Infection by the CoDiRO Strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 17, 358. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and Functional Xylem Anatomy in Olea europaea: Revisiting a Tale of Plant–Pathogen Interaction. AoB Plants 2021, 13, plab027. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.C.; White, S.M.; McKay Fletcher, D.; Ruiz, S.A.; Rankin, K.E.; De Stradis, A.; Saponari, M.; Williams, K.A.; Petroselli, C.; Roose, T. The Impact of Xylem Geometry on Olive Cultivar Resistance to Xylella fastidiosa: An Image-based Study. Plant Pathol. 2023, 72, 521–535. [Google Scholar] [CrossRef]
- Godefroid, M.; Cruaud, A.; Streito, J.-C.; Rasplus, J.-Y.; Rossi, J.-P. Xylella fastidiosa: Climate Suitability of European Continent. Sci. Rep. 2019, 9, 8844. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and Transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Coldiretti Xyella: Infettato Il 40% Della Puglia, Addio a 21 Mln di Ulivi. Available online: https://www.coldiretti.it/economia/xyella-infettato-il-40-della-puglia-addio-a-21-mln-di-ulivi (accessed on 20 June 2023).
- Schneider, K.; Van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Oude Lansink, A. Impact of Xylella fastidiosa subspecies pauca in European Olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef]
- Ali, B.M.; van der Werf, W.; Lansink, A.O. Assessment of the Environmental Impacts of Xylella fastidiosa subsp. pauca in Puglia. Crop. Prot. 2021, 142, 105519. [Google Scholar] [CrossRef]
- Boudet, A.-M. Evolution and Current Status of Research in Phenolic Compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts with Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 3906. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Ramezani, F.; Rahmani, F.; Dehestani, A. Exogenous Potassium Phosphite Application Improved PR-Protein Expression and Associated Physio-Biochemical Events in Cucumber Challenged by Pseudoperonospora cubensis. Sci. Hortic. 2018, 234, 335–343. [Google Scholar] [CrossRef]
- Najdabbasi, N.; Mirmajlessi, S.M.; Dewitte, K.; Mänd, M.; Landschoot, S.; Haesaert, G. Combination of Potassium Phosphite and Reduced Doses of Fungicides Encourages Protection against Phytophthora infestans in Potatoes. Agriculture 2022, 12, 189. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant Activity of Phosphite in Horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Block, E. The Chemistry of Garlic and Onions. Sci. Am. 1985, 252, 114–121. [Google Scholar] [CrossRef]
- Goncagul, G.; Ayaz, E. Antimicrobial Effect of Garlic (Allium sativum). Recent Pat. Anti-Infect. Drug Disc. 2010, 5, 91–93. [Google Scholar] [CrossRef]
- Anwar, A.; Gould, E.; Tinson, R.; Groom, M.; Hamilton, C.J. Think Yellow and Keep Green—Role of Sulfanes from Garlic in Agriculture. Antioxidants 2016, 6, 3. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green Extracts from Coratina Olive Cultivar Leaves: Antioxidant Characterization and Biological Activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Ghasemi, S.; Koohi, D.E.; Emmamzadehhashemi, M.S.B.; Khamas, S.S.; Moazen, M.; Hashemi, A.K.; Amin, G.; Golfakhrabadi, F.; Yousefi, Z.; Yousefbeyk, F. Investigation of Phenolic Compounds and Antioxidant Activity of Leaves Extracts from Seventeen Cultivars of Iranian Olive (Olea europaea L.). J. Food Sci. Technol. 2018, 55, 4600–4607. [Google Scholar] [CrossRef]
- Di Meo, M.C.; De Cristofaro, G.A.; Imperatore, R.; Rocco, M.; Giaquinto, D.; Palladino, A.; Zotti, T.; Vito, P.; Paolucci, M.; Varricchio, E. Microwave-Assisted Extraction of Olive Leaf from Five Italian Cultivars: Effects of Harvest-Time and Extraction Conditions on Phenolic Compounds and In vitro Antioxidant Properties. ACS Food Sci. Technol. 2021, 2, 31–40. [Google Scholar] [CrossRef]
- International Olive Council. Determination of Biophenols in Olive Oils by HPLC; IOC: Madrid, Spain, 2009. [Google Scholar]
