Simple Summary
Although the use of antiretroviral treatment (ART) is effective in reducing the risk of HIV transmission to the foetus, there are concerns of possible adverse effects of in utero exposure on offspring’s cardio-metabolic health. This paper systematically reviewed the effects of HIV/ART exposure during pregnancy on the cardio-metabolic health of offspring. Reports from 35 eligible studies showed that HIV-exposed uninfected (HEU) children’s cardio-metabolic health was negatively impacted by in utero exposure to ART. A few studies showed direct cardiometabolic risk factors including increased blood pressure and lipids, reduced insulin, oxidative stress, cardiac damage and vascular and myocardial dysfunction among HEU children compared to their HIV-unexposed uninfected (HUU) children while most studies reported indirect cardiovascular risk factors including reduced head circumference, low birth weight, and altered mitochondrial content in HEU children. These findings suggest that in utero exposure of ART may affect foetal health predisposing them to cardiometabolic diseases later in life.
Abstract
Background: Antiretroviral treatment (ART) use during pregnancy continues to rise as it is known to decrease the likelihood of HIV transmission from mother to child. However, it is still unknown whether foetal exposure to (ART) may affect the foetal environment, predisposing the offspring to cardiometabolic risk. Therefore, the aim of this study was to systematically review the cardio-metabolic effects of in utero exposure to HIV/ART on offspring. Methods: We carried out a systematic review and obtained literature from the Google scholar, PubMed, ProQuest, Web of Science, and Scopus databases. Two independent reviewers evaluated the titles, abstracts, and full-length English contents. Data from the eligible studies were included. Results: The search yielded 7596 records. After assessing all of these records, 35 of the full-length articles were included in this systematic review. Several studies showed that low birth weight, small head circumference, and altered mitochondrial content were more common among HIV-exposed uninfected (HEU) children compared to HIV-unexposed uninfected children (HUU). A few studies demonstrated elevated triglyceride levels, lower levels of insulin, and increased blood pressure, oxidative stress, vascular dysfunction, cardiac damage, and myocardial dysfunction among HEU children compared with HUU children. Conclusion: Most findings showed that there were cardio-metabolic health risk factors among HEU children, indicating that maternal exposure to HIV and ART may negatively affect foetal health, which may lead to cardio-metabolic morbidity later in life.
1. Introduction
Human immuno-deficiency virus (HIV), which is known to cause acquired immuno-deficiency syndrome (AIDS), continues to be a significant problem with regard to global public health [1]. Over 38 million people worldwide were infected with HIV in 2021. Approximately 54% of these people were women and girls [2]. Before the advent of antiretroviral therapy (ART), women of reproductive age were discouraged from becoming pregnant due to concerns about HIV transmission to their unborn children [3]. However, now that ART is widely available, HIV-positive women of childbearing age can have children with little risk to themselves, their children and partners [4,5]. When used during pregnancy, ART has been reported to be the best intervention to lower the risk of mother-to-child transmission levels to below 5% [6]. This is made evident by the increasing number of HIV-exposed uninfected (HEU) children [7]. In fact, 1.4 million HEU children are born each year [8].
Despite the fact that ART has significantly decreased rates of vertical HIV transmission during pregnancy, mounting evidence is indicating that ART may increase the risk of undesirable pregnancy outcomes, including premature birth and intrauterine growth restriction among children who were exposed to HIV during gestation [9,10]. Intrauterine growth restriction is a known cause of low birth weight and small head size [11]. Additionally, it was reported to be a risk factor for the development of long-term cardiometabolic disease [12]. Further, a study documented that the perinatal environment determines the developmental stages of a foetus and defines their susceptibility to the onset of diseases such as hypertension, diabetes, and metabolic disorders including obesity, dyslipidaemia, insulin resistance, inflammation, and vascular dysfunction, among others, as well as oxidative stress [13,14,15,16]. These undesired pregnancy outcomes affecting offspring after birth may be a result of “foetal programming”, a condition whereby harm to the intrauterine environment results in structural and functional alterations in vital organs in postnatal life, increasing the likelihood of the emergence of numerous diseases in later life [17,18].
The intrauterine environment modulates the placenta [19], as it provides the foetus with blood that is rich in nutrition and oxygen during pregnancy. The placenta serves as a conduit for communication between the mother and the foetus. Most toxins and medications cannot pass through the placental barrier, which is made up of chorionic connective tissue, the trophoblastic epithelium covering the villi, and the foetal capillary endothelium. [20]. In some cases, drugs are made to readily cross the placental barrier, as they are beneficial to the foetus. For example, the transplacental passage of ART is known to prevent the perinatal transmission of HIV [21]. However, the chronic administration of ART, often conducted throughout pregnancy, may affect the in utero environment, predisposing the foetus to the risk of developing cardiometabolic diseases after birth. Moreover, maturation and functions of the placenta may be interrupted by persistent exposure to ART [22]. For instance, a study found that ART increases maternal endothelium dysfunction and placental dysfunction, which may increase the risk of pre-eclampsia in pregnant women on ART [23]. Another study carried out in Tygerberg, South Africa, found that children born to HIV-positive women inhabited smaller placentas than children born from healthy women [24]. These results indicate that HIV/ART may have adverse effects on the in utero environment, which may predispose the foetus to future postnatal chronic diseases. The impact of the in utero environment on the health of a child was clearly demonstrated by a Helsinki study conducted on adulthood among men who had low birth weight (LBW). This study found that these men were more susceptible to developing coronary heart disease [25].
Although the use of ART is beneficial to lowering the risk of vertical transmission, it is crucial to investigate any potential negative effects of in utero exposure on offspring. Therefore, the purpose of this study was to systematically review the cardio-metabolic effects of in utero exposure to HIV/ART on offspring born to HIV-infected mothers.
