Habitats and Biotopes in the German Baltic Sea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Mapping
2.2. Compiling the BHT Map (Benthic Broad Habitat Types according to EU Commission Decision 2017/848/EU)
2.3. Compiling the OHT Map (Benthic Other Habitat Types according to EU Commission Decision 2017/848/EU)
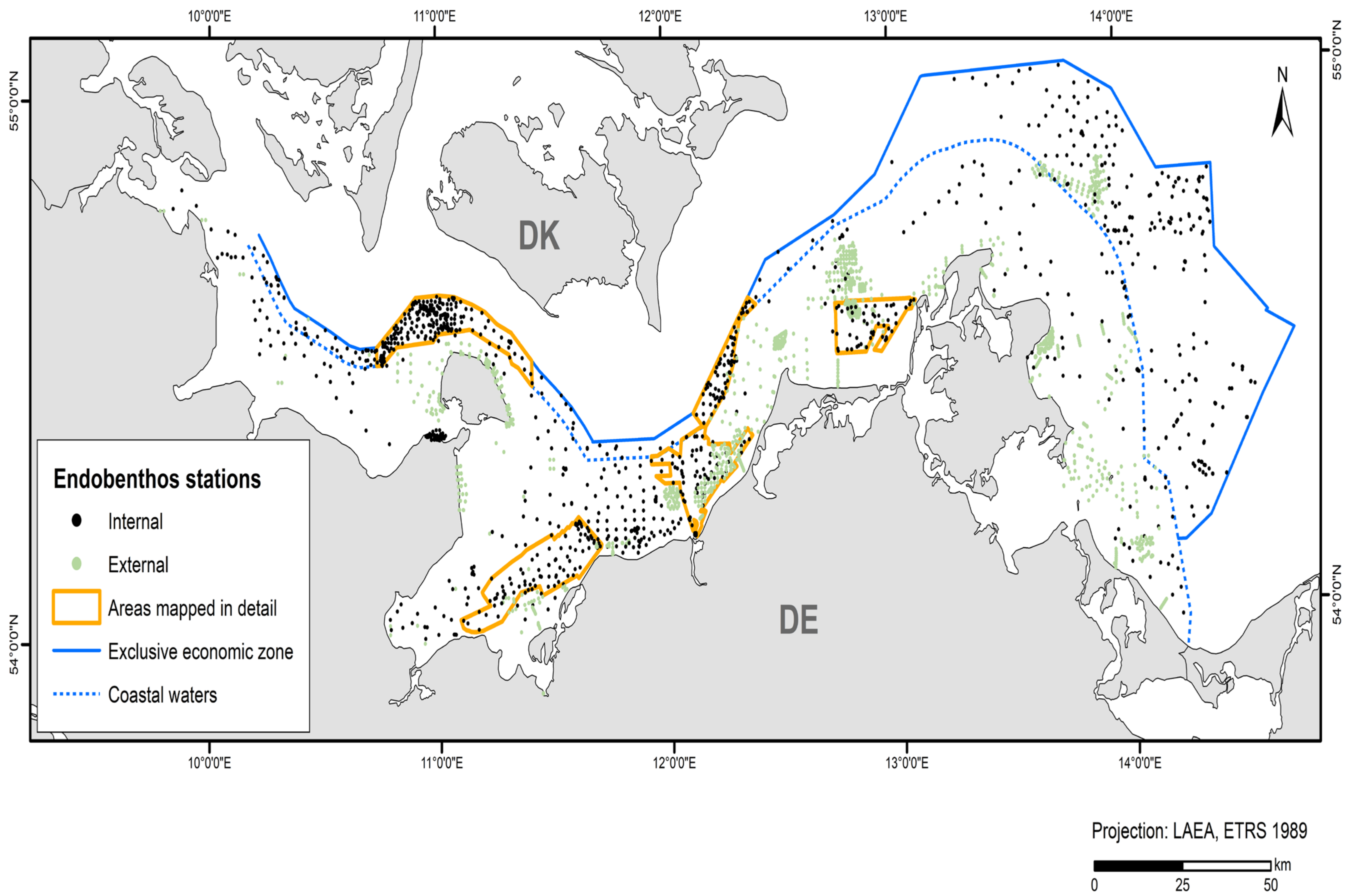
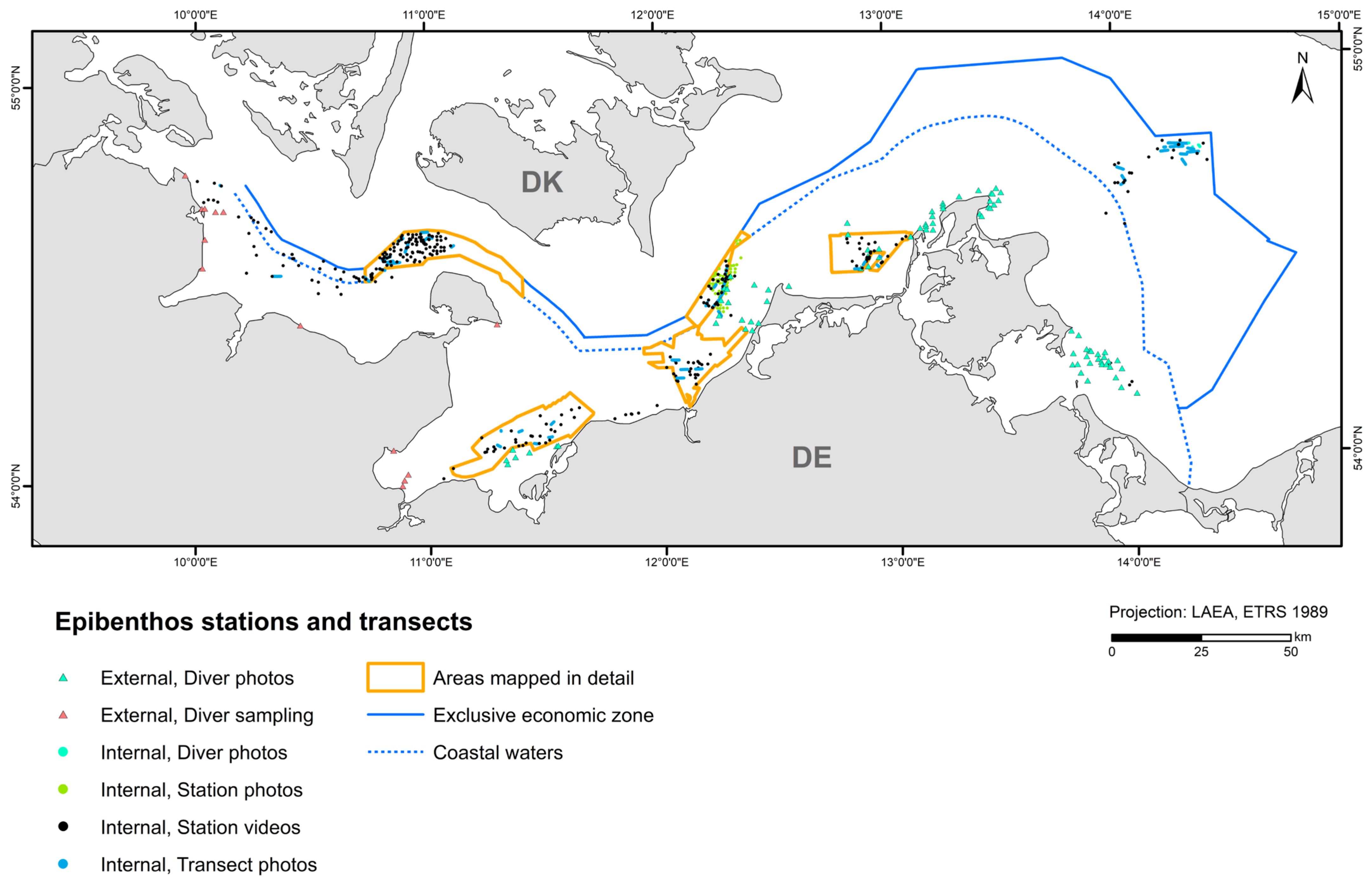
2.4. Biological Mapping
2.5. Data Basis for Modelling
2.6. Predictive Biotope Modelling
- Temperature, salinity, current velocity (in directions of north/south, east/west, without directional information), and bottom shear stress from the GETM model [65];
- Photosynthetically active radiation (PAR), oxygen concentration, number of hypoxic days, DOC, ammonium, nitrate, phosphate from the ERGOM model [64];
- Water depth and sediment type [32];
- Photic zonation (based on ERGOM model, [44]);
- Slope gradient (based on [32]).
2.7. HUB Map Modelling Limitations and Conventions
- Elimination of outliers:
- Before modelling the endobenthos in the whole German Baltic Sea, stations dominated by taxa that rarely occurred in the area and that accounted for max. 1% of the total number of stations were eliminated. Such outliers were Actiniaria and oligochaetes (in HELCOM HUB they are classified as meiofauna).
