Plant Hormone Modularity and the Survival-Reproduction Trade-Off
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Survival-Reproduction Trade-Off in Plants
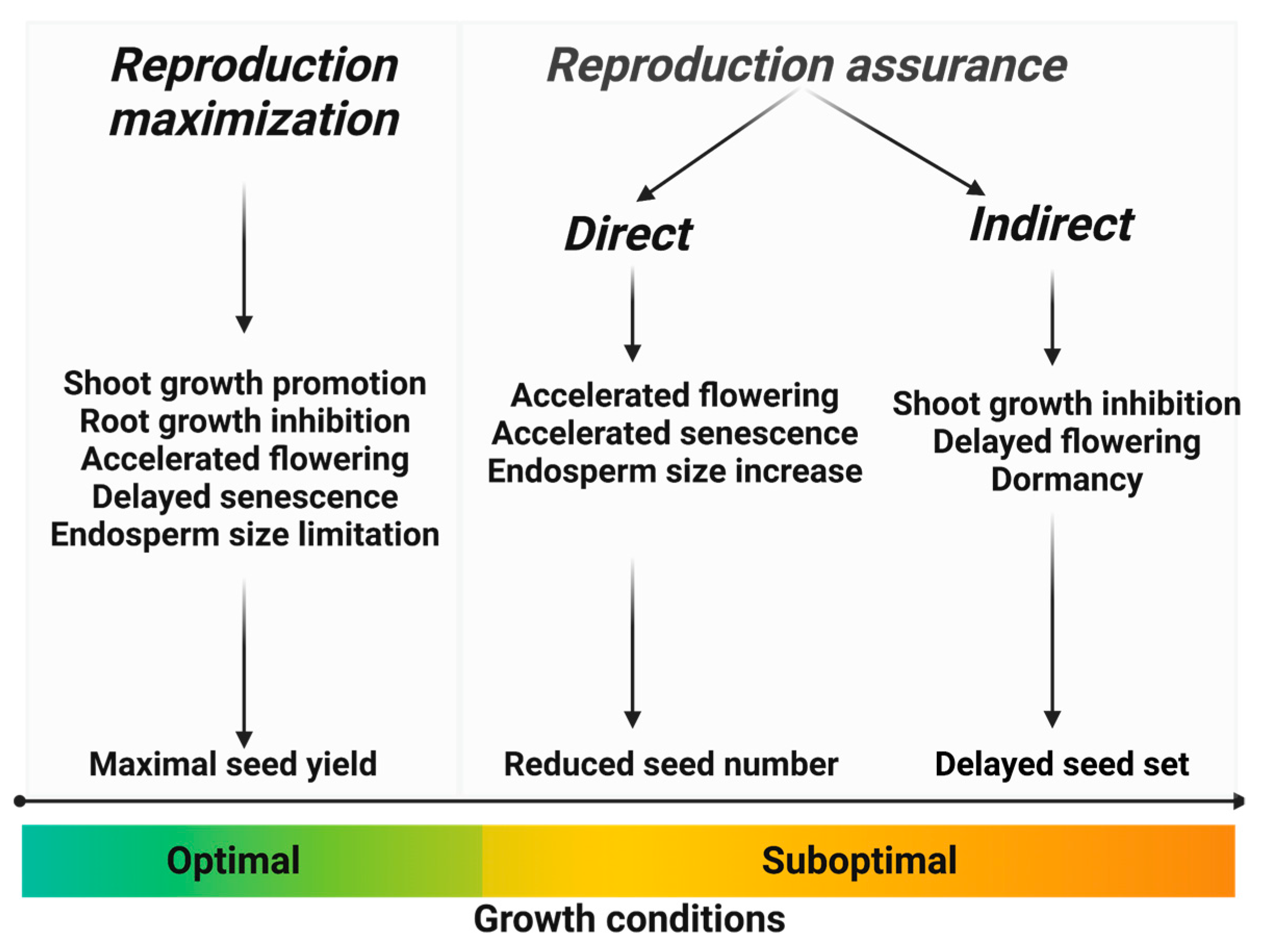
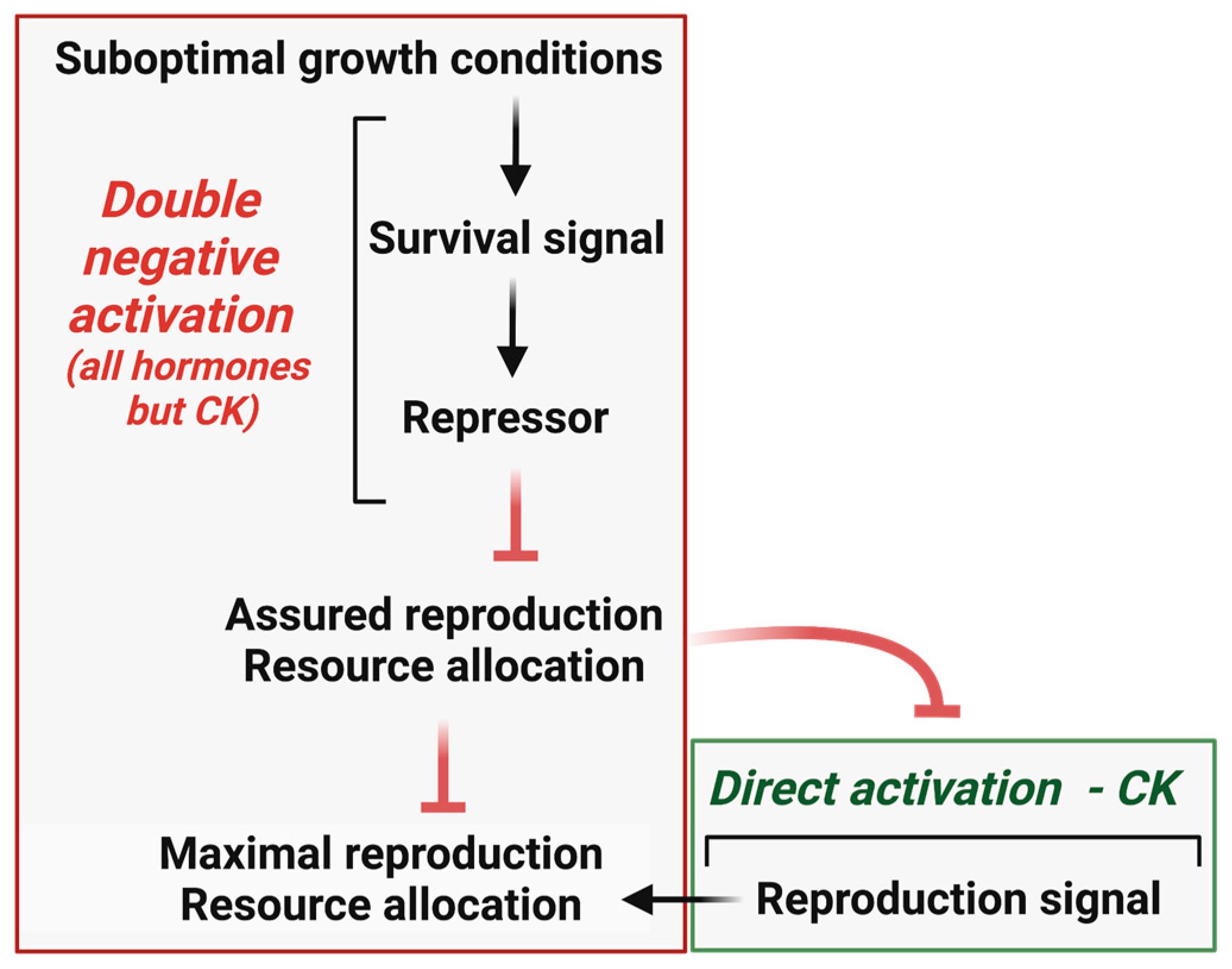
2.1. Direct Reproduction Assurance, Indirect Reproduction Assurance, and Reproduction Maximization—Definitions
2.2. Most Prominent Processes Involved in the Reproduction Assurance-Reproduction Maximization Trade-Off
2.2.1. Co-Regulation of Photosynthesis and Reproduction
2.2.2. Regulation of the Shoot-to-Root Growth Ratio
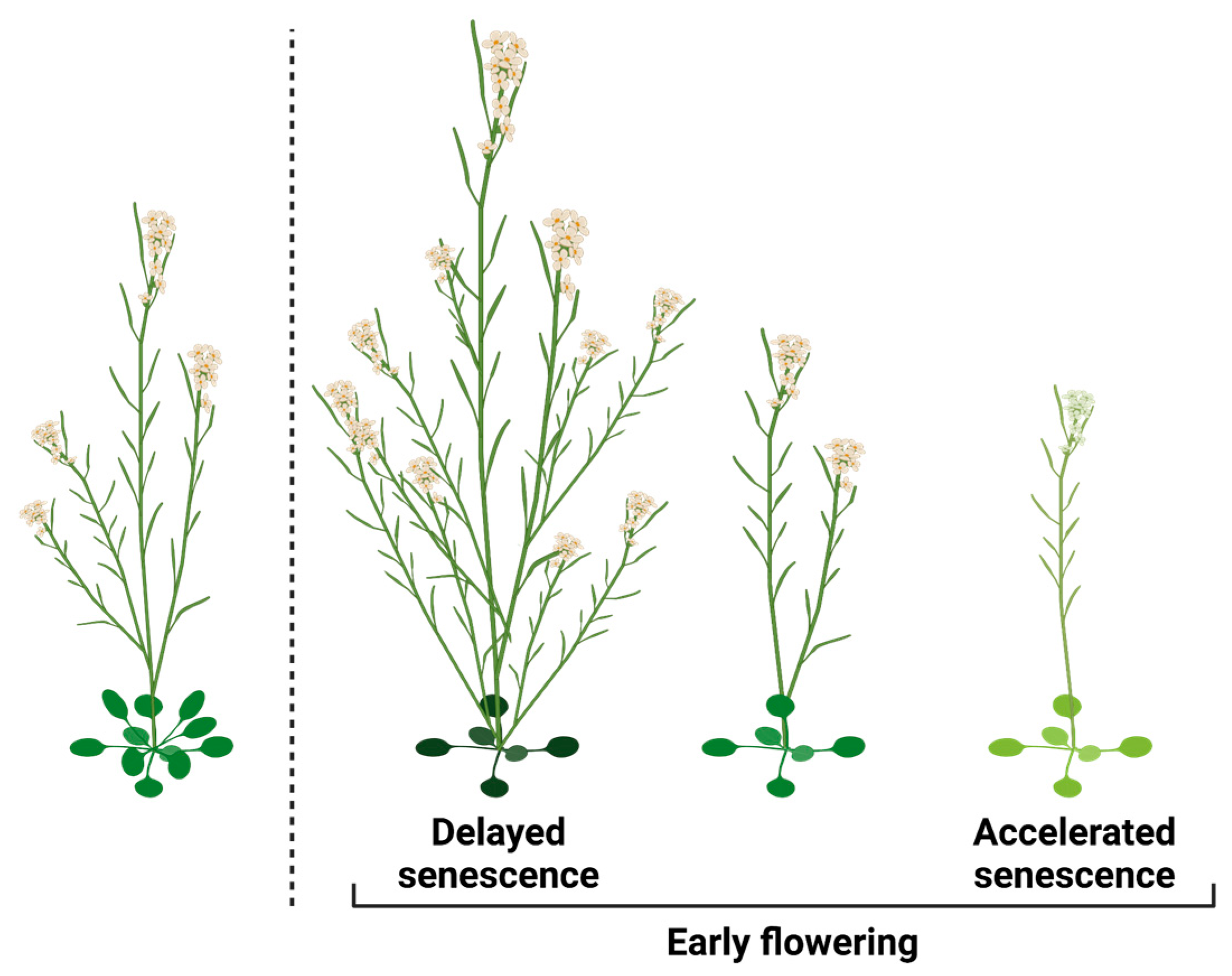
2.2.3. Timing of Senescence
2.2.4. Regulation of Flowering Time
2.2.5. Dormancy
2.2.6. Determination of Seed Size
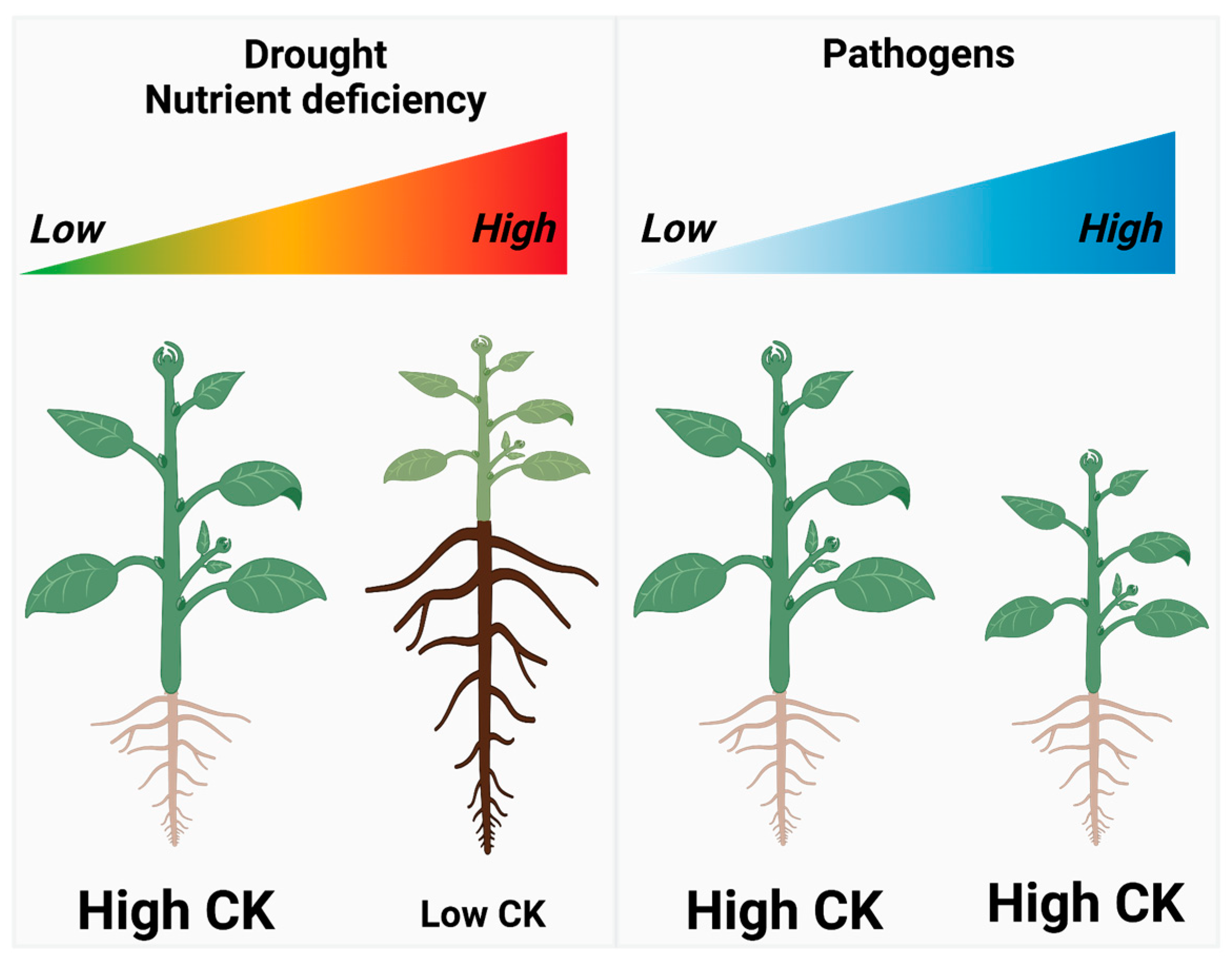
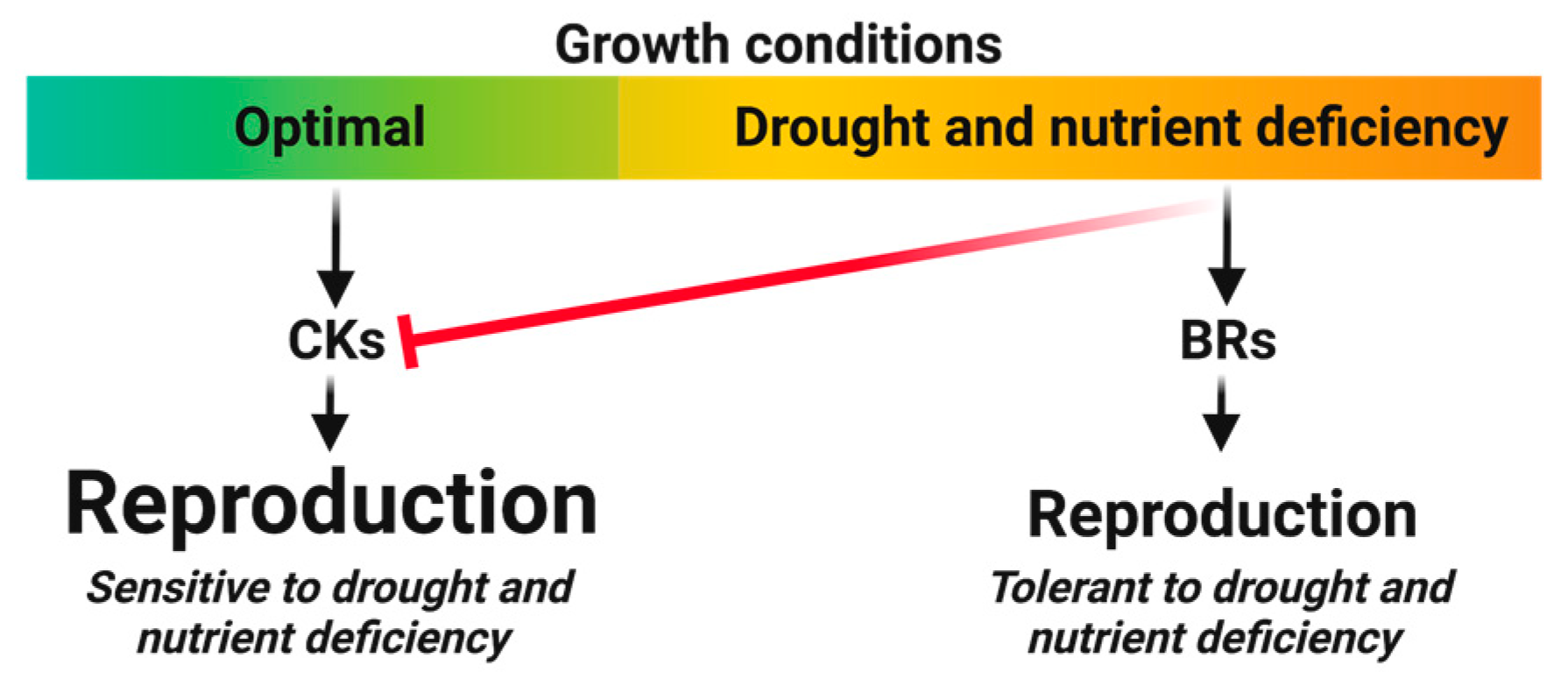
3. Cytokinins (CKs) and Reproduction Maximization
3.1. Reproduction Maximization
3.2. Direct Reproduction Assurances
4. Hormones with Reduced Reproduction Trade-Off Ratios
4.1. Brassinosteroids (BRs)
4.1.1. Reproduction Maximization
4.1.2. Direct Reproduction Assurance
4.2. Auxins
4.2.1. Direct Reproduction Assurance
4.2.2. Indirect Reproduction Assurance
4.3. Abscisic Acid (ABA)
4.3.1. Direct Reproduction Assurance
4.3.2. Indirect Reproduction Assurance
4.4. Gibberellic Acid (GA) and Direct Reproduction Assurance
4.5. Ethylene and Direct Reproduction Assurance
4.6. Strigolactones (SLs) and Reproduction Assurance
4.7. Jasmonate (JA)
4.7.1. Direct reproduction assurance
4.7.2. Indirect Reproduction Assurance
4.8. Salicylic Acid (SA) and Direct Reproduction Assurance
5. Hormone Response Pathways and the Reproduction Trade-Off
6. Origins and Evolution of Plant Hormone Modules
6.1. Origins
6.2. Evolution
7. Hormone Modules and Plant Domestication
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garland, T., Jr.; Downs, C.J.; Ives, A.R. Trade-offs (and constraints) in organismal biology. Physiol. Biochem. Zool. 2022, 95, 82–112. [Google Scholar] [CrossRef]
- Stearns, C.S. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Latta, L.C.; Tucker, K.N.; Haney, R.A. The relationship between oxidative stress, reproduction, and survival in a bdelloid rotifer. BMC Ecol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Aprison, E.Z.; Ruvinsky, I. Balanced trade-offs between alternative strategies shape the response of C. elegans reproduction to chronic heat stress. PLoS ONE 2014, 9, e105513. [Google Scholar] [CrossRef]