2. Methods
2.1. Search Strategy
This systematic review adheres to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for Systematic Reviews [26]. Online databases, namely, Google scholar, PubMed, ProQuest, and Scopus, were used to conduct a systematic search between March and June 2022. Full-length text case–control studies, longitudinal studies, prospective observational studies, prospective cohort studies, and retrospective observational studies reported in English were included in this systematic review. Studies that included appropriate controls for comparison were used to assess cardiovascular health. The terms “retrospective” and “prospective” describe the timing of research in relation to the acquisition of the results. In retrospective studies, each participant has already experienced the desired outcome (or not, as, for example, in controls) at the time of enrolment, and data are gathered either through records or by asking people to recall exposure. Conversely, in prospective research, individuals are monitored over time to ascertain the occurrence of outcomes because the outcome (and occasionally even the exposure or intervention) has not yet occurred at the initiation of the study [27]. Cross-sectional studies can be completed within a reasonable amount of time depending on the required sample size and accessibility to the study population. The main feature of this type of study design is that the results are generalised for the study population using a cross-sectional sample that is representative of the population [28]. A longitudinal study is the opposite of a cross-sectional study. In longitudinal studies, the same participants are frequently observed over time [29]. In randomized control trials, a homogeneous group of study participants is split into two groups at random. These two groups ought to be identical in every way, including with regard to measured and unmeasured confounders, if the randomization is successful [30].
The Population, Intervention, Comparison, and Outcome (PICO) tool used to develop the present research criteria is displayed in Table 1. The keywords searched for population included pregnant woman, pregnant women, and offspring. The intervention covered drugs used in HIV treatment, HIV, the environment of interest (i.e., in utero), and the period (namely, during pregnancy and the postnatal period). The intervention considered was compared with HIV/ART-free in utero and HIV-uninfected populations. The outcomes were categorised in terms of growth, cardiovascular risk, cardiac effects, and mitochondrial effects. The records resulting from the search were screened manually by two reviewers independently. Disagreements were resolved after a discussion so that extracted data could be merged. Secondary data such as editorials, reviews, and commentaries were not considered. Further, grey literature was not included.

Table 1.
Search criteria.
2.2. Data Extraction
The study titles were screened, and records of those that were eligible according to the inclusion criteria were exported to Microsoft (MS) Excel. Data that were extracted included the title of a study, its abstract, and its year of publication. Duplicate records and irrelevant studies were excluded, and full-length text articles were selected for this systematic review.
3. Results
A total of 7596 records were obtained from the systematic search. Of those records, 7292 were removed for several reasons, including ineligibility and duplication, among others. The remaining 304 articles were assessed based on their titles and abstracts, according to which 238 were excluded. Sixty-six articles were retrieved as full-length studies for comprehensive review. After removing articles based on the outcomes of interest, 35 articles met our eligibility criteria. The details of the study selection process are presented in Figure 1.
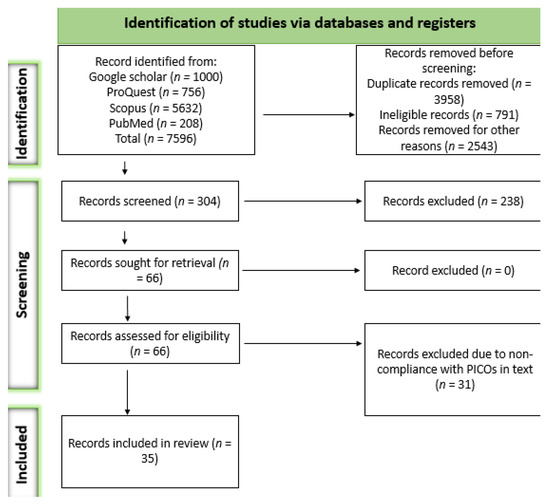
Figure 1.
Search and screening selection process based on PRISMA 2020 guidelines.
Presented below is a PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers only.
The characteristics of the included studies are displayed in Table 2. A few studies reported the onset of ART administration. All the studies published between 2011 and 2022, consisting of original data, and reported in English were included.

Table 2.
Characteristics of the included studies.
Six studies found that infants exposed to HIV/ART in utero had lower birth weights (LBWs) compared to HUU children [31,32,40,48,57,61]. In addition, infants exposed to ART in the first trimester had lower birth weights compared to those exposed later in pregnancy or not at all according to a study carried out in Brazil [36]. Another study carried out in Cape Town, South Africa, reported a higher prevalence of LBW among HEU children (14%) compared to HUU children (9%) [41]. Studies carried out in Uganda and Botswana found that children exposed to HIV in utero were significantly underweight as compared to healthy unexposed, uninfected children [60,66]. In a study carried out in South Africa, the prevalence of underweight was 11.1% for children in the HEU group and 8.4% for children in the HUU group, respectively [57]. However, other studies observed similar birth weights among HEU and HUU newborns [56,58,59,66]. In addition, children in the HEU group in a study conducted in Harare and Gweru, Zimbabwe, had significantly lower weight-for-length z scores than those in the HUU group [37,42]. Another study carried out in South Africa reported that the length-for-age z scores of HEU children were lower compared to those of HUU children at 12 months [38].
The head circumferences (HCs) of infants whose mothers were HIV-positive and those whose mothers did not have HIV were similar in Mozambique and Kenya [58,66]. HC was significantly smaller among infants in the HEU group than those in the HUU group in studies conducted in Nigeria, Romania, South Africa, and Uganda [32,35,39,40].
Height was significantly greater among HUU children compared to HEU children in Zimbabwe, Romania, and Cameroon [32,40,42]. However, studies conducted in Kenya and Mozambique found that the lengths of HEU children and HUU children were comparable [58,66]. An HIV-positive status for mothers was associated with lower mean birth weight and height of their offspring from birth to 6 months of life in Tanzania [43].
Low birth weight among HEU children was directly associated with the in vivo production of foetal TNF-α in a Brazilian study indicating increased pro-inflammatory cytokine levels among neonates born to HIV-positive mothers [51].