- Ophelia spp./Travisia spp. could not be separated from other communities by the random forest (RF) model and therefore were not reliably predicted, so stations with dominant Ophelia spp./Travisia spp. were also deleted.
- Less frequent dominant taxa were assigned to a higher category:
- Dominant Mya arenaria and Astarte spp. were assigned to the community with multiple infaunal bivalve species, because being a part of the overarching community, they were poorly separable from each other. Because the polychaete communities (partly with dominating Scoloplos armiger, Marenzelleria spp., Pygospio elegans, and Hediste diversicolor) were difficult to separate from the other communities; they were grouped together as the community with macroscopic infaunal biotic structures (HUB Level 4), as were stations ending at HUB level 5 (e.g., dominant bivalves/polychaetes/crustaceans). Therefore, the community with macroscopic infaunal biotic structures includes not only communities without dominant taxa, but also those previously mentioned that are too unspecific in their occurrence, leading to improved model performance.
- Non-dominant communities were indicated as dominant:
- Epibenthos-dominated stations that ended up at HUB level 5 were indicated as HUB level 6 (e.g., foliose red algae were treated as dominant even though they had < 50% cover), because the model cannot separate dominant and non-dominant communities, in order for those stations to be included in the model. This means that in areas where epibenthic communities are predicted, they do not need to be dominant, but they are more likely to occur than other communities.
- Mixed communities were indicated as non-mixed communities:
- Mixed communities that are very similar in species composition (e.g., foliose red algae, foliose red algae/sponges, foliose red algae/filamentous red algae, foliose red algae/bryozoans, and foliose red algae/sponges/kelp) cannot be clearly delineated by the model. Therefore, these mixed communities were assigned to those taxa that play a superior role in the biotope function (structuring, long-lived, and geographically dominant). For example, the classes listed above were assigned to dominant foliose red algae. This means that epibenthic mixed communities can always occur, even when indicated otherwise. Transitions cannot be modelled with the procedure chosen here because the model considers each class as distinct.
3. Results
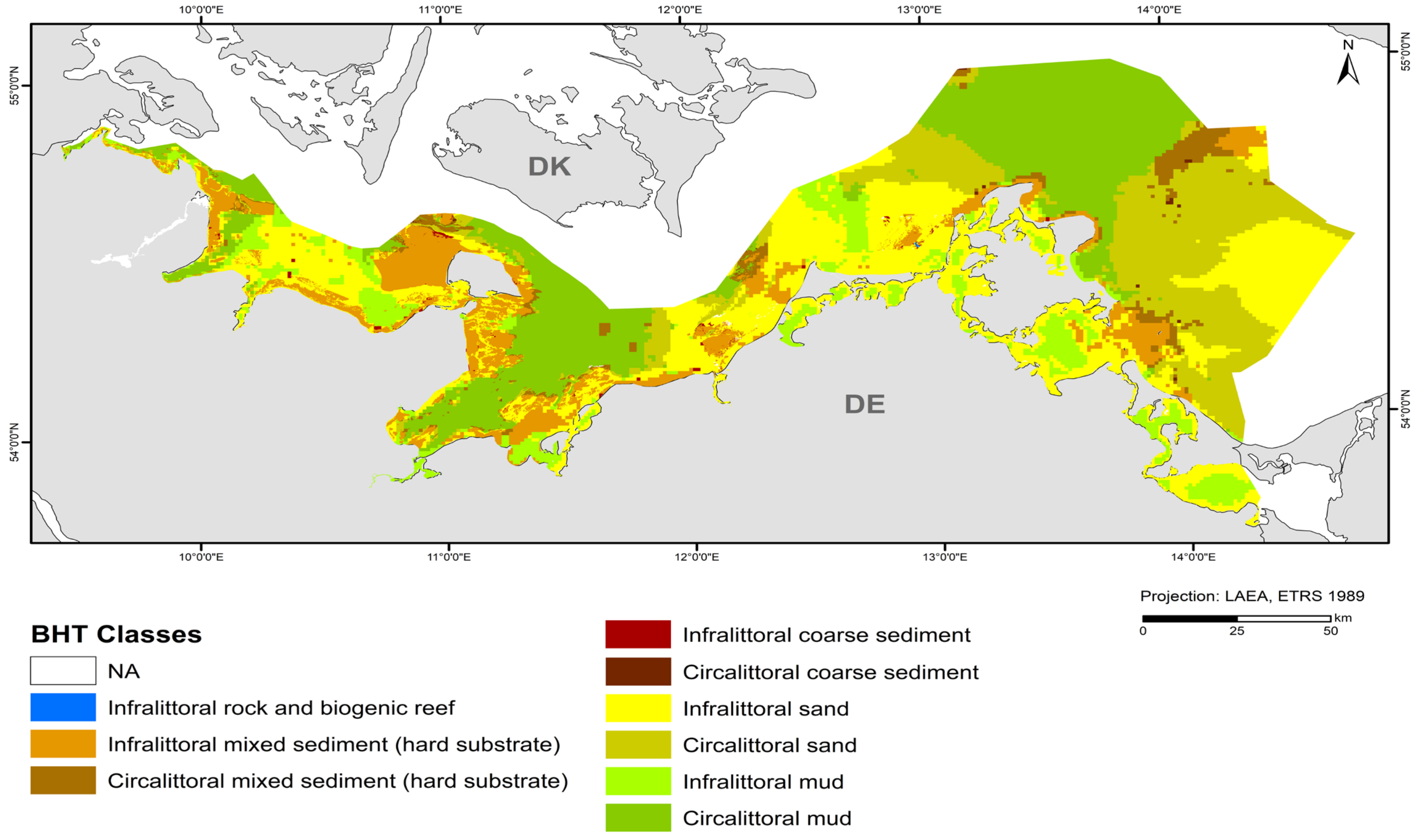
3.1. Broad Habitat Type (BHT) Map
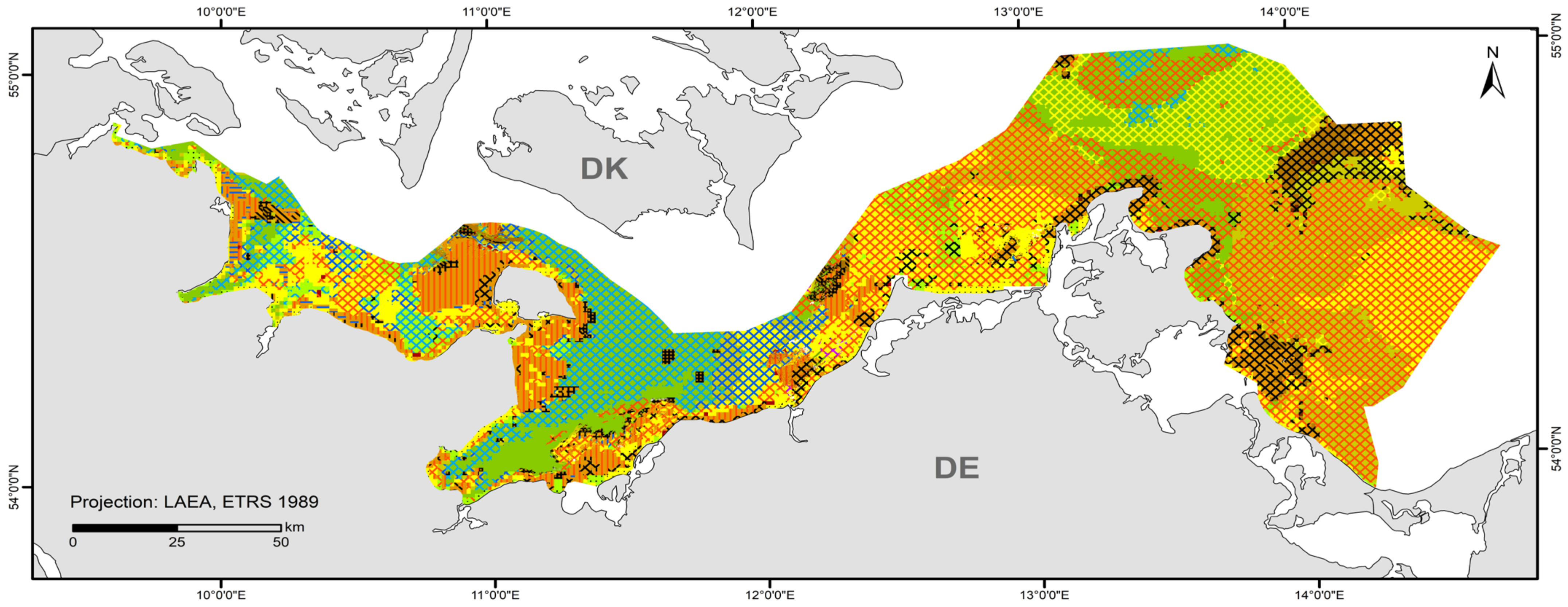
3.2. Other Habitat Type (OHT) Map
3.3. HELCOM HUB Map
4. Discussion
4.1. Modelling Biotope Distributions
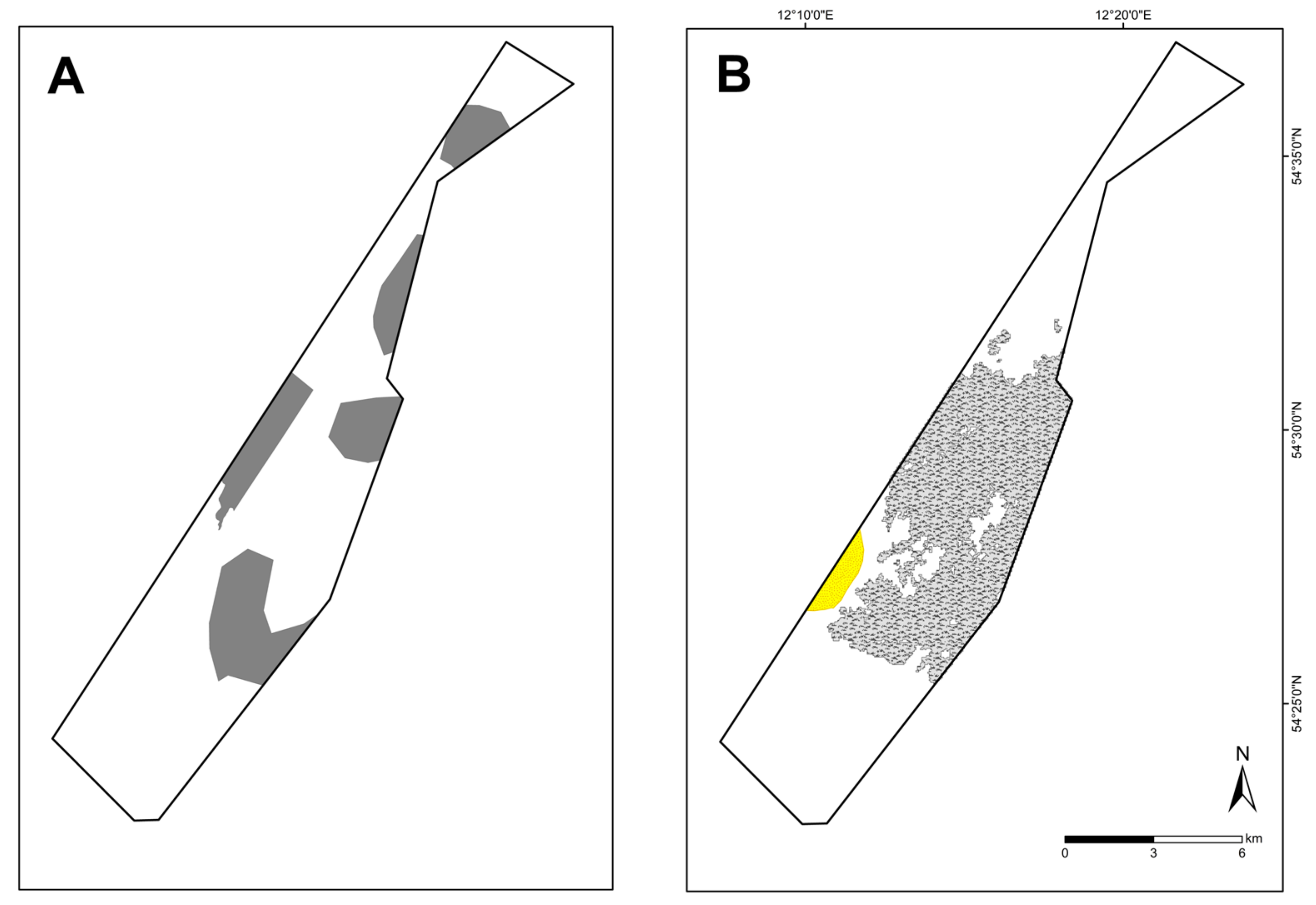
4.2. OHT “Species-Rich Areas of Gravel, Coarse-Sand and Shell-Gravel Areas”
4.3. Methodological Review
4.4. Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogalecka, M.; Kołowrocki, K. The Baltic Sea circumstances significant for its critical infrastructure networks. J. Polish Saf. Reliab. Assoc. Summer Saf. Reliab. Semin. 2016, 7, 37–42. [Google Scholar]
- Snoeijs-Leijonmalm, P.; Andrén, E. Why is the Baltic Sea so special to live in? In Biological Oceanography of the Baltic Sea; Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 23–84. [Google Scholar]
- Österblom, H.; Hansson, S.; Larsson, U.; Hjerne, O.; Wulff, F.; Elmgren, R.; Folke, C. Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 2007, 10, 877–889. [Google Scholar] [CrossRef]