- French, S.S.; DeNardo, D.F.; Moore, M.C. Trade-offs between the reproductive and immune systems: Facultative responses to resources or obligate responses to reproduction? Am. Nat. 2007, 170, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Giaimo, S.; Traulsen, A. Generation time measures the trade-off between survival and reproduction in a life cycle. Am. Nat. 2019, 194, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef]
- Bókony, V.; Lendvai, A.Z.; Liker, A.; Angelier, F.; Wingfield, J.C.; Chastel, O. Stress response and the value of reproduction: Are birds prudent parents? Am. Nat. 2009, 173, 589–598. [Google Scholar] [CrossRef]
- Billard, B.; Vigne, P.; Braendle, C. A natural mutational event uncovers a life history trade-off via hormonal pleiotropy. Curr. Biol. 2020, 30, 4142–4154.e4149. [Google Scholar] [CrossRef]
- Hau, M.; Wingfield, J.C. Hormonally-regulated trade-offs: Evolutionary variability and phenotypic plasticity in testosterone signaling pathways. In Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs; Flatt, T., Heyland, A., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 349–361. [Google Scholar]
- Zera, A.J.; Potts, J.; Kobus, K. The physiology of life-history trade-offs: Experimental analysis of a hormonally induced life-history trade-off in Gryllus assimilis. Am. Nat. 1998, 152, 7–23. [Google Scholar] [CrossRef]
- Melo, D.; Porto, A.; Cheverud, J.M.; Marroig, G. Modularity: Genes, development, and evolution. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 463–486. [Google Scholar] [CrossRef]
- Clune, J.; Mouret, J.B.; Lipson, H. The evolutionary origins of modularity. Proc. Biol. Sci. 2013, 280, 20122863. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Takeuchi, T.; Benning, C. Nitrogen-dependent coordination of cell cycle, quiescence and TAG accumulation in Chlamydomonas. Biotechnol. Biofuels 2019, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Saggere, R.M.S.; Lee, C.W.J.; Chan, I.C.W.; Durnford, D.G.; Nedelcu, A.M. A life-history trade-off gene with antagonistic pleiotropic effects on reproduction and survival in limiting environments. Proc. Biol. Sci. 2022, 289, 20212669. