The Role of the CPT Family in Cancer: Searching for New Therapeutic Strategies
Simple Summary
Abstract
1. Introduction
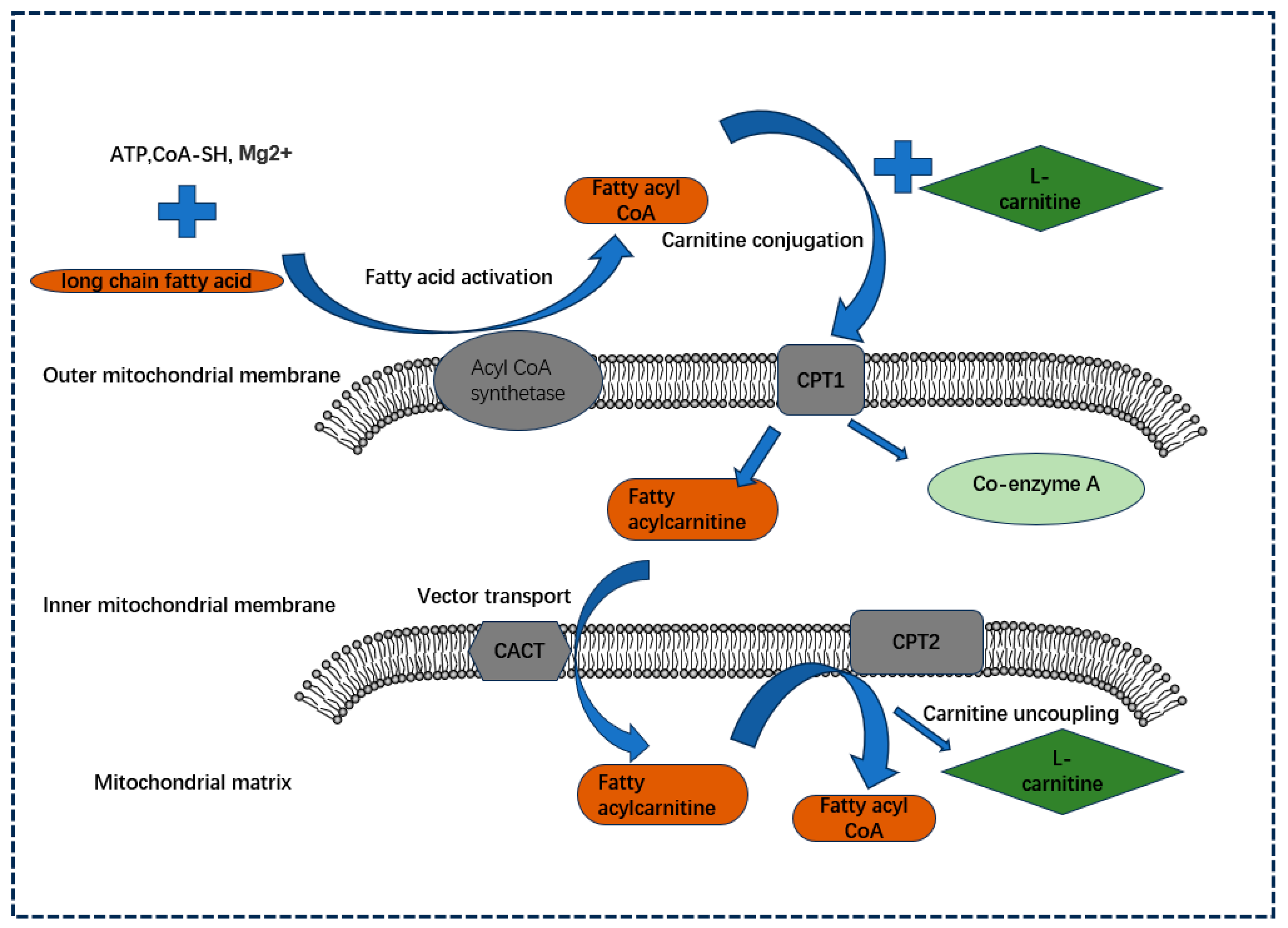
2. Molecular Structural Features of the CPT Subfamily
2.1. CPT1A
2.2. CPT1B
2.3. CPT1C
2.4. CPT2
| Type | CPT1A | CPT1B | CPT1C | CPT2 |
|---|---|---|---|---|
| Alternative name | CPT1-L, CPT1 liver subtype | CPT1-M, CPT1 muscle subtype | CPT1-B, CPT1 brain subtype | none |
| Localization of chromosomes | 11q1p32 | 22q13.3 | 19q13.33 | lp32 |
| Number of amino acids | 773 | 772 | 803 | 658 |
| Distribution of major organizations | Liver, kidneys, lungs, brain, intestines, lungs, ovaries, pancreas, spleen, etc. [31,32]. | Heart, skeletal muscle, adipocytes, and white adipocytes, among others [36]. | Brain, testicles, etc. [43]. | Heart, liver, skeletal muscle, etc. [23]. |
| Functional positioning | Mitochondrial outer membrane | Mitochondrial outer membrane | Endoplasmic reticulum [41]. | Inner mitochondrial membrane |
| Sensitivity to malonyl coenzyme A | Generally sensitive and regulated by insulin and thyroid hormones [21]. | Highly sensitive, about 100 times more sensitive than CPT1A [35]. | Capable of binding, but not enzymatically active in mitochondria [38]. | Very low sensitivity [21]. |
| Basic structural characteristics of proteins | A short N-terminal regulatory domain and a long C-terminal catalytic domain, two transmembrane fragments TM1 and TM2, and an intermembrane binding region connecting the two transmembrane fragments [55]. | Mitochondrial lead peptides, postulated membrane interaction regions, NT and CT domains [56]. | ||
| Key structural characteristics | TM1 and TM2 are responsible for anchoring the outer membrane, and the N-terminal domain can be transformed between Nα (M-CoA-sensitive) and Nβ (M-CoA-sensitive) conformations, while CPT1C is always in the Nα conformation [57]. | The TM region is absent and is anchored on the inner membrane by assuming the membrane [55] interaction zone. The mitochondrial leader peptide is cleaved before entering the inner membrane [58]. | ||
| Structural similarity | Nucleotide sequence and amino acid sequence similarity to rats were 82% and 88%, respectively [26]. | Nucleotide sequence and amino acid sequence similarity to rats were 84.6% and 85.9%, respectively [25]. | The amino acid sequence was 83.5%, similar to that of mouse [40]. | Nucleotide sequence and amino acid sequence similarity to the rat were 85% and 82% respectively [52]. |
| Sequence homology | CPT1A and CPT1B share 62% amino acid homology, CPT1A shares 54.5% amino acid homology with CPT1C, and CPT1B shares 52.7% amino acid homology with CPT1C [40]. | |||
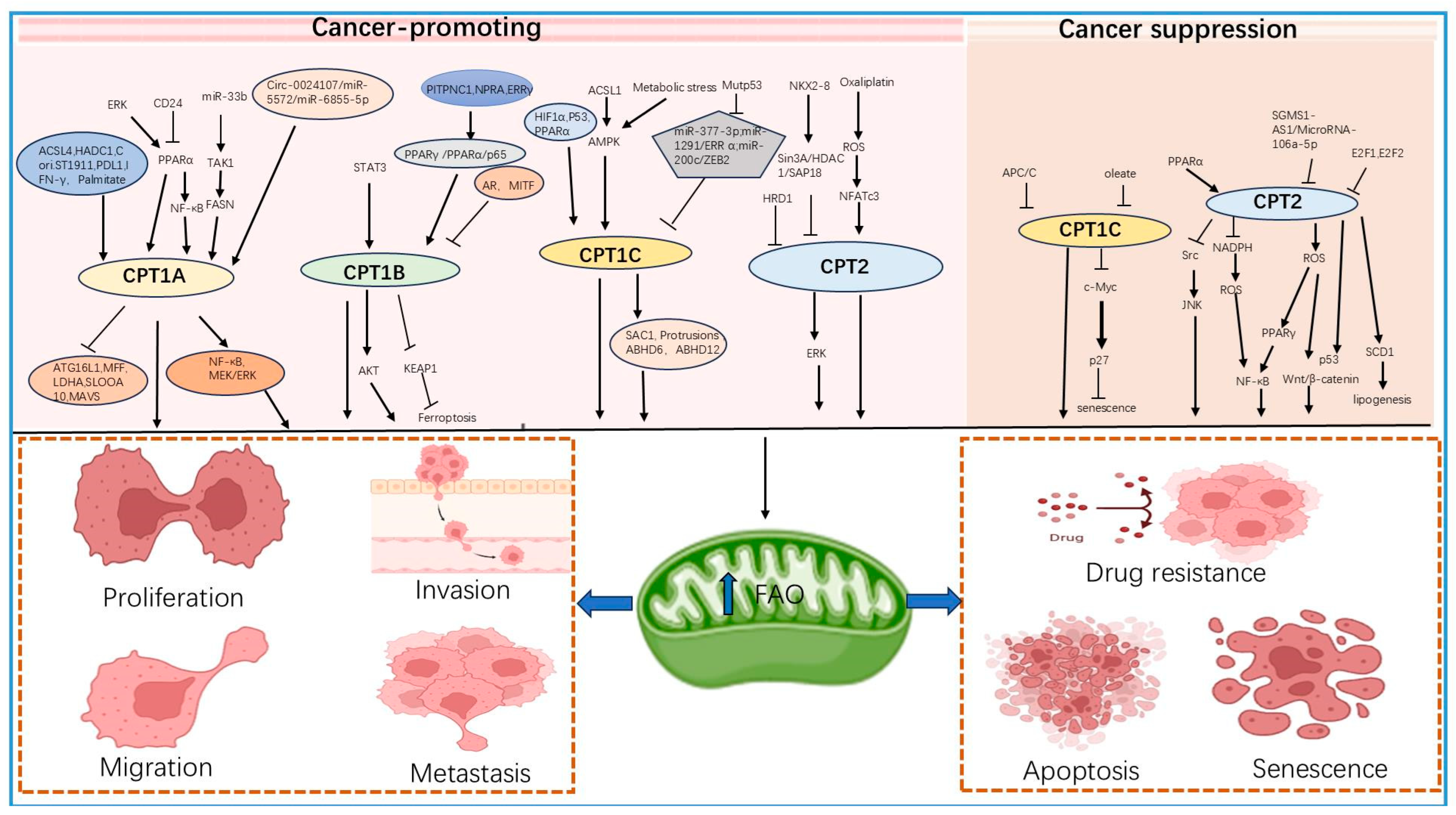
3. Role of the CPT Subfamily in Tumors
3.1. Role of the CPT1 Subfamily in Tumors
3.1.1. CPT1A and Tumors
3.1.2. CPT1B and Tumors
3.1.3. CPT1C and Tumors
3.2. Dual Role of CPT2 in Tumors
3.2.1. Carcinogenesis
3.2.2. Cancer Inhibition
| CPT Members | Functionality | Type of Cancer |
|---|---|---|
| CPT1A | cancer-promoting | Breast cancer [62,65,73]; Colorectal cancer [68,136]; Ovarian cancer [71,74]; Squamous cell carcinoma of the hypopharynx [72]; Gastric cancer [78,79,81]; lung cancer [84]. |
| cancer prevention | Leukemia [87,88,89]. | |
| CPT1B | cancer-promoting | Breast cancer [91,93]; Gastric cancer [90,94]; Prostate cancer [92]; Adenocarcinoma of the lungs [95,99]. |
| cancer prevention | Bladder cancer [96]; leukemia [88,97]. | |
| CPT1C | cancer-promoting | Colorectal cancer [104]; Gastric cancer [102,106,118]; Endometrial carcinoma [105]; Thyroid carcinoma [107]; Hepatocellular carcinoma [103]; Esophageal squamous cell carcinoma [20]; pancreatic [110,116]; Breast cancer [103,108,109,114]. |
| CPT2 | cancer-promoting | Leukemia [10]; Ovarian cancer [11]; Breast cancer [122,123]; Gastric cancer [94]. |
| cancer prevention | Ovarian cancer r [126]; Colorectal cancer [128,129,130]; Clear cell renal carcinoma [127]; Hepatocellular carcinoma [131,132,134]. |
4. Progress of Research on CPTs in Targeted Therapy
4.1. Targeted Inhibitors of CPTs
4.1.1. Etomoxir
4.1.2. ST1326 ([R]-N-[Tetradecylcarbamoyl]-aminocarnitine)
4.1.3. Perhexiline
4.1.4. Amiodarone
4.1.5. Other Drugs
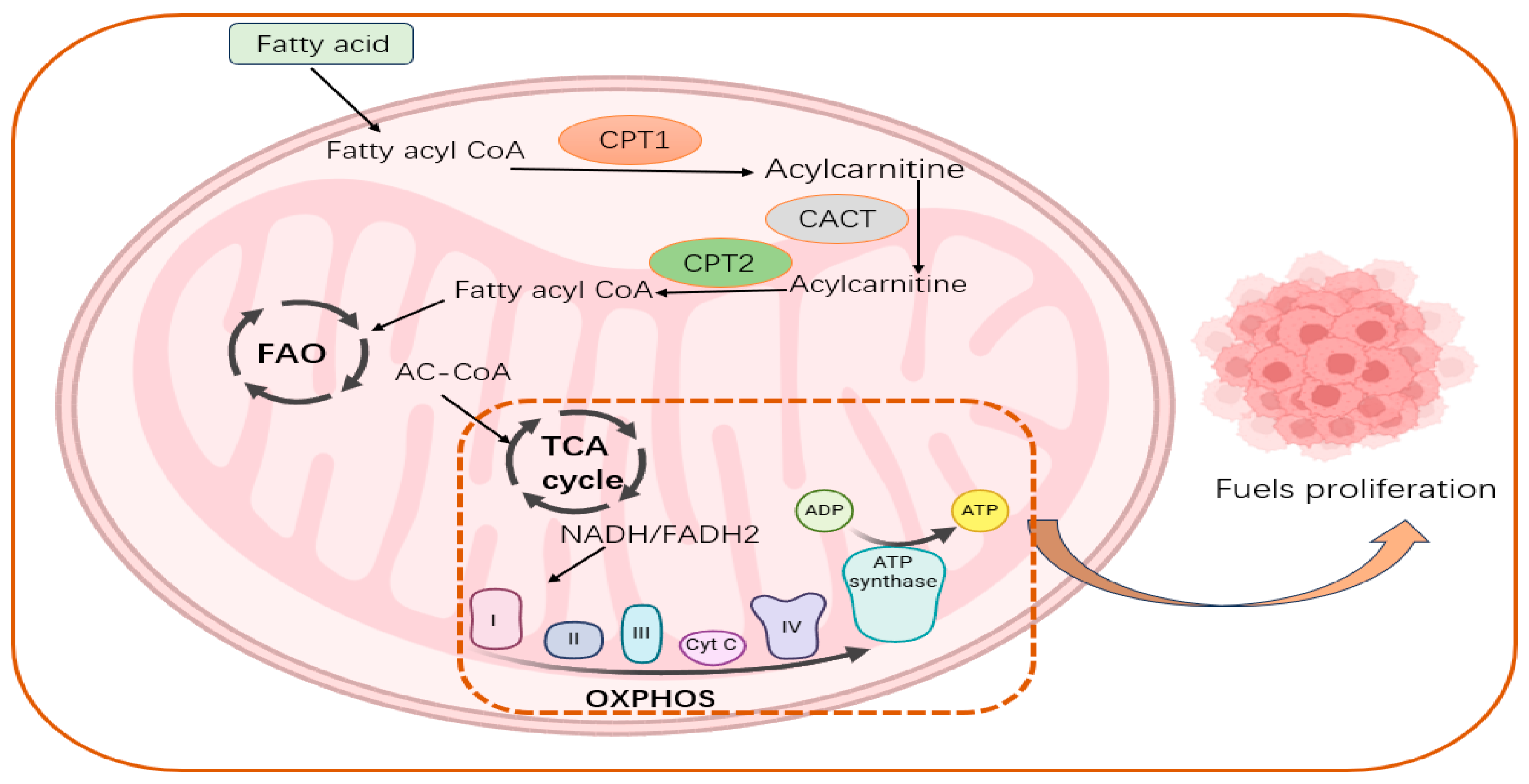
5. Potential Importance of CPTs in FAO, Reprogramming of Tumor Energy Metabolism
5.1. CPTs Can Promote FAO and Activate Oxidative Phosphorylation
5.2. CPTs Are Important Regulators of Energy Metabolic Reprogramming
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Gao, P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 2022, 13, 877–919. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Nguyen, N.T.; Bhutia, Y.D.; Sivaprakasam, S.; Ganapathy, V. Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth. Cancers 2024, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef]
- Hofmanová, J.; Slavík, J.; Ciganek, M.; Ovesná, P.; Tylichová, Z.; Karasová, M.; Zapletal, O.; Straková, N.; Procházková, J.; Bouchal, J.; et al. Complex Alterations of Fatty Acid Metabolism and Phospholipidome Uncovered in Isolated Colon Cancer Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 6650. [Google Scholar] [CrossRef]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1438–1450. [Google Scholar] [CrossRef]
- Deep, G.; Schlaepfer, I.R. Aberrant Lipid Metabolism Promotes Prostate Cancer: Role in Cell Survival under Hypoxia and Extracellular Vesicles Biogenesis. Int. J. Mol. Sci. 2016, 17, 1061. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Rider, L.; Rodrigues, L.U.; Gijón, M.A.; Pac, C.T.; Romero, L.; Cimic, A.; Sirintrapun, S.J.; Glodé, L.M.; Eckel, R.H.; et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol. Cancer Ther. 2014, 13, 2361–2371. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, J.; Jiang, W.Q.; Carew, J.S.; Ogasawara, M.A.; Pelicano, H.; Croce, C.M.; Estrov, Z.; Xu, R.H.; Keating, M.J.; et al. Elimination of chronic lymphocytic leukemia cells in stromal microenvironment by targeting CPT with an antiangina drug perhexiline. Oncogene 2016, 35, 5663–5673. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, G.; Song, L.; Cao, L.; Tan, Z.; Tang, M.; Li, Z.; Shi, D.; Zhang, S.; Li, J. NKX2-8 deletion-induced reprogramming of fatty acid metabolism confers chemoresistance in epithelial ovarian cancer. eBioMedicine 2019, 43, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Abe, M.; Yang, Y.; Cui, D.; Seki, T.; Nakamura, M.; Hosaka, K.; Lim, S.; Wu, J.; He, X.; et al. Cancer Lipid Metabolism Confers Antiangiogenic Drug Resistance. Cell Metab. 2018, 28, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sun, X.; Dong, H.; Wang, M.; Yao, L.; Wang, M.; Xu, L.; Xu, Y. ACSL3 regulates breast cancer progression via lipid metabolism reprogramming and the YES1/YAP axis. Cancer Biol. Med. 2024, 21, 606–635. [Google Scholar] [CrossRef]