- Tomczak, M.T.; Müller-Karulis, B.; Blenckner, T.; Ehrnsten, E.; Eero, M.; Gustafsson, B.; Norkko, A.; Otto, S.A.; Timmermann, K.; Humborg, C. Reference state, structure, regime shifts, and regulatory drivers in a coastal sea over the last century: The Central Baltic Sea case. Limnol. Oceanogr. 2022, 67, S266–S284. [Google Scholar] [CrossRef]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008; Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, L 164, 19–40. [Google Scholar]
- Salomidi, M.; Katsanevakis, S.; Borja, A.; Braeckman, U.; Damalas, D.; Galparsoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: A stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci. 2012, 13, 49–88. [Google Scholar] [CrossRef]
- European Commission. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Off. J. Eur. Union 1992, 206, 50. [Google Scholar]
- Heinicke, K.; Bildstein, T.; Reimers, H.-C.; Boedeker, D. Leitfaden zur großflächigen Abgrenzung und Kartierung des Lebensraumtyps “Riffe” in der deutschen Ostsee (EU-Code 1170; Untertyp: Geogene Riffe). BfN-Skripten 2021, 612, 46. [Google Scholar]
- HELCOM. HELCOM HUB—Technical Report on the HELCOM Underwater Biotope and Habitat Classification; Baltic Sea Environment Proceedings No. 139. 2013. Available online: https://www.academia.edu/32554320/HELCOM_HUB_Technical_Report_on_the_HELCOM_Underwater_Biotope_and_habitat_classifi_cation (accessed on 12 December 2023).
- European Commission. MSFD CIS Guidance Document No. 19; Article 8 MSFD; European Commission: Brussels, Belgium, 2022.
- European Commission. Commission Decision (EU) 2017/848 of 17 May 2017 Laying down Criteria and Methodological Standards on Good Environmental Status of Marine Waters and Specifications and Standardised Methods for Monitoring and Assessment, and Repealing Decision 2010/477/EU. Off. J. Eur. Union 2017, L 125, 43–74. [Google Scholar]
- Evans, D.; Aish, A.; Boon, A.; Condé, S.; Connor, D.; Gelabert, E.; Michez, N.; Parry, M.; Richard, D.; Salvati, E.; et al. Revising the Marine Section of the EUNIS Habitat Classification—Report of a Workshop Held at the European Topic Centre on Biological Diversity, 12 & 13 May 2016; ETC/BD Report to the EEA. 2016. Available online: https://www.eionet.europa.eu/etcs/etc-bd/products/etc-bd-reports/revising_marine_section_eunis_hab_classification (accessed on 12 December 2023).
- HELCOM. Red List of Baltic Sea Underwater Biotopes, Habitats and Biotope Complexes; Baltic Sea Environment Proceedings No. 138; HELCOM: Helsinki, Finland, 2013. [Google Scholar]
- Torn, K.; Herkül, K.; Martin, G.; Oganjan, K. Assessment of quality of three marine benthic habitat types in northern Baltic Sea. Ecol. Indic. 2017, 73, 772–783. [Google Scholar] [CrossRef]
- Sokołowski, A.; Jankowska, E.; Balazy, P.; Jędruch, A. Distribution and extent of benthic habitats in Puck Bay (Gulf of Gdańsk, southern Baltic Sea). Oceanologia 2021, 63, 301–320. [Google Scholar] [CrossRef]
- Olenin, S.; Daunys, D. Coastal typology based on benthic biotope and community data: The Lithuanian case study. Coastline Rep. 2004, 4, 65–83. [Google Scholar]
- Weslawski, J.M.; Kryla-Straszewska, L.; Piwowarczyk, J.; Urbański, J.; Warzocha, J.; Kotwicki, L.; Wlodarska-Kowalczuk, M.; Wiktor, J. Habitat modelling limitations—Puck Bay, Baltic Sea—A case study. Oceanologia 2013, 55, 167–183. [Google Scholar] [CrossRef]
- Wikström, S.A.; Enhus, C.; Fyhr, F.; Näslund, J.; Sundblad, G. Distribution of Biotopes, Habitats and Biological Values at Holmöarna and in the Kvarken Archipelago. 2013. Available online: https://aquabiota.se/wp-content/uploads/ABWR_Report2013-06_SUPERB.pdf (accessed on 12 December 2023).