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Friends in arms: Flavonoids and the auxin/cytokinin balance in terrestrialization. Plants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Smalle, J.A. Auxin/cytokinin antagonistic control of the shoot/root growth ratio and its relevance for adaptation to drought and nutrient deficiency stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Silva, A.C.d.; Lima, M.d.F.; Eloy, N.B.; Thiebaut, F.; Montessoro, P.; Hemerly, A.S.; Ferreira, P.C.G. The Yin and Yang in plant breeding: The trade-off between plant growth yield and tolerance to stresses. Biotechnol. Res. Innov. 2019, 3, 73–79. [Google Scholar] [CrossRef]
- Mayta, M.L.; Hajirezaei, M.R.; Carrillo, N.; Lodeyro, A.F. Leaf senescence: The chloroplast connection comes of age. Plants 2019, 8, 495. [Google Scholar] [CrossRef]
- Thomas, H.; Huang, L.; Young, M.; Ougham, H. Evolution of plant senescence. BMC Evol. Biol. 2009, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Takio, S.; Hiwatashi, Y.; Kumar, M. Environmental stress-promoting responses in algae. Front. Mar. Sci. 2021, 8, 797613. [Google Scholar] [CrossRef]
- Bentsink, L.; Koornneef, M. Seed dormancy and germination. Arab. Book 2008, 6, e0119. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H. Seed dormancy and germination-emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Bhalerao, R.P. Plant dormancy in the perennial context. Trends Plant Sci. 2007, 12, 217–223. [Google Scholar] [CrossRef]
- Muller-Landau, H.C. The tolerance-fecundity trade-off and the maintenance of diversity in seed size. Proc. Natl. Acad. Sci. USA 2010, 107, 4242–4247. [Google Scholar] [CrossRef]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, X. Mechanisms controlling seed size by early endosperm development. Seed Biol. 2023, 2, 1. [Google Scholar] [CrossRef]
- Noguchi, T.; Fujioka, S.; Choe, S.; Takatsuto, S.; Yoshida, S.; Yuan, H.; Feldmann, K.A.; Tax, F.E. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 1999, 121, 743–752. [Google Scholar] [CrossRef]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377. [Google Scholar] [CrossRef]
- Prigge, M.J.; Platre, M.; Kadakia, N.; Zhang, Y.; Greenham, K.; Szutu, W.; Pandey, B.K.; Bhosale, R.A.; Bennett, M.J.; Busch, W.; et al. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. eLife 2020, 9, e54740. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.Y.; Hsing, Y.I.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 1995, 270, 1986–1988. [Google Scholar] [CrossRef] [PubMed]