A South African study reported that the levels of cytokines such as interferon gamma and interleukin-1 beta (p < 0.01) were significantly decreased among HEU infants at 6 to 10 weeks old [34], while peroxidised lipids generated by reactive oxygen species (ROS) were elevated among infants exposed to ART/HIV in Netherlands [44]. This study further reported increased levels of triglycerides among HEU children [44]. A study carried out in Cameroon reported that HEU newborns had lower preprandial insulin levels than HUU neonates at 6 weeks of age [46]. Indirect measurements, including mitochondrial content, were not consistent in studies carried out in Spain. For example, in [49], the authors reported that there was increased mitochondrial content among HIV/ART-exposed children compared to those not exposed, while another study reported decreased mitochondrial content [62]. Elevated adenine–cytosine/thymine–guanine mutations were observed among HEU children compared to HUU infants, though the difference was insignificant (p = 0.09) [47]. Elsewhere, a negative relationship between complex IV (CIV) activity levels and mitochondrial DNA (mtDNA) function was observed among HEU children [52].
A direct measure such as myocardial wall thickness was reported to be increased among HEU children in studies conducted in Spain [49,50,53]. On the contrary, a study carried out in the United States reported decreased septal wall thickness among HEU infants [54]. Echocardiographic measurements were similar in Spain and in the United States [31,36]. Other studies showed decreased Left-ventricular (LV) mass among HEU children in the United States [45,54]. In addition, LV mass was significantly lower among girls of the same group compared to boys [54], while signs of systolic dysfunction, diastolic dysfunction, and increased cIMT were observed among HEU children [50]. Another study conducted in the United States reported that HEU children had higher heart rates [33]. In addition, a study conducted in the United States reported that HIV-negative children aged 8–12 years exposed to ART in utero had reduced left ventricular diastolic function compared to HUU children of the same age [65].
A study conducted in Portugal documented significantly decreased myocardial peak systolic velocities in HIV-exposed children [64]. Significant lower mitral late diastolic inflow velocities and higher adjusted mean LV mass-to-volume-ratio Z-scores were observed among HEU children and adolescents compared to HUU children and adolescents aged 7–16 years old in the United States [55]. A study carried out in Spain reported that the left isovolumic relaxation time was significantly longer in HEU foetuses than that of the HUU foetuses [49], while the foetal ratio between early (E) and late (atrial—A) ventricular filling velocity was significantly higher among HEU children compared to HUU children in Spain [31]. No discernible difference was observed between cardiac damage and perinatal exposure to ART among 3-year-old HEU children and 3-year-old HUU children in a study carried out in the United States [45]. Correlations between cardio biomarkers, LV mass, LV function, and inflammatory markers were observed among HEU youth in the United States [63].
4. Discussion
This study systematically reviewed the cardio-metabolic health of children exposed to HIV/ART in utero. Most studies were from Africa, where many children are exposed to HIV/ART in utero due to the high prevalence of HIV infection. Despite not being infected with HIV, HEU children are exposed to ART in utero during the crucial phase of their cardiovascular system development [35,55]. In this review, some studies were focused on LBW, alter mitochondrial content and HC as indirect cardiovascular risk factors, while others assessed the direct cardio-metabolic risk factors, including insulin resistance, lipid disorders, inflammation, oxidative stress, vascular dysfunction, cardiac damage and cardiac dysfunction following in utero exposure to HIV and ART. Selected cardiovascular disease markers were assessed in some studies, while mitochondrial DNA changes relating to cardiac health were assessed in others.
Pregnancy causes physiological changes that differently affect how all drugs are absorbed, distributed, metabolised, and eliminated in pregnant compared to non-pregnant women [67]. Even though the primary goal of drug therapy during pregnancy is to treat maternal problems, dose regimens are nearly always decided without taking into account the major modifications of drug handling by the pregnant woman. Furthermore, the majority of drugs cross the placenta easily, exposing the foetus to treatments. In most global guidelines and clinical settings, nucleoside reverse transcriptase inhibitors (NRTIs) are known to be the backbone of ART for pregnant women [67,68]. They prevent the virus from replicating itself. However, exposure to NRTI is known to be associated with mitochondrial toxicity [69]. For example, a study conducted in Spain found that the mitochondrial content of HEU children was significantly increased [49]. Loss of mitochondrial function, which was observed in HEU children exposed to ART in utero [47,49,52,62], may lead to an increase in the release of lipids into circulation, where they reduce nitric oxide, which is an important vasodilator that acts by indirectly mediating oxidative stress and downregulating insulin signalling [70,71]. Increased concentrations of insulin were observed in HEU children compared with those of HUU children [46]. In a study conducted in the Netherlands, the authors reported increased triglyceride levels as well as production of ROS-catalysed peroxidation metabolites among HEU infants [44]. This suggests that in utero exposure to ART may affect mitochondrial function, which may lead to dyslipidaemia, hyperglycaemia, and oxidative stress, affecting their metabolic health and posing a possible risk of CVD events in future [46].