- Vasquez, M.; Allen, H.; Manca, E.; Castle, L.; Lillis, H.; Agnesi, S.; Al Hamdani, Z.; Annunziatellis, A.; Askew, N.; Bekkby, T.; et al. EUSeaMap 2021. A European Broad-Scale Seabed Habitat Map; D1.13 EASME/EMFF/2018/1.3.1.8/Lot2/SI2.810241—EMODnet Thematic Lot n° 2—Seabed Habitats EUSeaMap 2021—Technical Report. 2021. Available online: https://archimer.ifremer.fr/doc/00723/83528/ (accessed on 12 December 2023).
- Schiele, K.S.; Darr, A.; Zettler, M.L.; Friedland, R.; Tauber, F.; von Weber, M.; Voss, J. Biotope map of the German Baltic Sea. Mar. Pollut. Bull. 2015, 96, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zettler, M.L.; Darr, A. Benthic Habitats and Their Inhabitants. In Southern Baltic Coastal Systems Analysis; Ecological Studies; Schubert, H., Müller, F., Eds.; Springer: Cham, Switzerland, 2023; Volume 246, pp. 97–102. ISBN 9783031136825. [Google Scholar]
- BSH. Anleitung zur Kartierung des Meeresbodens Mittels Hochauflösender Sonare in den Deutschen Meeresgebieten. BSH Nr. 7201. 2016. Available online: https://www.bsh.de/download/Kartierung-des-Meeresboden.pdf (accessed on 12 December 2023).
- Feldens, P.; Darr, A.; Feldens, A.; Tauber, F. Detection of boulders in side scan sonar mosaics by a neural network. Geoscience 2019, 9, 159. [Google Scholar] [CrossRef]
- Feldens, P.; Westfeld, P.; Valerius, J.; Feldens, A.; Papenmeier, S. Automatic detection of boulders by neural networks: A comparison of multibeam echo sounder and side-scan sonar performance. Hydrogr. Nachrichten 2021, 119, 6–17. [Google Scholar] [CrossRef]
- Feldens, A.; Marx, D.; Herbst, A.; Darr, A.; Papenmeier, S.; Hinz, M.; Zettler, M.L.; Feldens, P. Distribution of boulders in coastal waters of Western Pomerania, German Baltic Sea. Front. Earth Sci. 2023, 11, 1155765. [Google Scholar] [CrossRef]
- Darr, A.; Heinicke, K.; Meier, F.; Papenmeier, S.; Richter, P.; Schwarzer, K.; Valerius, J.; Boedeker, D. Die Biotope des Meeresbodens im Naturschutzgebiet Fehmarnbelt. BfN-Schriften 2022, 636, 1–99. [Google Scholar]
- Marx, D.; Romoth, K.; Papenmeier, S.; Valerius, J.; Darr, A.; Eisenbarth, S.; Heinicke, K. Die Biotope des Meeresbodens im Naturschutzgebiet “Kadetrinne”. 2024; in preparation. [Google Scholar]
- Richter, P.; Schwarzer, K.; Feldens, A.; Valerius, J.; Thiesen, M.; Mulckau, A. Map of Sediment Distribution in the German EEZ (1:10.000). 2020. Available online: www.geoseaportal.de (accessed on 12 December 2023).
- Richter, P.; Höft, D.; Feldens, A.; Schwarzer, K.; Diesing, M.; Valerius, J.; Mulckau, A. Map of Sediment Distribution in the German EEZ (1:10.000). 2021. Available online: https://www.geoseaportal.de/mapapps/resources/apps/sedimentverteilung_auf_dem_meeresboden/index.html?lang=de (accessed on 12 December 2023).
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landforms 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Folk, R.L. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar] [CrossRef]
- Tauber, F. Seabed Sediments and Seabed Relief in the German Baltic Sea: Data Positions and Sheet Index, Map no. 2930 [Map] 1: 550 000, 54° N; Federal Maritime and Hydrographic Agency: Hamburg, Germany, 2012.
- Diesing, M.; Schwarzer, K. Identification of submarine hard-bottom substrates in the German North Sea and Baltic Sea EEZ with high-resolution acoustic seafloor imaging. In Progress in Marine Conservation in Europe: NATURA 2000 Sites in German Offshore Waters; Von Nordheim, H., Boedeker, D., Krause, J.C., Eds.; Springer: Berlin, Germany; New York, NY, USA, 2006; pp. 111–125. ISBN 9783540332909. [Google Scholar]
- Bohling, B.; May, H.; Mosch, T.; Schwarzer, K. Regeneration of submarine hard-bottom substrate by natural abrasion in the western Baltic Sea. Marbg. Geogr. Schr. 2009, 145, 66–79. [Google Scholar]
- Schwarzer, K.; Bohling, B.; Heinrich, C. Submarine hard-bottom substrates in the western Baltic Sea—Human impact versus natural development. J. Coast. Res. 2014, 70, 145–150. [Google Scholar] [CrossRef]
- Figge, K. Sedimentverteilung in der Deutschen Bucht (Blatt: 2900, Maßstab: 1:250.000); Deutsches Hydrographisches Institut: Hamburg, Germany, 1981. [Google Scholar]
- Papenmeier, S.; Darr, A.; Feldens, P.; Michaelis, R. Hydroacoustic mapping of geogenic hard substrates: Challenges and review of German approaches. Geosci. 2020, 10, 100. [Google Scholar] [CrossRef]
- LANIS-SH, Schleswig-Holstein State Office for the Environment. Maritime Daten Ostsee LRT 1110 und 1170, State: December 2021. Available online: https://opendata.schleswig-holstein.de/dataset/maritime-daten-ostsee-lrt-1110-und-1170 (accessed on 12 December 2023).
- LUNG MV. State Office for the Environment Nature Conservation and Geology Mecklenburg-Western Pomerania: HELCOM HOLAS III Data Natura Habitats MV, State: 10.06.2022; LUNG MV: Güstrow, Germany, 2022.
- Bobsien, I.C.; Hukriede, W.; Schlamkow, C.; Friedland, R.; Dreier, N.; Schubert, P.R.; Karez, R.; Reusch, T.B.H. Modeling eelgrass spatial response to nutrient abatement measures in a changing climate. Ambio 2021, 50, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Schubert, H.; Schygulla, C. Abschlussbericht des Fachprojektes: “Die Erfassung rezenter Zostera-Bestände und weiterer Makrophyten in den Küstengewässern MV” (ZOSINF). Im Auftrag des Landesamtes für Umwelt, Naturschutz und Geologie Mecklenburg-Vorpommern (LUNG 100G-30.15/16); Landesamt für Umwelt, Naturschutz u. Geologie: Güstrow, Germany, 2017; p. 76.
- BfN. Artenreiche Kies-, Grobsand- und Schillgründe im Meeres- und Küstenbereich; Definition und Kartieranleitung; BfN: Bonn, Germany, 2011. [Google Scholar]
- EUNIS Marine Habitat Classification 2022 including Crosswalks. Available online: https://www.eea.europa.eu/data-and-maps/data/eunis-habitat-classification-1/eunis-marine-habitat-classification-review-2022/eunis-marine-habitat-classification-2022 (accessed on 4 October 2023).
- Friedland, R.; Neumann, T.; Schernewski, G. Climate change and the Baltic Sea action plan: Model simulations on the future of the western Baltic Sea. J. Mar. Syst. 2012, 105–108, 175–186. [Google Scholar] [CrossRef]
- Zustand der Deutschen Ostseegewässer 2018. Aktualisierung der Anfangsbewertung nach § 45c, der Beschreibung des Guten Zustands der Meeresgewässer nach § 45d und der Festlegung von Zielen nach § 45e des Wasserhaushaltsgesetzes zur Umsetzung der Meeresstrategie-Rahmenrichtlinie; BMU Hintergrunddokument: Methodik der Benthosbewertung in den Deutschen Ostseegewässern. 2018. Available online: https://dmkn.de/wp-content/uploads/2018/03/2018_Zustand_Ostsee_Entwurf.pdf (accessed on 12 December 2023).
- Boedeker, D.; Krause, J.C.; von Nordheim, H. Interpretation, identification and ecological assessment of the NATURA 2000 habitats “sandbank” and “reef.”. In Progress in Marine Conservation in Europe; Von Nordheim, H., Boedeker, D., Krause, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-33290-9. [Google Scholar]
- Gogina, M.; Zettler, A.; Zettler, M.L. Weight-to-weight conversion factors for benthic macrofauna: Recent measurements from the Baltic and the North seas. Earth Syst. Sci. Data 2022, 14, 1–4. [Google Scholar] [CrossRef]
- Bundesamt für Seeschifffahrt und Hydrographie (BSH). Standard Untersuchung der Auswirkungen von Offshore-Windenergieanlagen auf die Meeresumwelt (StUK 4); BSH: Munich, Germany, 2013. [Google Scholar]
- HELCOM Balsam Project 2013–2015. In Recommendations and Guidelines for Benthic Habitat Monitoring with Method Descriptions for Two Methods for Monitoring of Biotope and Habitat Extent; HELCOM: Helsinki, Finland, 2015; Available online: https://helcom.fi/wp-content/uploads/2019/08/Recommendations-and-guidelines-for-benthic-habitat-monitoring-in-the-Baltic-Sea.pdf (accessed on 12 December 2023).