- Bartrina, I.; Jensen, H.; Novák, O.; Strnad, M.; Werner, T.; Schmülling, T. Gain-of-function mutants of the cytokinin receptors AHK2 and AHK3 regulate plant organ size, Fflowering rime and plant longevity. Plant Physiol. 2017, 173, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S. Exploring the role of a cytokinin-activating enzyme LONELY GUY in unicellular microalga Chlorella variabilis. Front. Plant Sci. 2021, 11, 611871. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Plunkert, M.; Luo, X.; Liu, Z. Developmental regulation of stolon and rhizome. Curr. Opin. Plant Biol. 2021, 59, 101970. [Google Scholar] [CrossRef]
- Eviatar-Ribak, T.; Shalit-Kaneh, A.; Chappell-Maor, L.; Amsellem, Z.; Eshed, Y.; Lifschitz, E. A cytokinin-activating enzyme promotes tuber formation in tomato. Curr. Biol. 2013, 23, 1057–1064. [Google Scholar] [CrossRef]
- Ma, X.; Xu, Q.; Meyer, W.A.; Huang, B. Hormone regulation of rhizome development in tall fescue (Festuca arundinacea) associated with proteomic changes controlling respiratory and amino acid metabolism. Ann. Bot. 2016, 118, 481–494. [Google Scholar] [CrossRef]
- Lomin, S.N.; Myakushina, Y.A.; Kolachevskaya, O.O.; Getman, I.A.; Savelieva, E.M.; Arkhipov, D.V.; Deigraf, S.V.; Romanov, G.A. Global view on the cytokinin regulatorysystem in potato. Front. Plant Sci. 2020, 11, 613624. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Cytokinin-induced growth in the duckweeds Lemna gibba and Spirodela polyrhiza. Plant Growth Regul. 2018, 86, 477–486. [Google Scholar] [CrossRef]
- Karunadasa, S.; Kurepa, J.; Smalle, J.A. Gain-of-function of the cytokinin response activator ARR1 increases heat shock tolerance in Arabidopsis thaliana. Plant Signal. Behav. 2022, 17, 2073108. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Argueso, C.T. Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann. Bot. 2017, 119, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Jiang, W.B.; Lin, W.H. Brassinosteroid functions in Arabidopsis seed development. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Li, Z.; He, Y. Roles of brassinosteroids in plant reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Zu, S.H.; Jiang, Y.T.; Chang, J.H.; Zhang, Y.J.; Xue, H.W.; Lin, W.H. Interaction of brassinosteroid and cytokinin promotes ovule initiation and increases seed number per silique in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Asami, T. Suppression of Wolffia arrhiza growth by brassinazole, an inhibitor of brassinosteroid biosynthesis and its restoration by endogenous 24-epibrassinolide. Phytochemistry 2005, 66, 1787–1796. [Google Scholar] [CrossRef]