- Kemp, F.; Braverman, E.L.; Byersdorfer, C.A. Fatty acid oxidation in immune function. Front. Immunol. 2024, 15, 1420336. [Google Scholar] [CrossRef]
- Gobin, S.; Thuillier, L.; Jogl, G.; Faye, A.; Tong, L.; Chi, M.; Bonnefont, J.-P.; Girard, J.; Prip-Buus, C. Functional and structural basis of carnitine palmitoyltransferase 1A deficiency. J. Biol. Chem. 2003, 278, 50428–50434. [Google Scholar] [CrossRef]
- Patel, B.V.; Yao, F.; Howenstine, A.; Takenaka, R.; Hyatt, J.A.; Sears, K.E.; Shewchuk, B.M. Emergent Coordination of the CHKB and CPT1B Genes in Eutherian Mammals: Implications for the Origin of Brown Adipose Tissue. J. Mol. Biol. 2020, 432, 6127–6145. [Google Scholar] [CrossRef]
- Ceccarelli, S.M.; Chomienne, O.; Gubler, M.; Arduini, A. Carnitine palmitoyltransferase (CPT) modulators: A medicinal chemistry perspective on 35 years of research. J. Med. Chem. 2011, 54, 3109–3152. [Google Scholar] [CrossRef]
- Violante, S.; Ijlst, L.; van Lenthe, H.; de Almeida, I.T.; Wanders, R.J.; Ventura, F.V. Carnitine palmitoyltransferase 2: New insights on the substrate specificity and implications for acylcarnitine profiling. Biochim. Biophys. Acta 2010, 1802, 728–732. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, X.; Yan, L.; Mi, F.; Wang, W.; Hu, Y.; Liu, X.; Fan, Y.; Min, Q.; Wang, Y.; et al. APC/C-regulated CPT1C promotes tumor progression by upregulating the energy supply and accelerating the G1/S transition. Cell Commun. Signal 2024, 22, 283. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont, J.-P.; Djouadi, F.; Prip-Buus, C.; Gobin, S.; Munnich, A.; Bastin, J. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol. Aspects Med. 2004, 25, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Woeltje, K.F.; Esser, V.; Weis, B.C.; Cox, W.F.; Schroeder, J.G.; Liao, S.T.; Foster, D.W.; McGarry, J.D. Inter-tissue and inter-species characteristics of the mitochondrial carnitine palmitoyltransferase enzyme system. J. Biol. Chem. 1990, 265, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Esser, V.; Britton, C.H.; Weis, B.C.; Foster, D.W.; McGarry, J.D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J. Biol. Chem. 1993, 268, 5817–5822. [Google Scholar] [CrossRef] [PubMed]
- Britton, C.H.; Mackey, D.W.; Esser, V.; Foster, D.W.; Burns, D.K.; Yarnall, D.P.; Froguel, P.; McGarry, J.D. Fine chromosome mapping of the genes for human liver and muscle carnitine palmitoyltransferase I (CPT1A and CPT1B). Genomics 1997, 40, 209–211. [Google Scholar] [CrossRef]
- Britton, C.H.; Schultz, R.A.; Zhang, B.; Esser, V.; Foster, D.W.; McGarry, J.D. Human liver mitochondrial carnitine palmitoyltransferase I: Characterization of its cDNA and chromosomal localization and partial analysis of the gene. Proc. Natl. Acad. Sci. USA 1995, 92, 1984–1988. [Google Scholar] [CrossRef]
- Samanta, S.; Situ, A.J.; Ulmer, T.S. Structural characterization of the regulatory domain of brain carnitine palmitoyltransferase 1. Biopolymers 2014, 101, 398–405. [Google Scholar] [CrossRef]
- Jackson, V.N.; Zammit, V.A.; Price, N.T. Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within the amino termini of rat liver- and muscle-type carnitine palmitoyltransferase I. J. Biol. Chem. 2000, 275, 38410–38416. [Google Scholar] [CrossRef]
- López-Viñas, E.; Bentebibel, A.; Gurunathan, C.; Morillas, M.; de Arriaga, D.; Serra, D.; Asins, G.; Hegardt, F.G.; Gómez-Puertas, P. Definition by functional and structural analysis of two malonyl-CoA sites in carnitine palmitoyltransferase 1A. J. Biol. Chem. 2007, 282, 18212–18224. [Google Scholar] [CrossRef]
- Faye, A.; Esnous, C.; Price, N.T.; Onfray, M.A.; Girard, J.; Prip-Buus, C. Rat liver carnitine palmitoyltransferase 1 forms an oligomeric complex within the outer mitochondrial membrane. J. Biol. Chem. 2007, 282, 26908–26916. [Google Scholar] [CrossRef]
- Weber, M.; Mera, P.; Casas, J.; Salvador, J.; Rodríguez, A.; Alonso, S.; Sebastián, D.; Soler-Vázquez, M.C.; Montironi, C.; Recalde, S.; et al. Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD. FASEB J. 2020, 34, 11816–11837. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wen, Y.-A.; Fairchild, R.; Zaytseva, Y.Y.; Weiss, H.L.; Evers, B.M.; Gao, T. Upregulation of CPT1A is essential for the tumor-promoting effect of adipocytes in colon cancer. Cell Death Dis. 2020, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- van der Leij, F.R.; Huijkman, N.C.; Boomsma, C.; Kuipers, J.R.; Bartelds, B. Genomics of the human carnitine acyltransferase genes. Mol. Genet. Metab. 2000, 71, 139–153. [Google Scholar] [CrossRef] [PubMed]
- van der Leij, F.R.; Takens, J.; van der Veen, A.Y.; Terpstra, P.; Kuipers, J.R. Localization and intron usage analysis of the human CPT1B gene for muscle type carnitine palmitoyltransferase I. Biochim. Biophys. Acta 1997, 1352, 123–128. [Google Scholar] [CrossRef]
- He, L.; Kim, T.; Long, Q.; Liu, J.; Wang, P.; Zhou, Y.; Ding, Y.; Prasain, J.; Wood, P.A.; Yang, Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation 2012, 126, 1705–1716. [Google Scholar] [CrossRef]
- Lee, J.; Ellis, J.M.; Wolfgang, M.J. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 2015, 10, 266–279. [Google Scholar] [CrossRef]
- Schreurs, M.; Kuipers, F.; van der Leij, F.R. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef]