- Beisiegel, K. Picturing the Seafloor: Shedding Light on Offshore Rock Habitats in the Baltic Sea using Imaging Systems. Ph.D. Thesis, The University of Rostock, Rostock, Germany, 2019. [Google Scholar]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- StALU WM. Kartierbericht für den Managementplan für das FFH-Gebiet DE 1934-303 “Erweiterung Wismarbucht”; StALU WM: Schwerin, Germany, 2017. [Google Scholar]
- StALU WM. Managementplan für das Gebiet von gemeinschaftlicher Bedeutung DE 1540-302 Darßer Schwelle; StALU WM: Schwerin, Germany, 2019. [Google Scholar]
- StALU WM. Managementplan für das Gebiet von gemeinschaftlicher Bedeutung DE 1345-301 Erweiterung Libben, Steilküste und Blockgründe Wittow und Arkona; StALU WM: Schwerin, Germany, 2019. [Google Scholar]
- StALU WM. Managementplan für das Gebiet von gemeinschaftlicher Bedeutung DE 1343-301 Plantagenetgrund; StALU WM: Schwerin, Germany, 2019. [Google Scholar]
- StALU WM. Managementplan für das Gebiet von gemeinschaftlicher Bedeutung (GGB) DE 1749-302 “Greifswalder Boddenrandschwelle und Teile der Pommerschen Bucht”; StALU WM: Schwerin, Germany, 2020. [Google Scholar]
- Hiebenthal, C.; Bock, G. Maßnahme: Meeresstrategie Rahmenrichtlinie (MSRL) Biodiversität Makrophyten Ostseeküste. [Aktenzeichen 0608-451219]—Abschlussbericht-.Im Auftrag des Landesamtes für Landwirtschaft, Umwelt und ländliche Räume (Schleswig-Holstein); GEOMAR: Kiel, Germany, 2013; p. 134. [Google Scholar]
- Hiebenthal, C. Forschungskooperation: “Konzept zum Monitoring der Entwicklung von Flachwasser-Hartbodengemeinschaften in der s.-h. Ostsee“ [Aktenzeichen 0608.451722]–Endbericht [Projektteile 1 und 2]—Im Auftrag des Landesamtes für Landwirtschaft, Umwelt und ländliche Räume (Schleswig-Holstein); GEOMAR: Kiel, Germany, 2021; p. 76. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Gonzalez-Mirelis, G.; Bergström, P.; Lindegarth, M. Interaction between classification detail and prediction of community types: Implications for predictive modelling of benthic biotopes. Mar. Ecol. Prog. Ser. 2011, 432, 31–44. [Google Scholar] [CrossRef]
- Miller, K.; Huettmann, F.; Norcross, B.; Lorenz, M. Multivariate random forest models of estuarine-associated fish and invertebrate communities. Mar. Ecol. Prog. Ser. 2014, 500, 159–174. [Google Scholar] [CrossRef]
- Galaiduk, R.; Radford, B.; Harries, S.; Case, M.; Williams, D.; Low Choy, D.; Smit, N. Technical Report: Darwin–Bynoe Harbours Predictive Mapping of Benthic Communities; Australian Institute of Marine Science: Perth, Australia, 2019; p. 42. Available online: https://www.researchgate.net/publication/340374082_Technical_Report_Darwin_-_Bynoe_Harbours_Predictive_Mapping_of_Benthic_Communities_httppidgeosciencegovaudatasetga127389 (accessed on 12 December 2023).
- Leipe, T.; Naumann, M.; Tauber, F.; Radtke, H.; Friedland, R.; Hiller, A.; Arz, H.W. Regional distribution patterns of chemical parameters in surface sediments of the south-western Baltic Sea and their possible causes. Geo-Mar. Lett. 2017, 37, 593–606. [Google Scholar] [CrossRef]
- Gräwe, U.; Naumann, M.; Mohrholz, V.; Burchard, H. Anatomizing one of the largest saltwater inflows into the Baltic Sea in December 2014. J. Geophys. Res. Oceans 2015, 120, 7676–7697. [Google Scholar] [CrossRef]
- Schiele, K. Benthic Community and Habitat Analysis towards an Application in Marine Management. Ph.D. Thesis, Universität Rostock, Rostock, Germany, 2014. [Google Scholar]
- Van Hoey, G.; Degraer, S.; Vincx, M. Macrobenthic community structure of soft-bottom sediments at the Belgian Continental Shelf. Estuar. Coast. Shelf Sci. 2004, 59, 599–613. [Google Scholar] [CrossRef]
- Coleman, N.; Cuff, W.; Moverley, J.; Gason, A.S.H.; Heislers, S. Depth, sediment type, biogeography and high species richness in shallow-water benthos. Mar. Freshw. Res. 2007, 58, 293–305. [Google Scholar] [CrossRef]
- Kröncke, I.; Reiss, H.; Eggleton, J.D.; Aldridge, J.; Bergman, M.J.N.; Cochrane, S.; Craeymeersch, J.A.; Degraer, S.; Desroy, N.; Dewarumez, J.M.; et al. Changes in North Sea macrofauna communities and species distribution between 1986 and 2000. Estuar. Coast. Shelf Sci. 2011, 94, 1–15. [Google Scholar] [CrossRef]
- Bonsdorff, E. Zoobenthic diversity-gradients in the Baltic Sea: Continuous post-glacial succession in a stressed ecosystem. J. Exp. Mar. Bio. Ecol. 2006, 330, 383–391. [Google Scholar] [CrossRef]
- Bonsdorff, E.; Laine, A.O.; Hänninen, J.; Vuorinen, I.; Norkko, A. Zoobenthos of the outer archipelago waters (N. Baltic Sea)—The importance of local conditions for spatial distribution patterns. Boreal Environ. Res. 2003, 8, 135–145. [Google Scholar]
- Nyström Sandman, A.; Wikström, S.A.; Blomqvist, M.; Kautsky, H.; Isaeus, M. Scale-dependent influence of environmental variables on species distribution: A case study on five coastal benthic species in the Baltic Sea. Ecography (Cop.) 2013, 36, 354–363. [Google Scholar] [CrossRef]
- Peterson, A.; Herkül, K. Mapping benthic biodiversity using georeferenced environmental data and predictive modeling. Mar. Biodivers. 2019, 49, 131–146. [Google Scholar] [CrossRef]
- Wicaksono, P.; Aryaguna, P.A.; Lazuardi, W. Benthic habitat mapping model and cross validation using machine-learning classification algorithms. Remote Sens. 2019, 11, 1279. [Google Scholar] [CrossRef]
- Janowski, L.; Wroblewski, R.; Dworniczak, J.; Kolakowski, M.; Rogowska, K.; Wojcik, M.; Gajewski, J. Offshore benthic habitat mapping based on object-based image analysis and geomorphometric approach. A case study from the Slupsk Bank, Southern Baltic Sea. Sci. Total Environ. 2021, 801, 149712. [Google Scholar] [CrossRef]
- Stephens, D.; Diesing, M. A comparison of supervised classification methods for the prediction of substrate type using multibeam acoustic and legacy grain-size data. PLoS ONE 2014, 9, e93950. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley Interscience: New York, NY, USA, 2000. [Google Scholar]