- Pandey, A.; Devi, L.L.; Singh, A.P. Emerging roles of brassinosteroid in nutrient foraging. Plant Sci. 2020, 296, 110474. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, Z.; Guo, H. New advances in the regulation of leaf senescence by classical and peptide hormones. Front. Plant Sci. 2022, 13, 923136. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Trieu, A.; Radhakrishnan, P.; Kwok, S.F.; Harris, S.; Zhang, K.; Wang, J.; Wan, J.; Zhai, H.; Takatsuto, S.; et al. Brassinosteroids regulate grain filling in rice. Plant Cell 2008, 20, 2130–2145. [Google Scholar] [CrossRef]
- Wang, H.; Mao, H. On the origin and evolution of plant brassinosteroid receptor kinases. J. Mol. Evol. 2014, 78, 118–129. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef]
- Ma, L.; Li, G. Auxin-dependent cell elongation during the shade avoidance response. Front. Plant Sci. 2019, 10, 914. [Google Scholar] [CrossRef]
- Lim, P.O.; Lee, I.C.; Kim, J.; Kim, H.J.; Ryu, J.S.; Woo, H.R.; Nam, H.G. Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J. Exp. Bot. 2010, 61, 1419–1430. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Martignago, D.; Siemiatkowska, B.; Lombardi, A.; Conti, L. Abscisic acid and flowering regulation: Many targets, different places. Int. J. Mol. Sci. 2020, 21, 9700. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhang, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and bud dormancy in perennials: Current knowledge and future perspective. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- MacMillan, J. Occurrence of gibberellins in vascular plants, fungi, and bacteria. J. Plant Growth Regul. 2001, 20, 387–442. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, H.; Wang, T.; Peng, C.; Wang, H.; Wu, H.; Wang, X. Gibberellin indirectly promotes chloroplast biogenesis as a means to maintain the chloroplast population of expanded cells. Plant J. 2012, 72, 768–780. [Google Scholar] [CrossRef]
- Harberd, N.P.; Belfield, E.; Yasumura, Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 2009, 21, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Djakovic-Petrovic, T.; de Wit, M.; Voesenek, L.A.; Pierik, R. DELLA protein function in growth responses to canopy signals. Plant J. 2007, 51, 117–126. [Google Scholar] [CrossRef]