- Wolfgang, M.J.; Kurama, T.; Dai, Y.; Suwa, A.; Asaumi, M.; Matsumoto, S.-I.; Cha, S.H.; Shimokawa, T.; Lane, M.D. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc. Natl. Acad. Sci. USA 2006, 103, 7282–7287. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Fosch, A.; Garcia-Chica, J.; Zagmutt, S.; Casals, N. Targeting carnitine palmitoyltransferase 1 isoforms in the hypothalamus: A promising strategy to regulate energy balance. J. Neuroendocrinol. 2023, 35, e13234. [Google Scholar] [CrossRef]
- Price, N.; van der Leij, F.; Jackson, V.; Corstorphine, C.; Thomson, R.; Sorensen, A.; Zammit, V. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics 2002, 80, 433–442. [Google Scholar] [CrossRef]
- Wolfgang, M.J.; Cha, S.H.; Millington, D.S.; Cline, G.; Shulman, G.I.; Suwa, A.; Asaumi, M.; Kurama, T.; Shimokawa, T.; Lane, M.D. Brain-specific carnitine palmitoyl-transferase-1c: Role in CNS fatty acid metabolism, food intake, and body weight. J. Neurochem. 2008, 105, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Lohse, I.; Reilly, P.; Zaugg, K. The CPT1C 5′UTR contains a repressing upstream open reading frame that is regulated by cellular energy availability and AMPK. PLoS ONE 2011, 6, e21486. [Google Scholar] [CrossRef]
- Wolfgang, M.J.; Lane, M.D. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS J. 2011, 278, 552–558. [Google Scholar] [CrossRef]
- Roa-Mansergas, X.; Fadó, R.; Atari, M.; Mir, J.F.; Muley, H.; Serra, D.; Casals, N. CPT1C promotes human mesenchymal stem cells survival under glucose deprivation through the modulation of autophagy. Sci. Rep. 2018, 8, 6997. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.D.; Wolfgang, M.; Cha, S.H.; Dai, Y. Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA. Int. J. Obes. 2008, 32 (Suppl. S4), S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.Y.; Gratacós, E.; Carrasco, P.; Clotet, J.; Ureña, J.; Serra, D.; Asins, G.; Hegardt, F.G.; Casals, N. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J. Biol. Chem. 2008, 283, 6878–6885. [Google Scholar] [CrossRef]
- Carrasco, P.; Sahún, I.; McDonald, J.; Ramírez, S.; Jacas, J.; Gratacós, E.; Sierra, A.Y.; Serra, D.; Herrero, L.; Acker-Palmer, A.; et al. Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J. Biol. Chem. 2012, 287, 21224–21232. [Google Scholar] [CrossRef]
- Ghadiminejad, I.; Saggerson, D. Cholate separates the catalytic and malonyl-CoA-binding components of carnitine palmitoyltransferase from liver outer mitochondrial membranes. Biochim. Biophys. Acta 1991, 1083, 166–172. [Google Scholar] [CrossRef]
- Gellera, C.; Verderio, E.; Floridia, G.; Finocchiaro, G.; Montermini, L.; Cavadini, P.; Zuffardi, O.; Taroni, F. Assignment of the human carnitine palmitoyltransferase II gene (CPT1) to chromosome 1p32. Genomics 1994, 24, 195–197. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Taroni, F.; Rocchi, M.; Martin, A.L.; Colombo, I.; Tarelli, G.T.; DiDonato, S. cDNA cloning, sequence analysis, and chromosomal localization of the gene for human carnitine palmitoyltransferase. Proc. Natl. Acad. Sci. USA 1991, 88, 661–665. [Google Scholar] [CrossRef]
- Rufer, A.C.; Thoma, R.; Hennig, M. Structural insight into function and regulation of carnitine palmitoyltransferase. Cell. Mol. Life Sci. 2009, 66, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont, J.P.; Demaugre, F.; Prip-Buus, C.; Saudubray, J.M.; Brivet, M.; Abadi, N.; Thuillier, L. Carnitine palmitoyltransferase deficiencies. Mol. Genet. Metab. 1999, 68, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Kohl, C.; McGarry, J.D.; Girard, J.; Prip-Buus, C. The N-terminal domain of rat liver carnitine palmitoyltransferase 1 mediates import into the outer mitochondrial membrane and is essential for activity and malonyl-CoA sensitivity. J. Biol. Chem. 1998, 273, 29896–29904. [Google Scholar] [CrossRef] [PubMed]
- Ghadiminejad, I.; Saggerson, E.D. Carnitine palmitoyltransferase (CPT2) from liver mitochondrial inner membrane becomes inhibitable by malonyl-CoA if reconstituted with outer membrane malonyl-CoA binding protein. FEBS Lett. 1990, 269, 406–408. [Google Scholar] [CrossRef]
- Liang, K. Mitochondrial CPT1A: Insights into structure, function, and basis for drug development. Front. Pharmacol. 2023, 14, 1160440. [Google Scholar] [CrossRef]
- Akieda, K.; Takegawa, K.; Ito, T.; Nagayama, G.; Yamazaki, N.; Nagasaki, Y.; Nishino, K.; Kosako, H.; Shinohara, Y. Unique Behavior of Bacterially Expressed Rat Carnitine Palmitoyltransferase 2 and Its Catalytic Activity. Biol. Pharm. Bull. 2024, 47, 23–27. [Google Scholar] [CrossRef]
- Rao, J.N.; Warren, G.Z.L.; Estolt-Povedano, S.; Zammit, V.A.; Ulmer, T.S. An environment-dependent structural switch underlies the regulation of carnitine palmitoyltransferase 1A. J. Biol. Chem. 2011, 286, 42545–42554. [Google Scholar] [CrossRef]
- Borthwick, K.; Jackson, V.N.; Price, N.T.; Zammit, V.A. The mitochondrial intermembrane loop region of rat carnitine palmitoyltransferase 1A is a major determinant of its malonyl-CoA sensitivity. J. Biol. Chem. 2006, 281, 32946–32952. [Google Scholar] [CrossRef]
- Tan, Z.; Zou, Y.; Zhu, M.; Luo, Z.; Wu, T.; Zheng, C.; Xie, A.; Wang, H.; Fang, S.; Liu, S.; et al. Carnitine palmitoyl transferase 1A is a novel diagnostic and predictive biomarker for breast cancer. BMC Cancer 2021, 21, 409. [Google Scholar] [CrossRef]
- Tian, T.; Lu, Y.; Lin, J.; Chen, M.; Qiu, H.; Zhu, W.; Sun, H.; Huang, J.; Yang, H.; Deng, W. CPT1A promotes anoikis resistance in esophageal squamous cell carcinoma via redox homeostasis. Redox Biol. 2022, 58, 102544. [Google Scholar] [CrossRef]