HEU children exposed to NRTI in utero had increased carotid intima–media thickness (cIMT) and systolic blood pressure (SBP) and diastolic blood pressure (DBP) dysfunction, which may indicate a higher cardiovascular risk in later life for HIV-negative infants exposed to ART during pregnancy [50]. Significant elevated levels of circulating inflammatory cytokines were observed among HEU neonates in Brazil and may be associated with foetal inflammatory response syndrome. There is a need for the long-term observation of these infants in order to ascertain the impact of ART administered during pregnancy on the cellular immune system’s response to different antigens in later life [51]. A study in the United States linked increased inflammatory biomarkers with left-ventricular (LV) mass, LV function, and LV wall stress in the corresponding HEU group. This suggests inflammation may affect the heart function of the exposed children, increasing their risk for CVDs. Exposure to highly active antiretroviral therapy (HAART) was positively correlated with LV fractional shortening among perinatal HIV-infected children when compared with HIV-infected children without HAART exposure [72]. In addition, a study documented that by late adolescence, pulse wave velocity (PWV) was higher in perinatal HIV-infected children than in their HIV-negative peers, and PWV was positively correlated with higher arterial pressure, a CVD risk factor [73]. Another study carried out in the Unted States, Australia, and Europe involving HIV patients documented that protease inhibitors were associated with myocardial infarction [74]. These studies suggest that inflammation, high blood pressure, and vascular dysfunction may result in cardiac damage and dysfunction, increasing the risk for CVDs. Exposure to ART during pregnancy has been linked to minor direct cardiac effects [55]. For example, studies carried out in Spain revealed that HEU foetuses exposed to Zidovudine exhibited cardiac remodelling [49,50,53]. This finding could account for cardiovascular alterations in childhood [53]. The LV dimension and myocardial peak velocities were decreased among HEU children in the United States and Portugal, respectively [54,64]. These changes suggest an overall loss of cardiac tissue associated with ART exposure and may lead to progressive systolic dysfunction later in life [54,64].
Some South African studies reported decreased weight among HEU children [38,41]. This finding is in agreement with studies conducted in other African countries such as Tanzania, Nigeria, and Zimbabwe [32,37,43]. This finding suggests that both the actual maternal viral infection and the use of ART could directly affect foetal growth [40,75]. Furthermore, children whose mothers started ART before conception birthed infants with higher preterm births compared to those who started ART after pregnancy, which might have led to low birth weight [57]. However, other studies, including those conducted in South Africa, Malawi, Uganda, Botswana, Cameroon, and Mozambique, reported similar weights among HEU children and HUU children [34,56,60,66]. Length was also not consistent in Africa. For instance, low length was reported among HEU children in South African studies as well as in Zimbabwe and Uganda [35,37,38,39], whereas other studies carried out in South Africa, Botswana, and Mozambique documented similar results between children in the HEU group and HUU group [34,60,66]. Moreover, a study reported that intrauterine growth restriction increased the amount of nutrients supplied to the nervous system at the expense of other important systems. As a result, this restriction may impact cardiovascular and postnatal metabolism later in life, as reported in a study on post-menopausal women, which revealed that their LBW was linked to a higher risk of CVD in their adulthood [76]. A study carried out in South Africa reported that preterm delivery appeared to be more common among women who conceived on ART compared to those initiating treatment during pregnancy, which may explain the low birth weight among these children [41]. Further, underweight among children was associated with maternal CD4 [56]. Rapid weight increase in the early postnatal years is frequently associated with restricted foetal growth that is reflected by LBW, which exacerbates harmful health effects, including obesity. [77]. HIV-positive individuals are more likely to be obese, and abnormalities in fat distribution persist even with modern ART [69]. However, children not exposed to HIV but exposed to nurturing environments have been shown to exhibit equally dramatic gains in length and weight among adolescents in Romania [78].
It is known that head circumference at birth reflects intrauterine growth, which could lead to further cardiovascular events in the future. A study documented that having a small head size at birth increases the risk of death from coronary heart disease (CHD) [79]. Another study reported that smaller head circumference at delivery as a result of restricted foetal development was linked to an elevated likelihood of cardiovascular death [18]. Further, in [80], the authors reported hypoplastic left heart syndrome, which is a cardiovascular malformation affecting infants born with small heads. ART has also been shown to be associated with the head size of children after birth. Studies carried out in Cameroon, Nigeria, South Africa, and Uganda reported significantly lower head circumferences among children in the HIV/ART in utero exposure group compared to children in the HUU group [32,35,39,40]. In addition, this study showed a robust relationship between small head circumference and efavirenz-based ART. Further, this study also showed protective effects of darunavir with respect to head size [81]. This finding suggests that different types of ART could have different effects on the physical growth of children, including head size, rendering them prone to cardiovascular risk in the future. Therefore, changes in the body’s structure, physiology, and metabolism as a result of ART exposure throughout the foetal phase could be the origin of CVD [18].
One of this review’s shortcomings is that the included studies did not focus on the in utero effect of specific ART drugs. Only a few studies reported the effect of individual classes of ART in HEU children. Thus, it is not clear whether the observed cardiovascular risk observed for children in the HEU group is related to the specific ART treatment or class. The cardiovascular effect observed could not be established based on the duration of the administration of ART for pregnant women, as most studies reported that women started ART at different times. This might have influenced the quality of the results reported.
5. Conclusions
In utero exposure to HIV/ART had an adverse effect on the cardio-metabolic health of HIV-exposed uninfected children. Although there were sufficient data on indirect cardiovascular risk factors including intrauterine growth restriction, low birth weight, head circumference, and altered mitochondrial content, there were few data on direct cardio-metabolic biomarkers including dyslipidaemia, insulin resistance, hypertension, oxidative stress, vascular and myocardial dysfunction and cardiac damage, which are major cardiovascular disease risk factors. Therefore, more studies, especially those focusing on direct cardiovascular risk factors are required to investigate and monitor the cardiometabolic health of children exposed to HIV and ART in utero.
Author Contributions
Conceptualization, G.A.E. and B.N.N.-C.; Data curation, E.N.M.; Methodology, E.N.M., M.M.M. and G.A.E.; Funding acquisition, B.N.N.-C.; Investigation, M.M.M. and G.A.E.; Supervision, G.A.E., N.G., C.R.S.-R. and B.N.N.-C.; Validation, M.M.M., C.R.S.-R., B.N.N.-C. and N.G.; Writing—original draft, E.N.M. and G.A.E.; Writing—review and editing, G.A.E., E.N.M., N.G., and B.N.N.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the South African National Research Foundation NRF-CPRR, Grant No: 129245 to Prof. Benedicta N. Nkeh-Chungag.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organisation (WHO). HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 13 November 2022).
- UNAIDS. Global HIV & AIDS statistics. 2022. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 1 November 2022).