- Landis, J.R.; Koch, G.G. Measurement of observed agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]



| Level A | Level B | Level C |
|---|---|---|
| Fine sediment (Fsed) | not specified * | not classified ** |
| mud (M) | not classified | |
| sandy mud (sM) | ||
| muddy sand (mS) | ||
| Sand (S) | sand (S) | not classified |
| fine sand (fSa) | ||
| medium sand (mSa) | ||
| mixed sand (mxSa) | ||
| coarse sand (cSa) | ||
| Coarse sediment (Csed) | not specified | not classified |
| gravelly sand (gS) | not classified | |
| sandy gravel (sG) | ||
| gravel (G) | ||
| Mixed sediments (MxSed) | not specified | not classified |
| gravelly mud (gM) | not classified | |
| gravelly muddy sand (msG) | ||
| muddy gravel (mG) | ||
| Peat | ||
| Lag sediment (LagSed) | not classified | not classified |
| Not specified | not specified | not specified |
| BHT | OHT | HUB | ||||
|---|---|---|---|---|---|---|
| Detail Areas | Outside of Detail Areas | Detail Areas | Outside of Detail Areas | Detail Areas | Outside of Detail Areas | |
| Overall resolution | 50 × 50 m | 1 × 1 km | 50 × 50 m and polygons | 1 × 1 km and polygons | 50 × 50 m | 1 × 1 km |
| Map basis for soft bottom | Sediment distribution maps from hydroacoustic surveys (gridded) | Tauber [32] (gridded) | Seagrass meadows and “species-rich areas of gravel, coarse-sand and shell-gravel areas” mapped according to hydroacoustic results; distribution area of “Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica)” modelled in this study | “Seagrass meadows” modelled by [40,41] (gridded); sandbanks as reported to HOLAS III (polygons); distribution area of “Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica)” modelled in this study | Sediment distribution maps from hydroacoustic surveys (gridded) | Tauber [32] (gridded) |
| Map basis for hard bottom | Boulder distribution maps from hydroacoustic surveys according to [8] (grids) | Reef areas as reported to HOLAS III (gridded) | Distribution area of “other marine macrophyte populations” modelled in this study; reefs mapped hydroacoustically in this study (gridded) | Reef areas as reported to HOLAS III (polygons) | Boulder distribution maps from hydroacoustic surveys according to [8] (grids) | Reef areas as reported to HOLAS III (gridded) |
| Hard bottom assignment | >5 boulders/50 × 50 m cell or lag sediment and >1 boulder/50 × 50 m cell (from boulder and sediment distribution maps) | Reef areas as reported to HOLAS III (gridded) | Reefs mapped according to [8] | Reef areas as reported to HOLAS III | >5 boulders/50 × 50 m cell or lag sediment and >1 boulder/50 × 50 m cell (from boulder and sediment distribution maps) | Reef areas as reported to HOLAS III (gridded) |
| Biotope classification schemes | EUNIS | EUNIS | “Species-rich areas of gravel, coarse-sand and shell-gravel areas” according to [42]; “Seagrass meadows and other marine macrophyte populations” and “Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica)” according to HUB; reefs according to [8] | “Seagrass meadows and other marine macrophyte populations” and “Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica)” according to HUB; reefs according to [8] | HUB | HUB |
| Predictors used for modelling | - | - | Only “Seagrass meadows and other marine macrophyte populations” and “Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica)” were modelled in this study; the former is equivalent in their spatial extent to HUB class “Baltic photic mixed substrate dominated by perennial non-filamentous corticated red algae” and “Baltic a-/photic mixed substrate/coarse sediment dominated by foliose red algae” (Zostera spp. and Fucus spp. were not modelled in this study) and only indicated outside the reef areas; for predictors, see HUB entries | See detail areas | Endobenthos: sediment distribution map (50 × 50 m), water depth (50 × 50 m), temperature, salinity, current velocity (in directions north/south, east/west, without directional information), bottom shear stress, oxygen concentration, number of hypoxic days, DOC, ammonium, nitrate, phosphate (600 × 600 m) Epibenthos: boulder distribution map, water depth, photic zonation, slope gradient (50 × 50 m), temperature, salinity, current velocity (in directions north/south, east/west, without directional information), bottom shear stress, photosynthetically active radiation (PAR), oxygen concentration, number of hypoxic days, DOC, ammonium, nitrate, phosphate (600 × 600 m) | See detail areas; Tauber [32] was used instead of the sediment distribution map for endobenthos modelling, and reef coverage (as reported to HOLAS III) was used instead of boulder distribution map for epibenthos modelling |
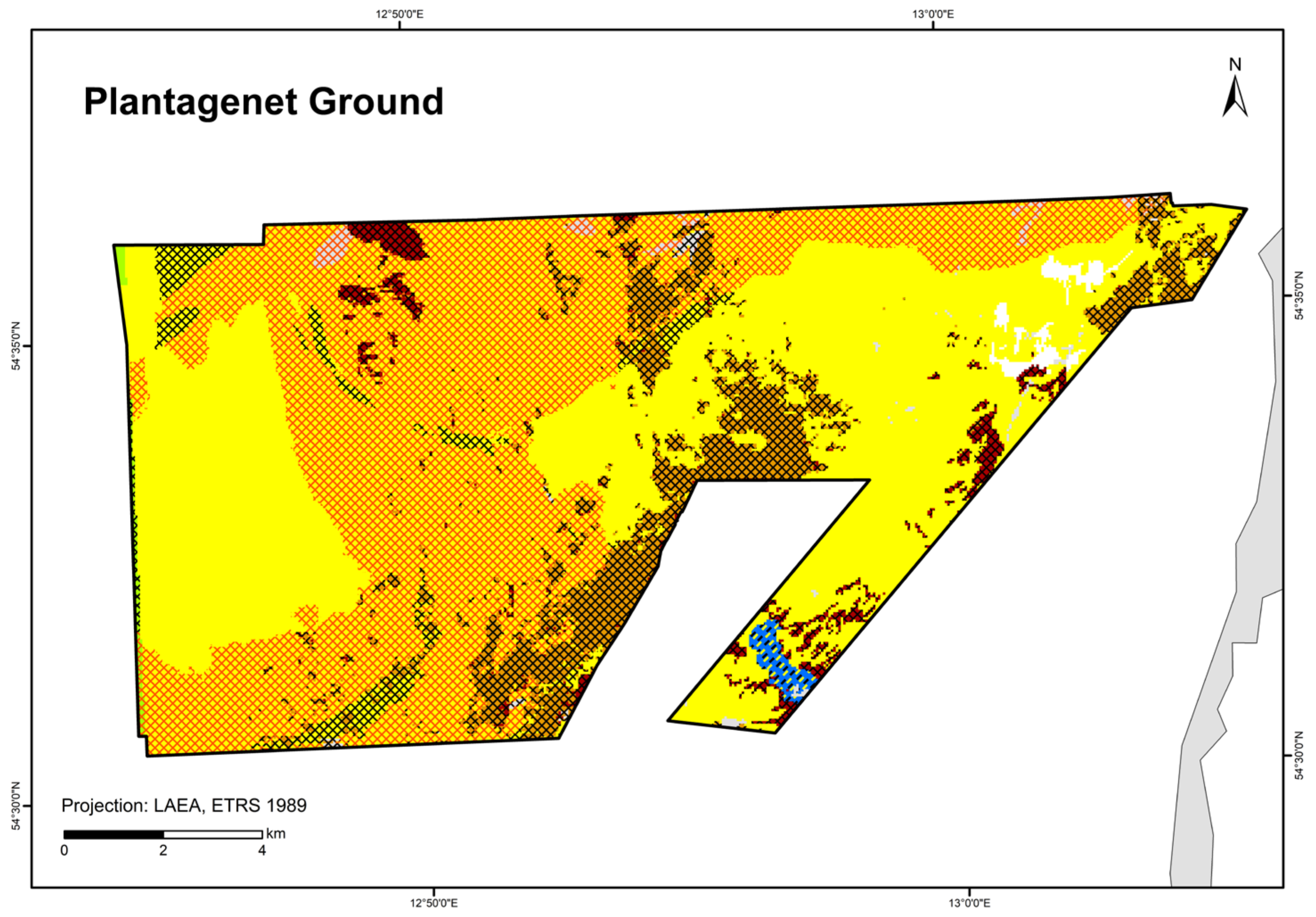
| “Seagrass meadows and other marine macrophyte populations” (paragraph §30 Federal Nature Conservation Act) | - | - | Seagrass mapped in the “Plantagenet Ground”; other macrophytes modelled in this study | Zostera spp. modelled in Schleswig-Holstein [40] and Mecklenburg-Western Pomerania [41]; Fucus spp. modelled in Schleswig-Holstein [40]; other macrophytes modelled in this study | See OHT | See OHT |
| Sediment Type Classified according to Tauber (2012) | Sediment Type Reclassified according to EUNIS |
|---|---|
| gravel, very coarse sand | coarse sediment |
| fine sand—coarse sand | sand |
| very fine mud—very fine sand | mud |
| clay, peat, lag sediment/till | mixed sediment (hard substrate) |
| Area | Number of Acquired Data Points | Sampling Instruments | References of Used Data | |
|---|---|---|---|---|
| Detail areas | Outer Wismar Bay | 85 (18) grab stations, 29 video stations, 6 video transects | Van Veen grab, SeaViewer, BaSIS | IOW, IfAÖ, LUNG, StALU WM, StALU MM |
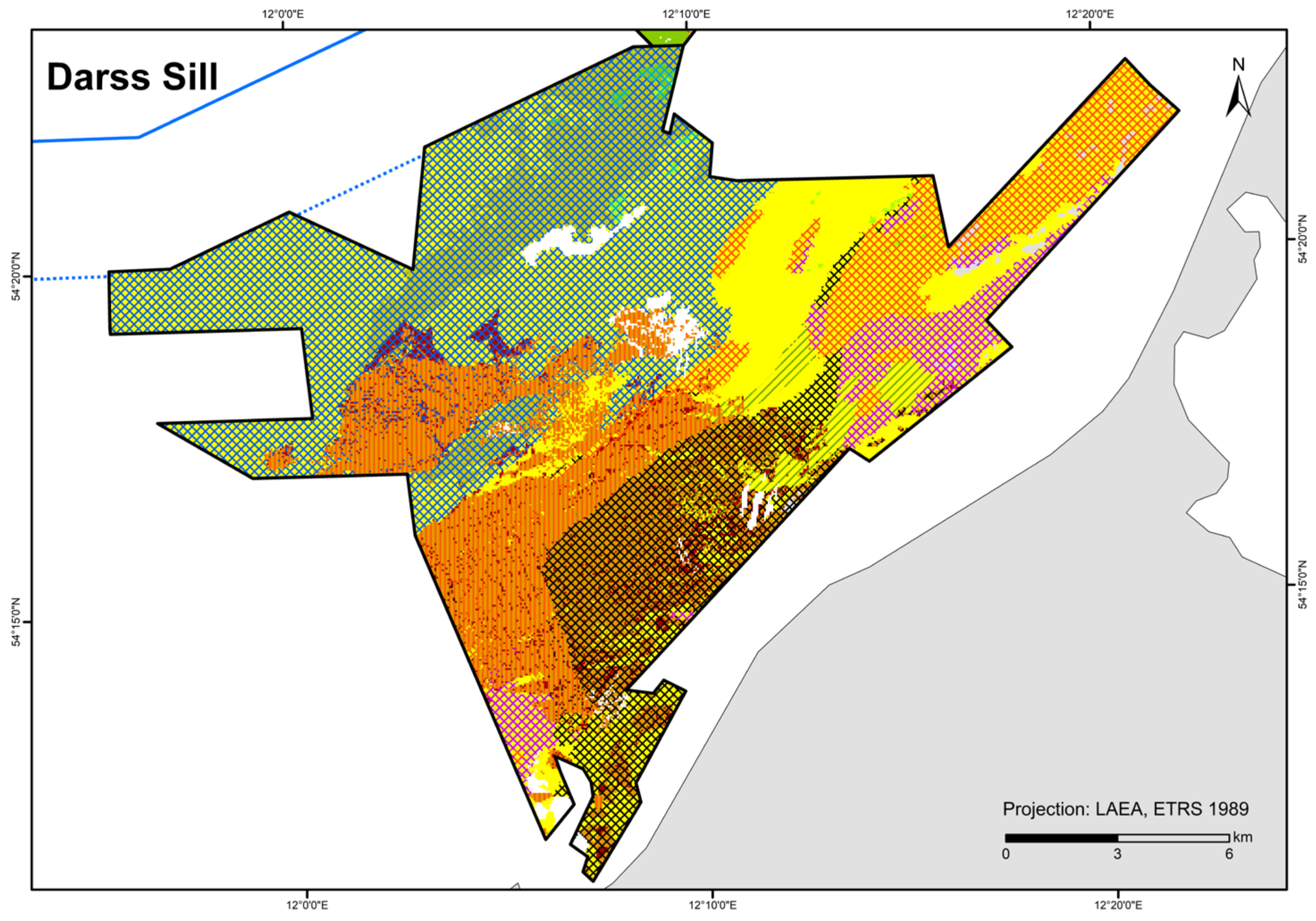