- Baldassarre, F.; Tatulli, G.; Vergaro, V.; Mariano, S.; Scala, V.; Nobile, C.; Pucci, N.; Dini, L.; Loreti, S.; Ciccarella, G. Sonication-Assisted Production of Fosetyl-Al Nanocrystals: Investigation of Human Toxicity and in vitro Antibacterial Efficacy against Xylella fastidiosa. Nanomaterials 2020, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.; Calvo-Alvarado, J.; Dohrenbusch, A. Calibration of LAI-2000 to Estimate Leaf Area Index (LAI) and Assessment of Its Relationship with Stand Productivity in Six Native and Introduced Tree Species in Costa Rica. For. Ecol. Manag. 2007, 247, 185–193. [Google Scholar] [CrossRef]
- Geng, J.; Yuan, G.; Chen, J.M.; Lyu, C.; Tu, L.; Fan, W.; Tian, Q.; Wu, Z.; Tao, T.; Yu, M. Error Analysis of LAI Measurements with LAI-2000 Due to Discrete View Angular Range Angles for Continuous Canopies. Remote Sens. 2021, 13, 1405. [Google Scholar] [CrossRef]
- Lombardo, L.; Trujillo, C.; Vanwalleghem, T.; Gómez, J.A. Organic Carbon Fluxes by Precipitation, Throughfall and Stemflow in an Olive Orchard in Southern Spain. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 1039–1047. [Google Scholar] [CrossRef]
- Gómez, J.A.; Zarco-Tejada, P.J.; García-Morillo, J.; Gama, J.; Soriano, M.A. Determining Biophysical Parameters for Olive Trees Using CASI-Airborne and Quickbird-Satellite Imagery. Agron. J. 2011, 103, 644–654. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Wickham, H.; Lionel, H.; Müller, K. Dplyr: A Grammar of Data Manipulation, Version 1. 2020. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 7 January 2023).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Kaitouni, L.B.D.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of Bioactive Compounds in Commercial Olive Leaf Extracts, and Olive Leaves and Their Infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef]
- Difonzo, G.; Crescenzi, M.A.; Piacente, S.; Altamura, G.; Caponio, F.; Montoro, P. Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season. Plants 2022, 11, 3321. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Malik, N.S.A.; Perez, J.L.; Brockington, J.E. Seasonal Changes of Individual Phenolic Compounds in Leaves of Twenty Olive Cultivars Grown in Texas. J. Agric. Sci. Technol. B 2012, 2, 242. [Google Scholar]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial Effects of the Olive Oil Phenolic Components Oleuropein and Hydroxytyrosol: Focus on Protection against Cardiovascular and Metabolic Diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The Phenolic Compounds of Olive Oil: Structure, Biological Activity and Beneficial Effects on Human Health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Zare, L.; Esmaeili-Mahani, S.; Abbasnejad, M.; Rasoulian, B.; Sheibani, V.; Sahraei, H.; Kaeidi, A. Oleuropein, Chief Constituent of Olive Leaf Extract, Prevents the Development of Morphine Antinociceptive Tolerance through Inhibition of Morphine-induced L-type Calcium Channel Overexpression. Phytother. Res. 2012, 26, 1731–1737. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) Leaf Extract Effective in Patients with Stage-1 Hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive Leaf Extract as a Hypoglycemic Agent in Both Human Diabetic Subjects and in Rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef]
- Abdel-Hamid, N.M.; El-Moselhy, M.A.; El-Baz, A. Hepatocyte Lysosomal Membrane Stabilization by Olive Leaves against Chemically Induced Hepatocellular Neoplasia in Rats. Int. J. Hepatol. 2011, 2011, 736581. [Google Scholar] [CrossRef]
- Mohagheghi, F.; Bigdeli, M.R.; Rasoulian, B.; Hashemi, P.; Pour, M.R. The Neuroprotective Effect of Olive Leaf Extract Is Related to Improved Blood–Brain Barrier Permeability and Brain Edema in Rat with Experimental Focal Cerebral Ischemia. Phytomedicine 2011, 18, 170–175. [Google Scholar] [CrossRef]