- Fleming, A.F. Tropical obstetrics and gynaecology. 1. Anaemia in pregnancy in tropical Africa. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Gutin, S.A.; Harper, G.W.; Bitsang, C.; Moshashane, N.; Ramogola-Masire, D.; Harries, J.; Morroni, C. Perspectives about childbearing and pregnancy planning amongst people living with HIV in Gaborone, Botswana. Cult. Health Sex. 2020, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Stringer, E.M.; Kendall, M.A.; Lockman, S.; Campbell, T.B.; Nielsen-Saines, K.; Sawe, F.; Cu-Uvin, S.; Wu, X.; Currier, J.S. Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS ONE 2018, 13, e0199555. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. UNAIDs info 2019. Available online: https://aidsinfo.unaids.org (accessed on 18 August 2022).
- Evans, C.; Jones, C.E.; Prendergast, A.J. HIV-exposed, uninfected infants: New global challenges in the era of paediatric HIV elimination. Lancet Infect. Dis. 2016, 16, e92–e107. [Google Scholar] [CrossRef] [PubMed]
- Ramokolo, V.; Goga, A.E.; Slogrove, A.L.; Powis, K.M. Unmasking the vulnerabilities of uninfected children exposed to HIV. BMJ 2019, 366, l4479. [Google Scholar] [CrossRef] [PubMed]
- Short, C.E.; Douglas, M.; Smith, J.H.; Taylor, G.P. Preterm delivery risk in women initiating antiretroviral therapy to prevent HIV mother-to-child transmission. HIV Med. 2014, 15, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Palacio, M.; Goncé, A.; Hernandez, S.; Barranco, F.J.; Garcia, L.; Loncà, M.; Coll, J.O.; Gratacós, E.; Figueras, F. Risk of intrauterine growth restriction among HIV-infected pregnant women: A cohort study. Eur. J. Clin. Microbiol. 2015, 34, 223–230. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine growth restriction: Antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016, 10, CMPed-S40070. [Google Scholar] [CrossRef]
- Joung, K.E.; Lee, J.; Kim, J.H. Long-term metabolic consequences of intrauterine growth restriction. Curr. Pediatr. Rep. 2020, 8, 45–55. [Google Scholar] [CrossRef]
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100, 153952. [Google Scholar] [CrossRef]
- Usui, N.; Shimada, S. Prenatal environment and neurodevelopmental disorders. Front. Endocrinol. 2022, 13, 407. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.N.; Rajindrajith, S. Fetal programming of obesity and type 2 diabetes. World J. Diabetes 2022, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Demicheva, E.; Crispi, F. Long-term follow-up of intrauterine growth restriction: Cardiovascular disorders. Fetal. Diagn. Ther. 2014, 36, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C.; Simmonds, S.J.; Wield, G.A. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 1993, 306, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E.; Charnock-Jones, D.S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 2009, 54, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.K.; Campbell, J.P. Placental structure, function and drug transfer. Crit. Care Pain Med. 2015, 15, 84–89. [Google Scholar] [CrossRef]
- Schalkwijk, S.; Greupink, R.; Colbers, A.P.; Wouterse, A.C.; Verweij, V.G.; van Drongelen, J.; Teulen, M.; van den Oetelaar, D.; Burger, D.M.; Russel, F.G. Placental transfer of the HIV integrase inhibitor dolutegravir in an ex vivo human cotyledon perfusion model. J. Antimicrob. Chemother. 2016, 71, 480–483. [Google Scholar] [CrossRef]
- Cerveny, L.; Murthi, P.; Staud, F. HIV in pregnancy: Mother-to-child transmission, pharmacotherapy, and toxicity. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166206. [Google Scholar] [CrossRef]
- Naidoo, N.; Moodley, J.; Naicker, T. Maternal endothelial dysfunction in HIV-associated preeclampsia comorbid with COVID-19: A review. Hypertens. Res. 2021, 44, 386–398. [Google Scholar] [CrossRef]
- Vermaak, A.; Theron, G.B.; Schubert, P.T.; Kidd, M.; Rabie, U.; Adjiba, B.M.; Wright, C.A. Morphologic changes in the placentas of HIV-positive women and their association with degree of immune suppression. Int. J. Gynaecol. Obstet. 2012, 119, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Kajantie, E.; Thornburg, K.L.; Osmond, C.; Barker, D.J. Mother’s body size and placental size predict coronary heart disease in men. Eur. Heart J. 2011, 32, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W-65. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Aggarwal, R. Study designs: Part 1–An overview and classification. Perspects Clin. Res. 2018, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Omair, A. Selecting the appropriate study design for your research: Descriptive study designs. J. Health Spec. 2015, 3, 153. [Google Scholar] [CrossRef]
- Thomas, L. Longitudinal Study|Definition, Approaches & Examples. Scribbr. Available online: https://www.scribbr.com/methodology/longitudinal-study/ (accessed on 19 November 2023).