- Su, W.; Xu, F.; Zhong, J.; Hu, R.; Wang, L.; Li, H.; Yang, Z.; Ge, S.; He, H.; Han, S.; et al. Screening of CPT1A-Targeting Lipid Metabolism Modulators Using Mitochondrial Membrane Chromatography. ACS Appl. Mater. Interfaces 2024, 16, 13234–13246. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, P.; Liu, W.; Liu, G.; Zhang, J.; Yan, M.; Duan, Y.; Yang, N. A positive feedback loop between ZEB2 and ACSL4 regulates lipid metabolism to promote breast cancer metastasis. eLife 2023, 12, RP87510. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Sivridis, E.; Fiska, A.; Koukourakis, M.I. The CD44+/CD24− phenotype relates to ‘triple-negative’ state and unfavorable prognosis in breast cancer patients. Med. Oncol. 2011, 28, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Camarda, R.; Zhou, A.Y.; Kohnz, R.A.; Balakrishnan, S.; Mahieu, C.; Anderton, B.; Eyob, H.; Kajimura, S.; Tward, A.; Krings, G.; et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016, 22, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Murthy, D.; Dutta, D.; Attri, K.S.; Samanta, T.; Yang, S.; Jung, K.H.; Latario, S.G.; Putluri, V.; Huang, S.; Putluri, N.; et al. CD24 negativity reprograms mitochondrial metabolism to PPARα and NF-κB-driven fatty acid β-oxidation in triple-negative breast cancer. Cancer Lett. 2024, 587, 216724. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Zeng, Z.-L.; Lu, J.; Wang, Y.; Liu, Z.-X.; He, M.-M.; Zhao, Q.; Wang, Z.-X.; Li, T.; Lu, Y.-X.; et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 2018, 37, 6025–6040. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, F.; Lin, X.; Li, Q.; Lu, Y.; Zhang, J.; Shen, X.; Tan, J.; Qin, Z.; Chen, J.; et al. Nuclear VCP drives colorectal cancer progression by promoting fatty acid oxidation. Proc. Natl. Acad. Sci. USA 2023, 120, e2221653120. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, H.; Xu, H.; Xia, H.; Zhang, C.; Ye, D.; Bi, F. Endogenous Coriobacteriaceae enriched by a high-fat diet promotes colorectal tumorigenesis through the CPT1A-ERK axis. NPJ Biofilms Microbiomes 2024, 10, 5. [Google Scholar] [CrossRef]
- Sawyer, B.T.; Qamar, L.; Yamamoto, T.M.; McMellen, A.; Watson, Z.L.; Richer, J.K.; Behbakht, K.; Schlaepfer, I.R.; Bitler, B.G. Targeting Fatty Acid Oxidation to Promote Anoikis and Inhibit Ovarian Cancer Progression. Mol. Cancer Res. 2020, 18, 1088–1098. [Google Scholar] [CrossRef]
- Huang, D.; Chowdhury, S.; Wang, H.; Savage, S.R.; Ivey, R.G.; Kennedy, J.J.; Whiteaker, J.R.; Lin, C.; Hou, X.; Oberg, A.L.; et al. Multiomic analysis identifies CPT1A as a potential therapeutic target in platinum-refractory, high-grade serous ovarian cancer. Cell Rep. Med. 2021, 2, 100471. [Google Scholar] [CrossRef]
- Wang, X.; Yung, M.M.H.; Sharma, R.; Chen, F.; Poon, Y.-T.; Lam, W.-Y.; Li, B.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. Epigenetic Silencing of miR-33b Promotes Peritoneal Metastases of Ovarian Cancer by Modulating the TAK1/FASN/CPT1A/NF-κB Axis. Cancers 2021, 13, 4795. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Chen, L.; Gao, Z.; Xu, S.; Hu, C.; Fan, G.; Wang, B.; Feng, T.; Wang, W.; et al. CPT1A mediates chemoresistance in human hypopharyngeal squamous cell carcinoma via ATG16L1-dependent cellular autophagy. Cell Insight 2023, 2, 100127. [Google Scholar] [CrossRef] [PubMed]
- Winkelkotte, A.M.; Schulze, A. Palmitate paves the way to lung metastasis. Trends Cancer 2023, 9, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Li, Y.; Li, Z.; Kong, W.; Zhao, X.; Chen, S.; Yan, L.; Wang, L.; Tong, Y.; et al. Carnitine palmitoyltransferase 1A promotes mitochondrial fission by enhancing MFF succinylation in ovarian cancer. Commun. Biol. 2023, 6, 618. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Song, Y.; Yan, Z. Inhibition of carnitine palmitoyl transferase 1A-induced fatty acid oxidation suppresses cell progression in gastric cancer. Arch. Biochem. Biophys. 2020, 696, 108664. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, X.; Shen, J.; Xu, Y.; Shi, H.; Mu, X.; Pan, J.; Zhao, T.; Li, M.; et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J. Cell. Mol. Med. 2019, 23, 293–305. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhao, T.; Su, Z.; Li, M.; Hu, J.; Wen, J.; Shen, J.; Wang, C.; Pan, J.; et al. Lysine-222 succinylation reduces lysosomal degradation of lactate dehydrogenase a and is increased in gastric cancer. J. Exp. Clin. Cancer Res. 2020, 39, 172. [Google Scholar] [CrossRef]
- Wang, M.; Yu, W.; Cao, X.; Gu, H.; Huang, J.; Wu, C.; Wang, L.; Sha, X.; Shen, B.; Wang, T.; et al. Exosomal CD44 Transmits Lymph Node Metastatic Capacity Between Gastric Cancer Cells via YAP-CPT1A-Mediated FAO Reprogramming. Front. Oncol. 2022, 12, 860175. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wen, J.; Zhao, X.; Wu, C.; Wang, L.; Cao, X.; Dong, H.; Xu, X.; Huang, F.; et al. Gastric cancer cell-originated small extracellular vesicle induces metabolic reprogramming of BM-MSCs through ERK-PPARγ-CPT1A signaling to potentiate lymphatic metastasis. Cancer Cell Int. 2023, 23, 87. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Mou, K.; Peng, L.; Zhou, H.; Lei, Y.; Wang, H.; Zhao, Z.; Wang, J.; Wu, J.; et al. SDPR Inhibits TGF-β Induced Cancer Metastasis Through Fatty Acid Oxidation Regulation in Gastric Cancer. Int. J. Biol. Sci. 2023, 19, 2999–3014. [Google Scholar] [CrossRef]
- Wang, L.; Wu, C.; Xu, J.; Gong, Z.; Cao, X.; Huang, J.; Dong, H.; Zhu, W.; Huang, F.; Zhou, C.; et al. GC-MSC-derived circ_0024107 promotes gastric cancer cell lymphatic metastasis via fatty acid oxidation metabolic reprogramming mediated by the miR-5572/6855-5p/CPT1A axis. Oncol. Rep. 2023, 50, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-G.; Yang, J.; Zhu, Y.; Zhu, Q.; Pan, W.; Deng, S.; He, Y.; Zuo, D.; Wang, P.; Han, Y.; et al. The microprotein encoded by exosomal lncAKR1C2 promotes gastric cancer lymph node metastasis by regulating fatty acid metabolism. Cell Death Dis. 2023, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Wu, S.-Y.; Sharma, S.; Wu, K.; Zhao, D.; Deshpande, R.; Singh, R.; Li, W.; Topaloglu, U.; Ruiz, J.; et al. Exosomal miR-4466 from nicotine-activated neutrophils promotes tumor cell stemness and metabolism in lung cancer metastasis. Oncogene 2022, 41, 3079–3092. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, W.; Wang, W.; Ma, Y.; Wang, Y.; Drum, D.L.; Cai, J.; Blevins, H.; Lee, E.; Shah, S.; et al. CPT1A-mediated fatty acid oxidation confers cancer cell resistance to immune-mediated cytolytic killing. Proc. Natl. Acad. Sci. USA 2023, 120, e2302878120. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, P.; Tang, W.; Wang, Y.; Qiu, F.; An, J.; Zheng, Y.; Wu, D.; Zhou, J.; Neculai, D.; et al. CPT1A induction following epigenetic perturbation promotes MAVS palmitoylation and activation to potentiate antitumor immunity. Mol. Cell 2023, 83, 4370–4385. [Google Scholar] [CrossRef]