- Gong, D.; Geng, C.; Jiang, L.; Wang, L.; Yoshimura, H.; Zhong, L. Mechanisms of Olive Leaf Extract-Ameliorated Rat Arthritis Caused by Kaolin and Carrageenan. Phytother. Res. 2012, 26, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Lo Scalzo, R.; Scarpati, M.L.; Verzegnassi, B.; Vita, G. Olea Europaea Chemicals Repellent to Dacus oleae Females. J. Chem. Ecol. 1994, 20, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, A.; Mazzuca, S.; Chiappetta, F.F.; Parise, A.; Perri, E.; Innocenti, A.M. Oleuropein-Specific-β-Glucosidase Activity Marks the Early Response of Olive Fruits (Olea europaea) to Mimed Insect Attack. Agric. Sci. China 2008, 7, 703–712. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S. Assessment of the Antimicrobial Activity of Olive Leaf Extract against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef]
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds. Stud. Nat. Prod. Chem. 2018, 57, 41–77. [Google Scholar]
- Talhaoui, N.; Trabelsi, N.; Taamalli, A.; Verardo, V.; Gomez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Arraez-Roman, D. Olea europaea as Potential Source of Bioactive Compounds for Diseases Prevention. Stud. Nat. Prod. Chem. 2018, 57, 389–411. [Google Scholar]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.R.; Krishna, D.R. Bioflavonoids Classification, Pharmacological, Biochemical Effects and Therapeutic Potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Khalifa, T.I.; Muhtadi, F.J.; Hassan, M.M. Rutin. In Analytical Profiles of Drug Substances; Elsevier: Amsterdam, The Netherlands, 1983; Volume 12, pp. 623–681. ISBN 0099-5428. [Google Scholar]
- Nijveldt, R.J.; Van Nood, E.L.S.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Lavee, S. Changes in Phenolic Content of Olive during Maturation. Int. J. Food Sci. Technol. 1999, 34, 265–274. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Peng, W.-H.; Tsai, K.-D.; Hsu, S.-L. Luteolin Suppresses Inflammation-Associated Gene Expression by Blocking NF-ΚB and AP-1 Activation Pathway in Mouse Alveolar Macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Park, C.M.; Jin, K.-S.; Lee, Y.-W.; Song, Y.S. Luteolin and Chicoric Acid Synergistically Inhibited Inflammatory Responses via Inactivation of PI3K-Akt Pathway and Impairment of NF-ΚB Translocation in LPS Stimulated RAW 264.7 Cells. Eur. J. Pharmacol. 2011, 660, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; D’Antuono, I.; Marchi, G.; Mita, G. In vitro Activity of Antimicrobial Compounds against Xylella fastidiosa, the Causal Agent of the Olive Quick Decline Syndrome in Apulia (Italy). FEMS Microbiol. Lett. 2018, 365, fnx281. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial Activity of Commercial Olea europaea (Olive) Leaf Extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and Antimicrobial Activities of Individual and Combined Phenolics in Olea europaea Leaf Extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Guerrero-Hurtado, E.; Gutierrez-Docio, A.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive Leaf as a Source of Antibacterial Compounds Active against Antibiotic-Resistant Strains of Campylobacter jejuni and Campylobacter coli. Antibiotics 2022, 12, 26. [Google Scholar] [CrossRef]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Jež-Krebelj, A.; Rupnik-Cigoj, M.; Stele, M.; Chersicola, M.; Pompe-Novak, M.; Sivilotti, P. The Physiological Impact of GFLV Virus Infection on Grapevine Water Status: First Observations. Plants 2022, 11, 161. [Google Scholar] [CrossRef]
- Mezghani, M.A.; Hassouna, G.; Ibtissem, L.; Labidi, F. Leaf Area Index and Light Distribution in Olive Tree Canopies. Int. J. Agron. Agric. Res. 2016, 8, 60–65. [Google Scholar]
- Rener, L.D.S.F.; Marcelo, D.A.B.; Maria, J.A.W.; Patrícia, D.S.C.; Ivomberg, D.M.; Aldair, D.S.M.; Hugo, M.F.; Vinicius, M.; Alberto, S.D.M.; Franklin, A.D.A. Potassium Phosphite Reduction of Candidatus liberibacter spp. Population on Leaves of Ponkan Tangerines Tree with Huanglongbing. Afr. J. Microbiol. Res. 2018, 12, 248–253. [Google Scholar] [CrossRef]