- Thiese, M.S. Observational and interventional study design types; an overview. Biochem. Medica 2014, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- De la Calle, M.; Rodriguez, R.; Deirós, L.; Bartha, J.L. Fetal cardiac biometry and function in HIV-infected pregnant women exposed to HAART therapy. Prenat. Diagn. 2015, 35, 453–455. [Google Scholar] [CrossRef]
- Grant-Beurmann, S.; Jumare, J.; Ndembi, N.; Matthew, O.; Shutt, A.; Omoigberale, A.; Martin, O.A.; Fraser, C.M.; Charurat, M. Dynamics of the infant gut microbiota in the first 18 months of life: The impact of maternal HIV infection and breastfeeding. Microbiome 2022, 10, 61. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Sasaki, N.; Thompson, B.; Eidem, B.W.; Cheng, I.; Colan, S.D.; O’brien, S.E.; Amdani, S.M.; Shearer, W.T.; Orav, E.J.; et al. Left ventricular diastolic dysfunction in HIV-uninfected infants exposed in utero to antiretroviral therapy. AIDS 2020, 34, 529. [Google Scholar] [CrossRef]
- Sevenoaks, T.; Wedderburn, C.J.; Donald, K.A.; Barnett, W.; Zar, H.J.; Stein, D.J.; Naudé, P.J. Association of maternal and infant inflammation with neurodevelopment in HIV-exposed uninfected children in a South African birth cohort. Brain Behav. Immun. 2021, 91, 65–73. [Google Scholar] [CrossRef]
- White, M.; Feucht, U.D.; Duffley, E.; Molokoane, F.; Durandt, C.; Cassol, E.; Rossouw, T.; Connor, K.L. Does in utero HIV exposure and the early nutritional environment influence infant development and immune outcomes? Findings from a pilot study in Pretoria, South Africa. Pilot Feasibility Stud. 2020, 6, 192. [Google Scholar] [CrossRef]
- Hofer, C.B.; Keiser, O.; Zwahlen, M.; Lustosa, C.S.; CisneFrota, A.C.; de Oliveira, R.H.; Abreu, T.F.; Carvalho, A.W.; Araujo, L.E.; Egger, M. In utero exposure to antiretroviral drugs: Effect on birth weight and growth among HIV-exposed uninfected children in Brazil. Pediatr. Infect Dis. J. 2016, 35, 71. [Google Scholar] [CrossRef] [PubMed]
- Mabaya, L.; Matarira, H.T.; Tanyanyiwa, D.M.; Musarurwa, C.; Mukwembi, J. Growth trajectories of HIV exposed and HIV unexposed infants. a prospective study in Gweru, Zimbabwe. Glob. Pediatr. Health 2021, 8, 2333794X21990338. [Google Scholar] [CrossRef] [PubMed]
- le Roux, S.M.; Abrams, E.J.; Donald, K.A.; Brittain, K.; Phillips, T.K.; Nguyen, K.K.; Zerbe, A.; Kroon, M.; Myer, L. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: A prospective study. Lancet Child Adolesc. 2019, 3, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Aizire, J.; Sikorskii, A.; Ogwang, L.W.; Kawalazira, R.; Mutebe, A.; Familiar-Lopez, I.; Mallewa, M.; Taha, T.; Boivin, M.J.; Fowler, M.G.; et al. Decreased growth among antiretroviral drug and HIV exposed uninfected versus unexposed children in Malawi and Uganda. AIDS 2020, 34, 215. [Google Scholar] [CrossRef] [PubMed]
- Sofeu, C.L.; Warszawski, J.; Ateba Ndongo, F.; Penda, I.C.; Tetang Ndiang, S.; Guemkam, G.; Makwet, N.; Owona, F.; Kfutwah, A.; Tchendjou, P.; et al. Low birth weight in perinatally HIV-exposed uninfected infants: Observations in urban settings in Cameroon. PLoS ONE 2014, 9, e93554. [Google Scholar] [CrossRef] [PubMed]
- Malaba, T.R.; Phillips, T.; Le Roux, S.; Brittain, K.; Zerbe, A.; Petro, G.; Ronan, A.; McIntyre, J.A.; Abrams, E.J.; Myer, L. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int. J. Epidemiol. 2017, 46, 1678–1689. [Google Scholar] [CrossRef]
- Omoni, A.O.; Ntozini, R.; Evans, C.; Prendergast, A.J.; Moulton, L.H.; Christian, P.S.; Humphrey, J.H. Child growth according to maternal and child HIV status in Zimbabwe. J. Pediatr. Infect. 2017, Dis.36, 869. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Pedersen, S.H.; Urassa, M.; Michael, D.; Todd, J.; Kinung’hi, S.; Changalucha, J.; McDermid, J.M. Associations between gestational anthropometry, maternal HIV, and fetal and early infancy growth in a prospective rural/semi-rural Tanzanian cohort, 2012–2013. BMC Pregnancy Childbirth 2015, 15, 277. [Google Scholar] [CrossRef]
- Schoeman, J.C.; Moutloatse, G.P.; Harms, A.C.; Vreeken, R.J.; Scherpbier, H.J.; Van Leeuwen, L.; Kuijpers, T.W.; Reinecke, C.J.; Berger, R.; Hankemeier, T.; et al. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1–and Combination Antiretroviral Therapy–Exposed Infants. J. Infect. Dis. 2017, 216, 436–446. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Williams, P.L.; Zeldow, B.; Wilkinson, J.D.; Seage, G.R., III; Dooley, L.B.; Kaltman, J.R.; Siberry, G.K.; Mofenson, L.M.; Shearer, W.T.; et al. Cardiac Effects of in utero Exposure to Antiretroviral Therapy in HIV-Uninfected Children Born to HIV-Infected Mothers. AIDS 2015, 29, 91. [Google Scholar] [CrossRef] [PubMed]