- Shi, J.; Fu, H.; Jia, Z.; He, K.; Fu, L.; Wang, W. High Expression of CPT1A Predicts Adverse Outcomes: A Potential Therapeutic Target for Acute Myeloid Leukemia. eBioMedicine 2016, 14, 55–64. [Google Scholar] [CrossRef]
- Karlic, H.; Louda, N.; Pfeilstöcker, M.; Keil, F.; Lohninger, A.; Pittermann, E.; Paukovits, J. Effect of the hemoregulatory peptide (pEEDCK)2 (pyroGlu-Glu-Asp-Cys-Lys)2 and MIP-1alpha is reduced in bone marrow cultures from patients with chronic myeloid leukemia (CML). Stem Cells 2001, 19, 321–328. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Rahbar Saadat, Y.; Ozkan, T.; Bozkurt, S.; Karadag Gurel, A. Identification of common genes and pathways underlying imatinib and nilotinib treatment in CML: A Bioinformatics Study. Nucleosides Nucleotides Nucleic Acids 2024, 43, 664–684. [Google Scholar] [CrossRef]
- Tan, Y.; Lin, K.; Zhao, Y.; Wu, Q.; Chen, D.; Wang, J.; Liang, Y.; Li, J.; Hu, J.; Wang, H.; et al. Adipocytes fuel gastric cancer omental metastasis via PITPNC1-mediated fatty acid metabolic reprogramming. Theranostics 2018, 8, 5452–5468. [Google Scholar] [CrossRef]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.-J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, M.; Zhu, W.; Wang, Y.; Wang, J.; Xu, W.; Huang, Y.; Zhu, Y.; Shi, G.; Zhang, H.; Zhu, Y.; et al. Targeting CPT1B as a potential therapeutic strategy in castration-resistant and enzalutamide-resistant prostate cancer. Prostate 2020, 80, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, L.; Zhou, J.; Lin, X.; Peng, Y.; Ge, L.; Chiang, C.-M.; Huang, H.; Wang, H.; He, W. N6-methyladenosine-induced ERRγ triggers chemoresistance of cancer cells through upregulation of ABCB1 and metabolic reprogramming. Theranostics 2020, 10, 3382–3396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.-H.; Wang, F.; Wang, Y.-N.; He, M.-M.; Wu, Q.-N.; Lu, Y.-X.; Yu, H.-E.; Chen, Z.-H.; Zhao, Q.; et al. Inhibition of fatty acid catabolism augments the efficacy of oxaliplatin-based chemotherapy in gastrointestinal cancers. Cancer Lett. 2020, 473, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tang, H.; Xu, S.; Yu, H.; Chen, Z. Transcription Factor MITF Inhibits the Transcription of CPT1B to Regulate Fatty Acid β-Oxidation and Thus Affects Stemness in Lung Adenocarcinoma Cells. Pharmacology 2024, 109, 52–64. [Google Scholar] [CrossRef]
- Vantaku, V.; Dong, J.; Ambati, C.R.; Perera, D.; Donepudi, S.R.; Amara, C.S.; Putluri, V.; Ravi, S.S.; Robertson, M.J.; Piyarathna, D.W.B.; et al. Multi-omics Integration Analysis Robustly Predicts High-Grade Patient Survival and Identifies CPT1B Effect on Fatty Acid Metabolism in Bladder Cancer. Clin. Cancer Res. 2019, 25, 3689–3701. [Google Scholar] [CrossRef]
- Ling, Q.; Mao, S.; Pan, J.; Wei, W.; Qian, Y.; Li, F.; Huang, S.; Ye, W.; Lin, X.; Huang, J.; et al. CPT1B, a metabolic molecule, is also an independent risk factor in CN-AML. Cancer Biomark. 2023, 37, 133–145. [Google Scholar] [CrossRef]
- Cao, T.; Wang, S.; Qian, L.; Wu, C.; Huang, T.; Wang, Y.; Li, Q.; Wang, J.; Xia, Y.; Xu, L.; et al. NPRA promotes fatty acid metabolism and proliferation of gastric cancer cells by binding to PPARα. Transl. Oncol. 2023, 35, 101734. [Google Scholar] [CrossRef]
- Tuerhong, A.; Xu, J.; Wang, W.; Shi, S.; Meng, Q.; Hua, J.; Liu, J.; Zhang, B.; Yu, X.; Liang, C. CPT1B maintains redox homeostasis and inhibits ferroptosis to induce gemcitabine resistance via the KEAP1/NRF2 axis in pancreatic cancer. Surgery 2024, 175, 1264–1275. [Google Scholar] [CrossRef]
- Reilly, P.T.; Mak, T.W. Molecular pathways: Tumor cells Co-opt the brain-specific metabolism gene CPT1C to promote survival. Clin. Cancer Res. 2012, 18, 5850–5855. [Google Scholar] [CrossRef]
- Zaugg, K.; Yao, Y.; Reilly, P.T.; Kannan, K.; Kiarash, R.; Mason, J.; Huang, P.; Sawyer, S.K.; Fuerth, B.; Faubert, B.; et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes. Dev. 2011, 25, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Song, J.; Zhang, Y.; Zhu, Z. CPT1C promotes the potential of gastric cancer ovarian metastasis through up-regulating fatty acid oxidation. Acta Biochim Biophys Sin 2022, 54, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Liu, J.; Ma, Y.; Ye, Q.; Yan, X.; Ding, L. MicroRNA-377-3p inhibits hepatocellular carcinoma growth and metastasis through negative regulation of CPT1C-mediated fatty acid oxidation. Cancer Metab. 2022, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, W.; Wu, J.; Zhang, J.; Lv, B.; Li, W.; Liu, J.; Zhang, X.; Huang, T.; Luo, Z. CPT1C-mediated fatty acid oxidation facilitates colorectal cancer cell proliferation and metastasis. Acta Biochim. Biophys. Sin. 2023, 55, 1301–1309. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Chen, G.; Guo, X.; Gao, X.; Meng, J.; Xu, Y.; Zhou, N.; Zhang, B.; Zhou, X. ACSL1-Mediated Fatty Acid β-Oxidation Enhances Metastasis and Proliferation in Endometrial Cancer. Front. Biosci. (Landmark Ed.) 2024, 29, 66. [Google Scholar] [CrossRef]
- Chen, T.; Wu, G.; Hu, H.; Wu, C. Enhanced fatty acid oxidation mediated by CPT1C promotes gastric cancer progression. J. Gastrointest. Oncol. 2020, 11, 695–707. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, Y.; Su, D.; Gong, B.; He, X.; Zhou, X.; Pang, Z.; Cheng, L.; Chen, Y.; Yao, Z. Cpt1c regulated by AMPK promotes papillary thyroid carcinomas cells survival under metabolic stress conditions. J. Cancer 2017, 8, 3675–3681. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Han, F.; Zhao, Y.; Tu, M.; Wang, Y.; Huang, C.; Fan, S.; Chen, P.; Yao, X.; et al. A novel miR-1291-ERRα-CPT1C axis modulates tumor cell proliferation, metabolism and tumorigenesis. Theranostics 2020, 10, 7193–7210. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, C.-H.; Mai, R.-T.; Chen, T.-W.; Li, C.-W.; Chao, C.-H. Mutant p53-microRNA-200c-ZEB2-Axis-Induced CPT1C Elevation Contributes to Metabolic Reprogramming and Tumor Progression in Basal-Like Breast Cancers. Front. Oncol. 