- Yang, C.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef]
- Busov, V.B.; Brunner, A.M.; Strauss, S.H. Genes for control of plant stature and form. New Phytol. 2008, 177, 589–607. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.H.; Tucker, M.R.; Brewer, P.B. The strigolactone pathway is a target for modifying crop shoot architecture and yield. Biology 2023, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, S.; Wang, G. Strigolactones are common regulators in induction of stomatal closure in planta. Plant Signal. Behav. 2018, 13, e1444322. [Google Scholar] [CrossRef]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell. Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Chen, W.; Xu, Z.; Chen, M.; Wang, H.; Yu, D. The emerging role of jasmonate in the control of flowering time. J. Exp. Bot. 2022, 73, 11–21. [Google Scholar] [CrossRef]
- Cipollini, D. Consequences of the overproduction of methyl jasmonate on seed production, tolerance to defoliation and competitive effect and response of Arabidopsis thaliana. New Phytol. 2007, 173, 146–153. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, Y.S.; Park, S.H.; Koo, Y.J.; Choi, Y.D.; Chung, Y.Y.; Lee, I.J.; Kim, J.K. Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol. 2009, 149, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Noir, S.; Bömer, M.; Takahashi, N.; Ishida, T.; Tsui, T.L.; Balbi, V.; Shanahan, H.; Sugimoto, K.; Devoto, A. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol. 2013, 161, 1930–1951. [Google Scholar] [CrossRef]
- Swiatek, A.; Lenjou, M.; Van Bockstaele, D.; Inzé, D.; Van Onckelen, H. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol. 2002, 128, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Baek, K.H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M.T. Salicylic acid improves growth and physiological attributes and salt tolerance differentially in two bread wheat cultivars. Plants 2022, 11, 1853. [Google Scholar] [CrossRef]
- Fan, Y.; Lv, Z.; Li, Y.; Qin, B.; Song, Q.; Ma, L.; Wu, Q.; Zhang, W.; Ma, S.; Ma, C.; et al. Salicylic acid reduces wheat yield loss caused by high temperature stress by enhancing the photosynthetic performance of the flag leaves. Agronomy 2022, 12, 1386. [Google Scholar] [CrossRef]