- Jao, J.; Kirmse, B.; Yu, C.; Qiu, Y.; Powis, K.; Nshom, E.; Epie, F.; Tih, P.M.; Sperling, R.S.; Abrams, E.J.; et al. Lower preprandial insulin and altered fuel use in HIV/antiretroviral-exposed infants in Cameroon. J. Clin. Endocrinol. Metab. 2015, 100, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Jitratkosol, M.H.; Sattha, B.; Maan, E.J.; Gadawski, I.; Harrigan, P.R.; Forbes, J.C.; Alimenti, A.; van Schalkwyk, J.; Money, D.M.; Côté, H.C. Blood mitochondrial DNA mutations in HIV-infected women and their infants exposed to HAART during pregnancy. AIDS 2012, 26, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Nyemba, D.C.; Kalk, E.; Madlala, H.P.; Malaba, T.R.; Slogrove, A.L.; Davies, M.A.; Boulle, A.; Myer, L.; Powis, K.M. Lower birth weight-for-age and length-for-age z-scores in infants with in-utero HIV and ART exposure: A prospective study in Cape Town, South Africa. BMC Pregnancy Childbirth 2021, 21, 354. [Google Scholar] [CrossRef]
- García-Otero, L.; López, M.; Guitart-Mampel, M.; Morén, C.; Goncé, A.; Esteve, C.; Salazar, L.; Gómez, O.; Martínez, J.M.; Torres, B.; et al. Cardiac and mitochondrial function in HIV-uninfected fetuses exposed to antiretroviral treatment. PLoS ONE 2019, 14, e0213279. [Google Scholar] [CrossRef]
- García-Otero, L.; López, M.; Goncé, A.; Fortuny, C.; Salazar, L.; Valenzuela-Alcaraz, B.; Guirado, L.; César, S.; Gratacós, E.; Crispi, F. Cardiac remodeling and hypertension in HIV-uninfected infants exposed in utero to antiretroviral therapy. Clin. Infect. Dis. 2021, 73, 586–593. [Google Scholar] [CrossRef]
- Kasahara, T.M.; Hygino, J.; Blanco, B.; Xavier, L.; Araújo-Lima, C.F.; Guillermo, L.V.; Bittencourt, V.C.B.; Guimarães, V.; Andrade, A.F.; Bento, C.A. The impact of maternal anti-retroviral therapy on cytokine profile in the uninfected neonates. Hum. Immunol. 2013, 74, 1051–1056. [Google Scholar] [CrossRef]
- Noguera-Julian, A.; Morén, C.; Rovira, N.; Garrabou, G.; Catalán, M.; Sánchez, E.; Cardellach, F.; Miró, Ó.; Fortuny, C. Decreased mitochondrial function among healthy infants exposed to antiretrovirals during gestation, delivery and the neonatal period. Pediatr. Infect. Dis. J. 2015, 34, 1349–1354. [Google Scholar] [CrossRef]
- García-Otero, L.; López, M.; Gómez, O.; Goncé, A.; Bennasar, M.; Martínez, J.M.; Valenzuela-Alcaraz, B.; Rodriguez-López, M.; Sitges, M.; Loncà, M.; et al. Zidovudine treatment in HIV-infected pregnant women is associated with fetal cardiac remodelling. AIDS 2016, 30, 1393–1401. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Shearer, W.T.; Thompson, B.; Rich, K.C.; Cheng, I.; Orav, E.J.; Kumar, S.; Pignatelli, R.H.; Bezold, L.I.; LaRussa, P.; et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study). J. Am. Coll. Cardiol. 2011, 57, 76–85. [Google Scholar] [CrossRef]
- Guerra, V.; Leister, E.C.; Williams, P.L.; Starc, T.J.; Lipshultz, S.E.; Wilkinson, J.D.; Van Dyke, R.B.; Hazra, R.; Colan, S.D.; pediatric HIV/AIDS cohort study (PHACS). Long-term effects of in utero antiretroviral exposure: Systolic and diastolic function in HIV-exposed uninfected youth. AIDS Res. Hum. Retroviruses 2016, 32, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Muhangi, L.; Lule, S.A.; Mpairwe, H.; Ndibazza, J.; Kizza, M.; Nampijja, M.; Nakazibwe, E.; Kihembo, M.; Elliott, A.M.; Webb, E.L. Maternal HIV infection and other factors associated with growth outcomes of HIV-uninfected infants in Entebbe, Uganda. Public Health Nutr. 2013, 16, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Ramokolo, V.; Goga, A.E.; Lombard, C.; Doherty, T.; Jackson, D.J.; Engebretsen, I. October. In utero ART exposure and birth and early growth outcomes among HIV-exposed uninfected infants attending immunization services: Results from national PMTCT surveillance, South Africa. Open Forum Infect. Dis. 2017, 4, ofx187. [Google Scholar] [CrossRef] [PubMed]
- Esemu, L.F.; Yuosembom, E.K.; Fang, R.; Rasay, S.; Fodjo, B.A.; Nguasong, J.T.; Kidima, W.; Ekali, G.L.; Chen, J.J.; Ndhlovu, L.; et al. Impact of HIV-1 infection on the IGF-1 axis and angiogenic factors in pregnant Cameroonian women receiving antiretroviral therapy. PLoS ONE 2019, 14, e0215825. [Google Scholar] [CrossRef] [PubMed]
- Pintye, J.; Langat, A.; Singa, B.; Kinuthia, J.; Odeny, B.; Katana, A.; Nganga, L.; John-Stewart, G.; McGrath, C.J. Maternal tenofovir disoproxil fumarate use in pregnancy and growth outcomes among HIV-exposed uninfected infants in Kenya. Infect. Dis. Obstet. Gynecol. 2015, 2015, 276851. [Google Scholar] [CrossRef]
- Chalashika, P.; Essex, C.; Mellor, D.; Swift, J.A.; Langley-Evans, S. Birthweight, HIV exposure and infant feeding as predictors of malnutrition in Botswanan infants. J. Hum. Nutr. Diet. 2017, 30, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Catalán-García, M.; Morén, C.; García-Otero, L.; López, M.; Guitart-Mampel, M.; Milisenda, J.; Coll, O.; Cardellach, F.; Gratacós, E.; et al. Placental mitochondrial toxicity, oxidative stress, apoptosis, and adverse perinatal outcomes in HIV pregnancies under antiretroviral treatment containing zidovudine. J. Acquir. Immune Defic. Syndr. 2017, 75, e113–e119. [Google Scholar] [CrossRef]
- Hernandez, S.; Moren, C.; Lopez, M.; Coll, O.; Cardellach, F.; Gratacos, E.; Miro, O.; Garrabou, G. Perinatal outcomes, mitochondrial toxicity and apoptosis in HIV-treated pregnant women and in-utero-exposed newborn. AIDS 2012, 26, 419–428. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williams, P.L.; Yu, W.; Colan, S.D.; Mendez, A.; Zachariah, J.P.; Van Dyke, R.B.; Shearer, W.T.; Margossian, R.E.; Lipshultz, S.E.; et al. Cardiac and inflammatory biomarkers in perinatally HIV-infected and HIV-exposed uninfected children. AIDS 2018, 32, 1267–1277. [Google Scholar] [CrossRef]