2022, 12, 940402. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Guan, L.; Zhang, H.; Huang, Y.; Johnson, C.H.; Wu, Z.; Gonzalez, F.J.; Yu, A.; Huang, P.; et al. Carnitine palmitoyltransferase 1C regulates cancer cell senescence through mitochondria-associated metabolic reprograming. Cell Death Differ. 2018, 25, 735–748. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, T.; Zhou, Y.; Wang, S.; Zhou, X.; Wang, L.; Ou, T.; Chen, Y.; Zhou, Y.; Zhang, H.; et al. Carnitine palmitoyltransferase 1C contributes to progressive cellular senescence. Aging 2020, 12, 6733–6755. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Macedo, N.; Feng, J.; Faubert, B.; Chang, N.; Elia, A.; Rushing, E.J.; Tsuchihara, K.; Bungard, D.; Berger, S.L.; Jones, R.G.; et al. Depletion of the novel p53-target gene carnitine palmitoyltransferase 1C delays tumor growth in the neurofibromatosis type I tumor model. Cell Death Differ. 2013, 20, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Mascaró, C.; Acosta, E.; Ortiz, J.A.; Marrero, P.F.; Hegardt, F.G.; Haro, D. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 1998, 273, 8560–8563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Huang, Y.; Zeng, H.; Hu, B.; Guan, L.; Zhang, H.; Yu, A.-M.; Johnson, C.H.; Gonzalez, F.J.; et al. PPARα regulates tumor cell proliferation and senescence via a novel target gene carnitine palmitoyltransferase 1C. Carcinogenesis 2017, 38, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Chen, Y.; Wang, Y.; Zhang, H.; Fan, S.; Gao, Y.; Jiao, T.; Fu, K.; Sun, J.; Yu, A.; et al. Effects of carnitine palmitoyltransferases on cancer cellular senescence. J. Cell. Physiol. 2019, 234, 1707–1719. [Google Scholar] [CrossRef]
- Chen, P.; Tian, J.; Zhou, Y.; Chen, Y.; Zhang, H.; Jiao, T.; Huang, M.; Zhang, H.; Huang, P.; Yu, A.-M.; et al. Metabolic Flux Analysis Reveals the Roles of Stearate and Oleate on CPT1C-mediated Tumor Cell Senescence. Int. J. Biol. Sci. 2023, 19, 2067–2080. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Guan, L.; Chen, Y.; Chen, P.; Sun, J.; Gonzalez, F.J.; Huang, M.; Bi, H. Lipidomics reveals carnitine palmitoyltransferase 1C protects cancer cells from lipotoxicity and senescence. J. Pharm. Anal. 2021, 11, 340–350. [Google Scholar] [CrossRef]
- Wei, R.; Song, J.; Pan, H.; Liu, X.; Gao, J. CPT1C-positive cancer-associated fibroblast facilitates immunosuppression through promoting IL-6-induced M2-like phenotype of macrophage. Oncoimmunology 2024, 13, 2352179. [Google Scholar] [CrossRef]
- Fadó, R.; Zagmutt, S.; Herrero, L.; Muley, H.; Rodríguez-Rodríguez, R.; Bi, H.; Serra, D.; Casals, N. To be or not to be a fat burner, that is the question for cpt1c in cancer cells. Cell Death Dis. 2023, 14, 57. [Google Scholar] [CrossRef]
- Cirillo, A.; Di Salle, A.; Petillo, O.; Melone, M.A.B.; Grimaldi, G.; Bellotti, A.; Torelli, G.; De’ Santi, M.S.; Cantatore, G.; Marinelli, A.; et al. High grade glioblastoma is associated with aberrant expression of ZFP57, a protein involved in gene imprinting, and of CPT1A and CPT1C that regulate fatty acid metabolism. Cancer Biol. Ther. 2014, 15, 735–741. [Google Scholar] [CrossRef]
- Casals, N.; Zammit, V.; Herrero, L.; Fadó, R.; Rodríguez-Rodríguez, R.; Serra, D. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog. Lipid Res. 2016, 61, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wei, R.; Zhang, X.; Jiang, N.; Fan, M.; Huang, J.H.; Xie, B.; Zhang, L.; Miao, W.; Butler, A.C.-P.; et al. CPT1A/2-Mediated FAO Enhancement-A Metabolic Target in Radioresistant Breast Cancer. Front. Oncol. 2019, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.M.D.; Adamoski, D.; Ornitz Oliveira Souza, R.; Rodrigues Ascenção, C.F.; Sousa de Oliveira, K.R.; Corrêa-da-Silva, F.; Malta de Sá Patroni, F.; Meira Dias, M.; Consonni, S.R.; Mendes de Moraes-Vieira, P.M.; et al. Dual inhibition of glutaminase and carnitine palmitoyltransferase decreases growth and migration of glutaminase inhibition-resistant triple-negative breast cancer cells. J. Biol. Chem. 2019, 294, 9342–9357. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, A.; Wang, W.; Wang, Y.; Chen, H.; Liu, X.; Xia, T.; Zhang, A.; Chen, D.; Qi, H.; et al. HRD1 inhibits fatty acid oxidation and tumorigenesis by ubiquitinating CPT2 in triple-negative breast cancer. Mol. Oncol. 2021, 15, 642–656. [Google Scholar] [CrossRef]
- Lin, M.; Lv, D.; Zheng, Y.; Wu, M.; Xu, C.; Zhang, Q.; Wu, L. Downregulation of CPT2 promotes tumorigenesis and chemoresistance to cisplatin in hepatocellular carcinoma. Onco Targets Ther. 2018, 11, 3101–3110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Liu, S.; Li, J.; Wu, L.; Lv, X.; Xu, J.; Chen, B.; Zhao, S.; Yang, H. CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFκB pathway in ovarian cancer. Transl. Oncol. 2021, 14, 101023. [Google Scholar] [CrossRef]
- Zeng, K.; Li, Q.; Song, G.; Chen, B.; Luo, M.; Miao, J.; Liu, B. CPT2-mediated fatty acid oxidation inhibits tumorigenesis and enhances sorafenib sensitivity via the ROS/PPARγ/NF-κB pathway in clear cell renal cell carcinoma. Cell Signal 2023, 110, 110838. [Google Scholar] [CrossRef]
- Ruochen, Y.; Wenbin, J.; Chao, G.; Yuhua, Y.; Feng, Q. SGMS1-AS1/MicroRNA-106a-5p/CPT2 Axis as a Novel Target for Regulating Lactate Metabolism in Colon Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231212071. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Liu, J.; Lai, Y.; Huang, S.; Zheng, L.; Fan, N. CPT2 downregulation triggers stemness and oxaliplatin resistance in colorectal cancer via activating the ROS/Wnt/β-catenin-induced glycolytic metabolism. Exp. Cell Res. 2021, 409, 112892. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Yan, H.; Wu, J.; Yang, Y.; He, J.; Chen, J.; Jiang, Z.; Wu, F.; Jiang, Z. Downregulation of CPT2 promotes proliferation and inhibits apoptosis through p53 pathway in colorectal cancer. Cell Signal 2022, 92, 110267. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Enooku, K.; Kudo, Y.; Hayata, Y.; Nakatsuka, T.; Tanaka, Y.; Tateishi, R.; Hikiba, Y.; Misumi, K.; et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut 2018, 67, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- González-Romero, F.; Mestre, D.; Aurrekoetxea, I.; O’Rourke, C.J.; Andersen, J.B.; Woodhoo, A.; Tamayo-Caro, M.; Varela-Rey, M.; Palomo-Irigoyen, M.; Gómez-Santos, B.; et al. E2F1 and E2F2-Mediated Repression of CPT2 Establishes a Lipid-Rich Tumor-Promoting Environment. Cancer Res. 2021, 81, 2874–2887. [Google Scholar] [CrossRef] [PubMed]