| Darss Sill | 73 (106) grab stations, 26 video stations, 4 video transects | Van Veen grab, SeaViewer, BaSIS | IOW, IfAÖ, LUNG, StALU WM, StALU MM | |
| Plantagenet Ground | 49 (67) grab stations, 27 video stations, 4 video transects | Van Veen grab, SeaViewer, BaSIS | IOW, IfAÖ, LUNG, StALU WM, StALU MM | |
| Kadet Trench | 103 (17) grab stations, 37 video stations, 8 video transects, 36 photo stations | Van Veen grab, SeaViewer, BaSIS, BfN drop camera | IOW, CAU Kiel, BSH | |
| Fehmarn Belt | 339 grab stations, 134 video stations, 11 video transects | Van Veen grab, BaSIS | IOW, CAU Kiel, BSH | |
| German Baltic Sea | 1637 (1991) grab stations, 403 video stations, 47 video transects, 59 photo stations, (45) diver stations, 9 (82) diver photo stations | Van Veen grab, SeaViewer, BaSIS, BfN drop camera, diver scratch samples and photos | IOW, BfN, BSH, LFU, LUNG, StALU WM, StALU MM, WSA Stralsund, CAU Kiel, IfAÖ, Geomar |
| BHT | Area (km2) | Area (%) |
|---|---|---|
| Infralittoral rock and biogenic reef | 1.0 | 0.007 |
| Infralittoral mixed sediment (hard substrate) | 1785.3 | 11.6 |
| Circalittoral mixed sediment (hard substrate) | 488.3 | 3.2 |
| Infralittoral coarse sediment | 35.5 | 0.2 |
| Circalittoral coarse sediment | 16.2 | 0.1 |
| Infralittoral sand | 4600.0 | 29.8 |
| Circalittoral sand | 3010.4 | 19.5 |
| Infralittoral mud | 1393.8 | 9.0 |
| Circalittoral mud | 4115.1 | 26.6 |
| OHT | Area (km2) | Area within the German Baltic Sea (%) |
|---|---|---|
| Reefs (habitat type 1170) | 2183.5 | 14.1 |
| Sandbanks (habitat type 1110) | 875.6 | 5.7 |
| Seagrass meadows and other marine macrophyte populations | 321.4 | 2.1 |
| Species-rich areas of gravel, coarse-sand, and shell-gravel areas | 5.9 | 0.04 |
| Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica) | 1417.6 | 9.2 |
| Non-OHT | 10,641.6 | 68.9 |
| Colour Coding HUB Map | HUB Code | HUB Biotope | Area (km2) |
|---|---|---|---|
 | AA.? | Baltic photic benthos | 0.4 |
 | AB.? | Baltic aphotic benthos | 0.5 |
 | AA.?1E1 | Baltic photic unknown substrate dominated by Mytilidae | 0.5 |
 | AA.?3 | Baltic photic unknown substrate characterised by macroscopic infaunal biotic structures | 2.1 |
 | AA.?3L3 | Baltic photic unknown substrate dominated by ocean quahog (Arctica islandica) | 0.003 |
 | AB.?3L3 | Baltic aphotic unknown substrate dominated by ocean quahog (Arctica islandica) | 0.1 |
 | AA.?3L4 | Baltic photic unknown substrate dominated by sand gaper (Mya arenaria) | 0.4 |
 | AA.?3L9 | Baltic photic unknown substrate dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 2.4 |
 | AA.M | Baltic photic mixed substrate | 0.02 |
 | AB.M | Baltic aphotic mixed substrate | 0.04 |
 | AA.M1 | Baltic photic mixed substrate characterised by macroscopic epibenthic biotic structures | 14.4 |
 | AB.M1 | Baltic aphotic mixed substrate characterised by macroscopic epibenthic biotic structures | 15.3 |
 | AA.M1C1 | Baltic photic mixed substrate dominated by Fucus spp. | 102.8 |
 | AA.M1C2 | Baltic photic mixed substrate dominated by perennial non-filamentous corticated red algae | 16.3 |
 | AA.M1C3 | Baltic photic mixed substrate dominated by foliose red algae | 840.8 |
 | AB.M1C3 * | Baltic aphotic mixed substrate dominated by foliose red algae | 0.9 |
 | AA.M1C5 | Baltic photic mixed substrate dominated by perennial filamentous algae | 24.2 |
 | AA.M1E1 | Baltic photic mixed substrate dominated by Mytilidae | 540.6 |
 | AA.M1E1? | Baltic photic mixed substrate dominated by Mytilidae? | 0.2 |
 | AB.M1E1 | Baltic aphotic mixed substrate dominated by Mytilidae | 302.9 |
 | AA.M1G1 | Baltic photic mixed substrate dominated by hydroids (Hydrozoa) | 80.4 |
 | AB.M1G1 | Baltic aphotic mixed substrate dominated by hydroids (Hydrozoa) | 109.0 |
 | AA.M1H2 | Baltic photic mixed substrate dominated by erect moss animals (Flustra foliacea) | 0.02 |
 | AB.M1I1 | Baltic aphotic mixed substrate dominated by barnacles (Balanidae) | 0.01 |
 | AA.M1S1 | Baltic photic mixed substrate dominated by filamentous annual algae | 75.0 |
 | AA.M1V | Baltic photic mixed substrate characterised by mixed epibenthic macrocommunity | 0.1 |
 | AB.M1V | Baltic aphotic mixed substrate characterised by mixed epibenthic macrocommunity | 10.9 |
 | AA.M2T | Baltic photic mixed substrate characterised by sparse epibenthic macrocommunity | 36.0 |
 | AB.M2T | Baltic aphotic mixed substrate characterised by sparse epibenthic macrocommunity | 28.9 |
 | AB.M4U | Baltic aphotic mixed substrate characterised by no macrocommunity | 3.0 |
 | AA.G+AA.J1E1 | Baltic photic peat bottoms + Baltic photic sand dominated by Mytilidae | 1.0 |
 | AA.I | Baltic photic coarse sediment | 0.006 |
 | AA.I1E1 | Baltic photic coarse sediment dominated by Mytilidae | 11.4 |
 | AA.I1E1? | Baltic photic coarse sediment dominated by Mytilidae? | 0.7 |
 | AB.I1E1 | Baltic aphotic coarse sediment dominated by Mytilidae | 9.0 |
 | AA.I1C3 | Baltic photic coarse sediment dominated by foliose red algae | 0.1 |
 | AA.I3 | Baltic photic coarse sediment characterised by macroscopic infaunal biotic structures | 10.5 |
 | AB.I3 | Baltic aphotic coarse sediment characterised by macroscopic infaunal biotic structures | 3.9 |
 | AA.I3L3 * | Baltic photic coarse sediment dominated by ocean quahog (Arctica islandica) | 4.3 |
 | AB.I3L3 * | Baltic aphotic coarse sediment dominated by ocean quahog (Arctica islandica) | 2.0 |
 | AA.I3L4 * | Baltic photic coarse sediment dominated by sand gaper (Mya arenaria) | 0.2 |
 | AA.I3L9 * | Baltic photic coarse sediment dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 4.1 |
 | AB.I3L9 * | Baltic aphotic coarse sediment dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 1.7 |
 | AA.I3L10 | Baltic photic coarse sediment dominated by multiple infaunal bivalve species: Macoma calcarea, Mya truncata, Astarte spp., Spisula spp. | 4.8 |