- Lobato, M.C.; Olivieri, F.P.; Daleo, G.R.; Andreu, A.B. Antimicrobial Activity of Phosphites against Different Potato Pathogens. J. Plant Dis. Prot. 2010, 117, 102–109. [Google Scholar] [CrossRef]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; L’aurora, A.; Petriccione, M.; Zampella, L.; Mastrobuoni, F. A Zinc, Copper and Citric Acid Biocomplex Shows Promise for Control of Xylella fastidiosa subsp. pauca in Olive Trees in Apulia Region (Southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar]
- Scortichini, M.; Loreti, S.; Pucci, N.; Scala, V.; Tatulli, G.; Verweire, D.; Oehl, M.; Widmer, U.; Codina, J.M.; Hertl, P. Progress towards Sustainable Control of Xylella fastidiosa subsp. pauca in Olive Groves of Salento (Apulia, Italy). Pathogens 2021, 10, 668. [Google Scholar] [CrossRef]
- Blonda, P.; Tarantino, C.; Scortichini, M.; Maggi, S.; Tarantino, M.; Adamo, M. Satellite monitoring of bio-fertilizer restoration in olive groves affected by Xylella fastidiosa subsp. pauca. Sci. Rep. 2023, 13, 5695. [Google Scholar] [CrossRef] [PubMed]
- Surano, A.; Abou Kubaa, R.; Nigro, F.; Altamura, G.; Losciale, P.; Saponari, M.; Saldarelli, P. Susceptible and resistant olive cultivars show differential physiological response to Xylella fastidiosa infections. Front. Plant Sci. 2022, 13, 968934. [Google Scholar] [CrossRef]
- Lombardo, L.; Fila, G.; Lombardo, N.; Epifani, C.; Duffy, D.H., III; Godino, G.; Salimonti, A.; Zelasco, S. Uncovering Olive Biodiversity through Analysis of Floral and Fruiting Biology and Assessment of Genetic Diversity of 120 Italian Cultivars with Minor or Marginal Diffusion. Biology 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Santilli, E.; Lombardo, L.; Varlaro, M.E.; Nannelli, R.; Gagnarli, E.; BriccoliBati, C. Effectiveness of the GAEC cross-compliance Standard “Maintenance of olive groves in good vegetative condition” in avoiding the deterioration of habitats and land abandonment. Ital. J. Agron. 2011, 6, 107–120. [Google Scholar] [CrossRef]




| Substituted Phenols and Flavonoids | mg kg−1 |
|---|---|
| Oleuropein | 10,621 ± 121 |
| Catechol | 2903 ± 61 |
| Hydroxytyrosol | 662.71 ± 18.49 |
| Tyrosol | 678.89 ± 15.11 |
| Diosmetin | 569.94 ± 12.87 |
| Catechin | 480.90 ± 13.51 |
| o-Cumaric acid | 67.05 ± 10.12 |
| Ferulic acid | 41.91 ± 8.33 |
| Verbascoside | 39.11 ± 5.21 |
| Caffeico acid | 8.38 ± 4.02 |
| p-Cumaric acid | 4.47 ± 0.45 |
| Vanillic acid | 2.24 ± 0.85 |
| Homovanillic acid | 0.87 ± 0.04 |
| Luteolin | 2023 ± 31 |
| Luteolin-4-O-glucoside | 704.04 ± 25.41 |
| Luteolin-7-O-glucoside | 1430 ± 13.47 |
| Rutin | 64.26 ± 9.11 |
| Apigenin | 7.26 ± 1.03 |
| Apigenin-7-O-glucoside | 6.71 ± 0.96 |
| Total polar phenols | 23,300 ± 115 |
| Treatment | LAI (m2 m−2) | LAD (m2 m−3) |
|---|---|---|
| G | 1.02 ± 0.18 | 0.83 ± 0.09 |
| PE3 | 1.07 ± 0.34 | 0.93 ± 0.22 |
| KP | 1.18 ± 0.11 | 0.90 ± 0.06 |
| T | 1.12 ± 0.21 | 0.88 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizzarri, V.; Ienco, A.; Benincasa, C.; Perri, E.; Pucci, N.; Cesari, E.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Zaffina, F.; et al. Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees. Biology 2023, 12, 1141. https://doi.org/10.3390/biology12081141
Vizzarri V, Ienco A, Benincasa C, Perri E, Pucci N, Cesari E, Novellis C, Rizzo P, Pellegrino M, Zaffina F, et al. Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees. Biology. 2023; 12(8):1141. https://doi.org/10.3390/biology12081141
Chicago/Turabian StyleVizzarri, Veronica, Annamaria Ienco, Cinzia Benincasa, Enzo Perri, Nicoletta Pucci, Erica Cesari, Carmine Novellis, Pierluigi Rizzo, Massimiliano Pellegrino, Francesco Zaffina, and et al. 2023. "Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees" Biology 12, no. 8: 1141. https://doi.org/10.3390/biology12081141
APA StyleVizzarri, V., Ienco, A., Benincasa, C., Perri, E., Pucci, N., Cesari, E., Novellis, C., Rizzo, P., Pellegrino, M., Zaffina, F., & Lombardo, L. (2023). Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees. Biology, 12(8), 1141. https://doi.org/10.3390/biology12081141