- Deepa, P.R.; Vandhana, S.; Muthukumaran, S.; Umashankar, V.; Jayanthi, U.; Krishnakumar, S. Chemical inhibition of fatty acid synthase: Molecular docking analysis and biochemical validation in ocular cancer cells. J. Ocul. Biol. Dis. Inform. 2010, 3, 117–128. [Google Scholar] [CrossRef] [PubMed]
- You, B.-J.; Chen, L.-Y.; Hsu, P.-H.; Sung, P.-H.; Hung, Y.-C.; Lee, H.-Z. Orlistat Displays Antitumor Activity and Enhances the Efficacy of Paclitaxel in Human Hepatoma Hep3B Cells. Chem. Res. Toxicol. 2019, 32, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, X.; Zhang, Y.; Zhang, K.; Zhan, C.; Shi, X.; Li, Y.; Zhao, J.; Bai, Y.; Wang, Y.; et al. Metabolic profiling analysis upon acylcarnitines in tissues of hepatocellular carcinoma revealed the inhibited carnitine shuttle system caused by the downregulated carnitine palmitoyltransferase 2. Mol. Carcinog. 2019, 58, 749–759. [Google Scholar] [CrossRef]
- Hu, A.; Wang, H.; Xu, Q.; Pan, Y.; Jiang, Z.; Li, S.; Qu, Y.; Hu, Y.; Wu, H.; Wang, X. A novel CPT1A covalent inhibitor modulates fatty acid oxidation and CPT1A-VDAC1 axis with therapeutic potential for colorectal cancer. Redox Biol. 2023, 68, 102959. [Google Scholar] [CrossRef]
- Hegardt, F.G.; Serra, D.; Asins, G. Influence of etomoxir on the expression of several genes in liver, testis and heart. Gen. Pharmacol. 1995, 26, 897–904. [Google Scholar] [CrossRef]
- Rupp, H.; Maisch, B. Functional genomics of pressure-loaded cardiomyocytes: Etomoxir in heart failure? Herz 2002, 27, 166–173. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Wall, S.R.; Olley, P.M.; Davies, N.J. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ. Res. 1988, 63, 1036–1043. [Google Scholar] [CrossRef]
- Kruszynska, Y.T.; Sherratt, H.S. Glucose kinetics during acute and chronic treatment of rats with 2[6(4-chloro-phenoxy)hexyl]oxirane-2-carboxylate, etomoxir. Biochem. Pharmacol. 1987, 36, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Szczepaniak, L.S.; Bentley, B.; Esser, V.; Myhill, J.; McGarry, J.D. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 2001, 50, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Giannessi, F.; Pessotto, P.; Tassoni, E.; Chiodi, P.; Conti, R.; De Angelis, F.; Dell’Uomo, N.; Catini, R.; Deias, R.; Tinti, M.O.; et al. Discovery of a long-chain carbamoyl aminocarnitine derivative, a reversible carnitine palmitoyltransferase inhibitor with antiketotic and antidiabetic activity. J. Med. Chem. 2003, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Rufer, A.C.; Thoma, R.; Benz, J.; Stihle, M.; Gsell, B.; De Roo, E.; Banner, D.W.; Mueller, F.; Chomienne, O.; Hennig, M. The crystal structure of carnitine palmitoyltransferase 2 and implications for diabetes treatment. Structure 2006, 14, 713–723. [Google Scholar] [CrossRef]
- Pacilli, A.; Calienni, M.; Margarucci, S.; D’Apolito, M.; Petillo, O.; Rocchi, L.; Pasquinelli, G.; Nicolai, R.; Koverech, A.; Calvani, M.; et al. Carnitine-acyltransferase system inhibition, cancer cell death, and prevention of myc-induced lymphomagenesis. J. Natl. Cancer Inst. 2013, 105, 489–498. [Google Scholar] [CrossRef]
- Conti, R.; Mannucci, E.; Pessotto, P.; Tassoni, E.; Carminati, P.; Giannessi, F.; Arduini, A. Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes 2011, 60, 644–651. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foà, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the leukemia cell metabolism by the CPT1a inhibition: Functional preclinical effects in leukemias. Blood 2015, 126, 1925–1929. [Google Scholar] [CrossRef]
- Grønningsæter, I.S.; Reikvam, H.; Aasebø, E.; Bartaula-Brevik, S.; Tvedt, T.H.; Bruserud, Ø.; Hatfield, K.J. Targeting Cellular Metabolism in Acute Myeloid Leukemia and The Role of Patient Heterogeneity. Cells 2020, 9, 1155. [Google Scholar] [CrossRef]
- Mao, S.; Ling, Q.; Pan, J.; Li, F.; Huang, S.; Ye, W.; Wei, W.; Lin, X.; Qian, Y.; Wang, Y.; et al. Inhibition of CPT1a as a prognostic marker can synergistically enhance the antileukemic activity of ABT199. J. Transl. Med. 2021, 19, 181. [Google Scholar] [CrossRef]
- Dhakal, B.; Tomita, Y.; Drew, P.; Price, T.; Maddern, G.; Smith, E.; Fenix, K. Perhexiline: Old Drug, New Tricks? A Summary of Its Anti-Cancer Effects. Molecules 2023, 28, 3624. [Google Scholar] [CrossRef]
- Burns-Cox, C.J.; Chandrasekhar, K.P.; Ikram, H.; Peirce, T.H.; Pilcher, J.; Quinlan, C.D.; Rees, J.R. Clinical evaluation of perhexiline maleate in patients with angina pectoris. Br. Med. J. 1971, 4, 586–588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ananthakrishna, R.; Lee, S.L.; Foote, J.; Sallustio, B.C.; Binda, G.; Mangoni, A.A.; Woodman, R.; Semsarian, C.; Horowitz, J.D.; Selvanayagam, J.B. Randomized controlled trial of perhexiline on regression of left ventricular hypertrophy in patients with symptomatic hypertrophic cardiomyopathy (RESOLVE-HCM trial). Am. Heart J. 2021, 240, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Unger, S.A.; Horowitz, J.D. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem. Pharmacol. 1996, 52, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, E.; Seki, S.; Itagaki, S.; Matsuura, M.; Kobayashi, M.; Hirano, T.; Goto, Y.; Tadano, K.; Iseki, K. Efflux transport of N-monodesethylamiodarone by the human intestinal cell-line Caco-2 cells. Drug Metab. Pharmacokinet. 2007, 22, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kang, Y.J.; Yoon, M.J.; Kim, E.H.; Kim, S.U.; Kwon, T.K.; Kim, I.A.; Choi, K.S. Amiodarone sensitizes human glioma cells but not astrocytes to TRAIL-induced apoptosis via CHOP-mediated DR5 upregulation. Neuro Oncol. 2011, 13, 267–279. [Google Scholar] [CrossRef]