 | AB.I3L10 | Baltic aphotic coarse sediment dominated by multiple infaunal bivalve species: Macoma calcarea, Mya truncata, Astarte spp., Spisula spp. | 1.0 |
 | AA.I3L11 | Baltic photic coarse sediment dominated by multiple infaunal polychaete species including Ophelia spp. | 0.7 |
 | AB.I3M6 * | Baltic aphotic coarse sediment dominated by multiple infaunal polychaete species | 0.01 |
 | AA.J | Baltic photic sand | 0.1 |
 | AB.J | Baltic aphotic sand | 0.005 |
 | AA.J1B7 | Baltic photic sand dominated by common eelgrass (Zostera marina) | 223.1 |
 | AA.J1E1 | Baltic photic sand dominated by Mytilidae | 141.3 |
 | AB.J1E1 | Baltic aphotic sand dominated by Mytilidae | 196.2 |
 | AA.J1S | Baltic photic sand characterised by annual algae | 4.0 |
 | AA.J3 | Baltic photic sand characterised by macroscopic infaunal biotic structures | 425.9 |
 | AB.J3 | Baltic aphotic sand characterised by macroscopic infaunal biotic structures | 121.5 |
 | AA.J3L1 | Baltic photic sand dominated by Baltic tellin (Macoma balthica) | 8.2 |
 | AB.J3L1 | Baltic aphotic sand dominated by Baltic tellin (Macoma balthica) | 60.7 |
 | AA.J3L3 | Baltic photic sand dominated by ocean quahog (Arctica islandica) | 367.4 |
 | AB.J3L3 | Baltic aphotic sand dominated by ocean quahog (Arctica islandica) | 252.4 |
 | AA.J3L4 | Baltic photic sand dominated by sand gaper (Mya arenaria) | 15.7 |
 | AB.J3L4 | Baltic aphotic sand dominated by sand gaper (Mya arenaria) | 0.1 |
 | AA.J3L9 | Baltic photic sand dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 2338.7 |
 | AB.J3L9 | Baltic aphotic sand dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 2381.1 |
 | AA.J3L10 | Baltic photic sand dominated by multiple infaunal bivalve species: Macoma calcarea, Mya truncata, Astarte spp., Spisula spp. | 1.1 |
 | AB.J3L10 | Baltic aphotic sand dominated by multiple infaunal bivalve species: Macoma calcarea, Mya truncata, Astarte spp., Spisula spp. | 1.2 |
 | AA.J3L11 | Baltic photic sand dominated by multiple infaunal polychaete species including Ophelia spp. | 5.7 |
 | AA.J3M6* | Baltic photic sand dominated by multiple infaunal polychaete species | 0.005 |
 | AB.J3M6* | Baltic aphotic sand dominated by multiple infaunal polychaete species | 0.3 |
 | AA.H1B7 | Baltic photic muddy sediment dominated by common eelgrass (Zostera marina) | 69.0 |
 | AA.H1E1 | Baltic photic muddy sediment dominated by Mytilidae | 46.6 |
 | AB.H1E1 | Baltic aphotic muddy sediment dominated by Mytilidae | 15.0 |
 | AA.H1S | Baltic photic muddy sediment characterised by annual algae | 1.0 |
 | AA.H3 | Baltic photic muddy sediment characterised by macroscopic infaunal biotic structures | 65.2 |
 | AB.H3 | Baltic aphotic muddy sediment characterised by macroscopic infaunal biotic structures | 546.4 |
 | AB.H3L1 | Baltic aphotic muddy sediment dominated by Baltic tellin (Macoma balthica) | 1131.6 |
 | AA.H3L3 | Baltic photic muddy sediment dominated by ocean quahog (Arctica islandica) | 249.9 |
 | AB.H3L3 | Baltic aphotic muddy sediment dominated by ocean quahog (Arctica islandica) | 1435.5 |
 | AA.H3L4 * | Baltic photic muddy sediment dominated by sand gaper (Mya arenaria) | 0.003 |
 | AB.H3L4 * | Baltic aphotic muddy sediment dominated by sand gaper (Mya arenaria) | 0.005 |
 | AA.H3L9 * | Baltic photic muddy sediment dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 307.9 |
 | AB.H3L9 * | Baltic aphotic muddy sediment dominated by multiple infaunal bivalve species: Cerastoderma spp., Mya arenaria, Astarte borealis, Arctica islandica, Macoma balthica | 991.0 |
 | AA.H3L10 * | Baltic photic muddy sediment dominated by multiple infaunal bivalve species: Macoma calcarea, Mya truncata, Astarte spp., Spisula spp. | 0.003 |
 | AB.H3L10 * | Baltic aphotic muddy sediment dominated by multiple infaunal bivalve species: Macoma calcarea, Mya truncata, Astarte spp., Spisula spp. | 0.1 |
 | AB.H3M6 | Baltic aphotic muddy sediment dominated by multiple infaunal polychaete species | 3.2 |
| NA | 8.0 |
| Endobenthos | Epibenthos | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Overall Accuracy | 95% CI | AUC | Kappa | Most Important Variables | Overall Accuracy | 95% CI | AUC | Kappa | Most Important Variables | |
| Detail areas | Outer Wismar Bay | 0.393 | 0.215–0.594 | 0.758 | 0.035 | current velocity (10th percentile) | 0.98 | 0.893–1 | 0.975 | 0.96 | DOC (10th percentile), O2 (10th percentile) |
| Darss Sill | 0.564 | 0.423–0.7 | 0.648 | 0.453 | temperature (10th percentile) | 1 | 0.936–1 | 1 | 1 | DOC (mean) | |
| Plantagenet Ground | 0.719 | 0.533–0.863 | 0.797 | 0.559 | sediment | NA | NA | NA | NA | NA | |
| Kadet Trench | 0.759 | 0.565–0.9 | 0.786 | 0.576 | shear stress (mean), current velocity N/S (90 percentile) | 0.657 | 0.556–0.748 | 0.716 | 0.485 | depth | |
| Fehmarn Belt | 0.763 | NA | 0.788 | 0.563 | sediment, depth | 0.926 | NA | 0.915 | 0.83 | DOC (mean), depth | |
| German Baltic Sea | 0.666 | 0.636–0.695 | 0.704 | 0.535 | sediment | 0.797 | 0.770–0.821 | 0.805 | 0.712 | depth, salinity (mean) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marx, D.; Feldens, A.; Papenmeier, S.; Feldens, P.; Darr, A.; Zettler, M.L.; Heinicke, K. Habitats and Biotopes in the German Baltic Sea. Biology 2024, 13, 6. https://doi.org/10.3390/biology13010006
Marx D, Feldens A, Papenmeier S, Feldens P, Darr A, Zettler ML, Heinicke K. Habitats and Biotopes in the German Baltic Sea. Biology. 2024; 13(1):6. https://doi.org/10.3390/biology13010006
Chicago/Turabian StyleMarx, Denise, Agata Feldens, Svenja Papenmeier, Peter Feldens, Alexander Darr, Michael L. Zettler, and Kathrin Heinicke. 2024. "Habitats and Biotopes in the German Baltic Sea" Biology 13, no. 1: 6. https://doi.org/10.3390/biology13010006
APA StyleMarx, D., Feldens, A., Papenmeier, S., Feldens, P., Darr, A., Zettler, M. L., & Heinicke, K. (2024). Habitats and Biotopes in the German Baltic Sea. Biology, 13(1), 6. https://doi.org/10.3390/biology13010006