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Yan, S. Salicylic acid and ethylene coordinately promote leaf senescence. J. Integr. Plant Biol. 2021, 63, 823–827. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M.; Cao, J.; Cao, F.; Zhang, L. The role of salicylic acid in plant flower development. For. Res. 2022, 2, 14. [Google Scholar] [CrossRef]
- Filgueiras, C.C.; Martins, A.D.; Pereira, R.V.; Willett, D.S. The ecology of salicylic acid signaling: Primary, secondary and tertiary effects with applications in agriculture. Int. J. Mol. Sci. 2019, 20, 5851. [Google Scholar] [CrossRef]
- Keshishian, E.A.; Rashotte, A.M. Plant cytokinin signalling. Essays Biochem. 2015, 58, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, 149344. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Estelle, M. Diversity and specificity: Auxin perception and signaling through the TIR1/AFB pathway. Curr. Opin. Plant Biol. 2014, 21, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Sae-Seaw, J.; Wang, Z.Y. Brassinosteroid signalling. Development 2013, 140, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharmacol. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, H.; Yuan, K.; Liao, Z.; Meng, X.; Jing, Y.; Liu, G.; Chu, J.; Li, J. Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 14319–14324. [Google Scholar] [CrossRef]
- Zha, M.; Zhao, Y.; Wang, Y.; Chen, B.; Tan, Z. Strigolactones and cytokinin interaction in buds in the control of rice tillering. Front. Plant Sci. 2022, 13, 837136. [Google Scholar] [CrossRef]
- Greenboim-Wainberg, Y.; Maymon, I.; Borochov, R.; Alvarez, J.; Olszewski, N.; Ori, N.; Eshed, Y.; Weiss, D. Cross talk between gibberellin and cytokinin: The Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell 2005, 17, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Zubo, Y.O.; Schaller, G.E. Role of the cytokinin-activated type-B response regulators in hormone crosstalk. Plants 2020, 9, 166. [Google Scholar] [CrossRef]
- Frébort, I.; Kowalska, M.; Hluska, T.; Frébortová, J.; Galuszka, P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef]
- Wang, C.; Qi, M.; Guo, J.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The active phytohormone in microalgae: The characteristics, efficient detection, and their adversity resistance applications. Molecules 2021, 27, 46. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis. Arab. Book 2014, 12, e0173. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J. Exp. Bot. 2008, 59, 111–119. [Google Scholar] [CrossRef]