- Yan, J.; Xu, Y.; Zhu, Q. Case Report: Amiodarone-induced multi-organ toxicity. Front. Cardiovasc. Med. 2024, 11, 1401049. [Google Scholar] [CrossRef]
- Karkhanis, A.; Leow, J.W.H.; Hagen, T.; Chan, E.C.Y. Dronedarone-Induced Cardiac Mitochondrial Dysfunction and Its Mitigation by Epoxyeicosatrienoic Acids. Toxicol. Sci. 2018, 163, 79–91. [Google Scholar] [CrossRef]
- Steinberg, E.; Fluksman, A.; Zemmour, C.; Tischenko, K.; Karsch-Bluman, A.; Brill-Karniely, Y.; Birsner, A.E.; D’Amato, R.J.; Benny, O. Low dose amiodarone reduces tumor growth and angiogenesis. Sci. Rep. 2020, 10, 18034. [Google Scholar] [CrossRef]
- Ramadan, F.H.J.; Szabo, A.; Kovacs, D.; Takatsy, A.; Bognar, R.; Gallyas, F.; Bognar, Z. Involvement of Mitochondrial Mechanisms in the Cytostatic Effect of Desethylamiodarone in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 7436. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Horowitz, J.D. Effect of trimetazidine on carnitine palmitoyltransferase-1 in the rat heart. Cardiovasc. Drugs Ther. 1998, 12, 359–363. [Google Scholar] [CrossRef]
- Hamdan, M.; Urien, S.; Le Louet, H.; Tillement, J.P.; Morin, D. Inhibition of mitochondrial carnitine palmitoyltransferase-1 by a trimetazidine derivative, S-15176. Pharmacol. Res. 2001, 44, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G. Pharmacology at work for cardio-oncology: Ranolazine to treat early cardiotoxicity induced by antitumor drugs. J. Pharmacol. Exp. Ther. 2013, 346, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-S.; Lo, Y.-C.; Peng, H.; Hsu, T.-I.; Wu, S.-N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH(3) cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, B.R.; Skettino, S.L.; Parker, J.O.; Hanley, P.; Meluzin, J.; Kuch, J.; Pepine, C.J.; Wang, W.; Nelson, J.J.; Hebert, D.A.; et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J. Am. Coll. Cardiol. 2004, 43, 1375–1382. [Google Scholar] [CrossRef]
- Uslu, C.; Kapan, E.; Lyakhovich, A. Cancer resistance and metastasis are maintained through oxidative phosphorylation. Cancer Lett. 2024, 587, 216705. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Petricca, S.; Iorio, R.; Toniato, E.; Flati, V. Mitochondrial and metabolic alterations in cancer cells. Eur. J. Cell Biol. 2022, 101, 151225. [Google Scholar] [CrossRef]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.-Y.; Fang, X. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Zhang, X.; Ma, B.; Shi, Y.; Yin, Y.; Kong, W.; Zhang, W.; Li, J.; Yang, H. MARCH5-mediated downregulation of ACC2 promotes fatty acid oxidation and tumor progression in ovarian cancer. Free Radic. Biol. Med. 2024, 212, 464–476. [Google Scholar] [CrossRef]
- Ahn, S.; Park, J.H.; Grimm, S.L.; Piyarathna, D.W.B.; Samanta, T.; Putluri, V.; Mezquita, D.; Fuqua, S.A.W.; Putluri, N.; Coarfa, C.; et al. Metabolomic Rewiring Promotes Endocrine Therapy Resistance in Breast Cancer. Cancer Res. 2024, 84, 291–304. [Google Scholar] [CrossRef]
- Wu, H.; Huang, H.; Zhao, Y. Interplay between metabolic reprogramming and post-translational modifications: From glycolysis to lactylation. Front. Immunol. 2023, 14, 1211221. [Google Scholar] [CrossRef]
- Farahzadi, R.; Hejazi, M.S.; Molavi, O.; Pishgahzadeh, E.; Montazersaheb, S.; Jafari, S. Clinical Significance of Carnitine in the Treatment of Cancer: From Traffic to the Regulation. Oxidative. Med. Cell Longev. 2023, 2023, 9328344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; An, Y.; Ren, M.; Wang, H.; Bai, J.; Du, W.; Kong, D. The mechanisms of action of mitochondrial targeting agents in cancer: Inhibiting oxidative phosphorylation and inducing apoptosis. Front. Pharmacol. 2023, 14, 1243613. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, F.; Shi, P.; Song, A.; Huang, Z.; Zou, D.; Chen, Q.; Li, J.; Gao, X. Fatty acid oxidation promotes reprogramming by enhancing oxidative phosphorylation and inhibiting protein kinase C. Stem Cell Res. Ther. 2018, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Hernández-Esquivel, L.; Marín-Hernández, A.; El Hafidi, M.; Gallardo-Pérez, J.C.; Hernández-Reséndiz, I.; Rodríguez-Zavala, J.S.; Pacheco-Velázquez, S.C.; Moreno-Sánchez, R. Mitochondrial free fatty acid β-oxidation supports oxidative phosphorylation and proliferation in cancer cells. Int. J. Biochem. Cell Biol. 2015, 65, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J.A.; Ranea-Robles, P. Metabolic interactions between peroxisomes and mitochondria with a special focus on acylcarnitine metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165720. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, J.; Zhou, Q.; He, X.; Zheng, Z.; Wei, Y.; Zhou, K.; Lin, Y.; Yu, H.; Zhang, H.; et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef]
| Inhibitor | Target | Side Effects |
|---|---|---|
| Etomoxir | CPT1A, CPT1B [138]. | Hepatotoxicity, cardiac hypertrophy, etc. [139]. |
| ST1326 (teglicar) | CPT1A, CPT1B [143]; CACT [145]; CPT2 [56]. | Only transient toxic effects in the liver [145]. |
| Perhexiline | CPT1 [152]; CPT2 [150]. | Serious side effects such as hepatotoxicity and neurotoxicity [150]. |
| Amiodarone | CPT1 [157]. | Serious adverse reactions such as thyroid dysfunction, hepatic injury, and pulmonary toxicity [156]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Liu, J.; Li, A.; Liu, C.; Shu, G.; Yin, G. The Role of the CPT Family in Cancer: Searching for New Therapeutic Strategies. Biology 2024, 13, 892. https://doi.org/10.3390/biology13110892
Duan Y, Liu J, Li A, Liu C, Shu G, Yin G. The Role of the CPT Family in Cancer: Searching for New Therapeutic Strategies. Biology. 2024; 13(11):892. https://doi.org/10.3390/biology13110892
Chicago/Turabian StyleDuan, Yanxia, Jiaxin Liu, Ailin Li, Chang Liu, Guang Shu, and Gang Yin. 2024. "The Role of the CPT Family in Cancer: Searching for New Therapeutic Strategies" Biology 13, no. 11: 892. https://doi.org/10.3390/biology13110892
APA StyleDuan, Y., Liu, J., Li, A., Liu, C., Shu, G., & Yin, G. (2024). The Role of the CPT Family in Cancer: Searching for New Therapeutic Strategies. Biology, 13(11), 892. https://doi.org/10.3390/biology13110892