- Martins, P.; Pires, A.; Albuquerque, M.E.; Oliveira-Santos, M.; Santos, J.; Sena, C.; Seiça, R. Myocardial peak systolic velocity—A tool for cardiac screening of HIV-exposed uninfected children. Eur. J. Ped. 2020, 179, 395–404. [Google Scholar] [CrossRef]
- Cade, W.T.; Waggoner, A.D.; Hubert, S.; Krauss, M.J.; Singh, G.K.; Overton, E.T. Reduced diastolic function and left ventricular mass in HIV-negative pre-adolescent children exposed to antiretroviral therapy in utero. AIDS 2012, 26, 2053. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Rupérez, M.; Sevene, E.; Vala, A.; Maculuve, S.; Bulo, H.; Nhacolo, A.; Mayor, A.; Aponte, J.J.; Macete, E.; et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: A prospective cohort study after a decade of antiretroviral drugs roll out. PLoS ONE 2017, 12, e0178134. [Google Scholar] [CrossRef] [PubMed]
- Hodel, E.M.; Marzolini, C.; Waitt, C.; Rakhmanina, N. Pharmacokinetics, placental and breast milk transfer of antiretroviral drugs in pregnant and lactating women living with HIV. Curr. Pharm Des. 2019, 25, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Pariente, G.; Leibson, T.; Carls, A.; Adams-Webber, T.; Ito, S.; Koren, G. Pregnancy-associated changes in pharmacokinetics: A systematic review. PLoS Med. 2016, 13, e1002160. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; McComsey, G.A. Cardiometabolic complications in youth with perinatally acquired HIV in the era of antiretroviral therapy. Curr. HIV/AIDS Rep. 2021, 18, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Matjuda, E.N.; Engwa, G.A.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. An Overview of Vascular Dysfunction and Determinants: The Case of Children of African Ancestry. Front. Pediatr. 2021, 9, 769589. [Google Scholar] [CrossRef]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial dysfunction, insulin resistance, and potential genetic implications: Potential role of alterations in mitochondrial function in the pathogenesis of insulin resistance and type 2 diabetes. Endocrinology 2020, 161, bqaa017. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Wilkinson, J.D.; Thompson, B.; Cheng, I.; Briston, D.A.; Shearer, W.T.; Orav, E.J.; Westphal, J.A.; Miller, T.L.; Colan, S.D.; et al. Cardiac effects of highly active antiretroviral therapy in perinatally HIV-infected children: The CHAART-2 study. J. Am. Coll. Cardiol. 2017, 70, 2240–2247. [Google Scholar] [CrossRef]
- Mellin, J.; Le Prevost, M.; Kenny, J.; Sturgeon, K.; Thompson, L.C.; Foster, C.; Kessler, H.H.; Goswami, N.; Klein, N.; Judd, A.; et al. Arterial stiffness in a cohort of young people living with perinatal HIV and HIV negative young people in England. Front. Cardiovasc. Med. 2022, 9, 821568. [Google Scholar] [CrossRef]
- Worm, S.W.; Sabin, C.; Weber, R.; Reiss, P.; El-Sadr, W.; Dabis, F.; De Wit, S.; Law, M.; Monforte, A.D.A.; Friis-Møller, N.; et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: The data collection on adverse events of anti-HIV drugs (D: A: D) study. J. Infect. Dis. 2010, 201, 318–330. [Google Scholar] [CrossRef]
- Matasariu, D.R.; Onofriescu, M.; Mihalceanu, E.; Schaas, C.M.; Bujor, I.E.; Tibeica, A.M.; Cristofor, A.E.; Ursache, A. Impact of HAART Therapy and HIV Infection over Fetal Growth—An Anthropometric Point of View. Microorganisms 2022, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryckman, K.K.; Barnabei, V.M.; Howard, B.V.; Isasi, C.R.; Sarto, G.E.; Tom, S.E.; Van Horn, L.V.; Wallace, R.B.; Robinson, J.G. The impact of birth weight on cardiovascular disease risk in the Women’s Health Initiative. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Dasinger, J.H.; Intapad, S. Fetal programming and cardiovascular pathology. Compr. Physiol. 2015, 5, 997. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Slopen, N.; Nelson, C.A.; Zeanah, C.H.; Georgieff, M.K.; Fox, N.A. Catch-up growth, metabolic, and cardiovascular risk in post-institutionalized Romanian adolescents. Pediatr. Res. 2018, 84, 842–848. [Google Scholar] [CrossRef]
- Risnes, K.R.; Nilsen, T.I.; Romundstad, P.R.; Vatten, L.J. Head size at birth and long-term mortality from coronary heart disease. Int. J. Epidemiol. 2009, 38, 955–962. [Google Scholar] [CrossRef][Green Version]
- Hangge, P.T.; Cnota, J.F.; Woo, J.G.; Hinton, A.C.; Divanovic, A.A.; Manning, P.B.; Ittenbach, R.F.; Hinton, R.B. Microcephaly is associated with early adverse neurologic outcomes in hypoplastic left heart syndrome. Pediatr. Res. 2013, 74, 61–67. [Google Scholar] [CrossRef]
- Williams, P.L.; Yildirim, C.; Chadwick, E.G.; Van Dyke, R.B.; Smith, R.; Correia, K.F.; DiPerna, A.; Seage III, G.R.; Hazra, R.; Crowell, C.S. Association of maternal antiretroviral use with microcephaly in HIV-exposed uninfected children in the prospective surveillance monitoring for ART toxicities (SMARTT) cohort study. Lancet HIV 2020, 7, e49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).