- Sung, Y.; Yu, Y.C.; Han, J.M. Nutrient sensors and their crosstalk. Exp. Mol. Med. 2023, 55, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.A.; Demetriades, C. The multifaceted role of nutrient sensing and mTORC1 signaling in physiology and aging. Front. Aging 2021, 2, 707372. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, K.A.; Blommaert, L.; Ljung, K.; Beeckman, T.; De Clerck, O. Auxin function in the brown alga Dictyota dichotoma. Plant Physiol. 2019, 179, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Tounosu, N.; Sesoko, K.; Hori, K.; Shimojima, M.; Ohta, H. Cis-regulatory elements and transcription factors related to auxin signaling in the streptophyte algae Klebsormidium nitens. Sci. Rep. 2023, 13, 9635. [Google Scholar] [CrossRef]
- Fürst-Jansen, J.M.R.; de Vries, S.; de Vries, J. Evo-physio: On stress responses and the earliest land plants. J. Exp. Bot. 2020, 71, 3254–3269. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Holzinger, A. Understanding the algae to land plant transition. J. Exp. Bot. 2020, 71, 3241–3246. [Google Scholar] [CrossRef]
- de Vries, J.; Archibald, J.M. Plant evolution: Landmarks on the path to terrestrial life. New Phytol. 2018, 217, 1428–1434. [Google Scholar] [CrossRef]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, Z.; Wang, H.; Shen, R. Leaf angle: A target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotechnol. J. 2022, 20, 426–436. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-H. Designed manipulation of the brassinosteroid signal to enhance crop yield. Front. Plant Sci. 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurepa, J.; Smalle, J.A. Plant Hormone Modularity and the Survival-Reproduction Trade-Off. Biology 2023, 12, 1143. https://doi.org/10.3390/biology12081143
Kurepa J, Smalle JA. Plant Hormone Modularity and the Survival-Reproduction Trade-Off. Biology. 2023; 12(8):1143. https://doi.org/10.3390/biology12081143
Chicago/Turabian StyleKurepa, Jasmina, and Jan A. Smalle. 2023. "Plant Hormone Modularity and the Survival-Reproduction Trade-Off" Biology 12, no. 8: 1143. https://doi.org/10.3390/biology12081143
APA StyleKurepa, J., & Smalle, J. A. (2023). Plant Hormone Modularity and the Survival-Reproduction Trade-Off. Biology, 12(8), 1143. https://doi.org/10.3390/biology12081143




