Simple Summary
Although Lyme Borreliosis is endemic in countries of the Northern Hemisphere, climate change, migratory birds, and the availability of ticks have favored the survival of Borreliae Lyme Group in African countries bordering the Mediterranean Sea and the Indian Ocean.
Abstract
Lyme borreliosis (LB) is more common in the Northern Hemisphere. It is endemic mainly in North America, where the vectors are Ixodes scapularis and Ixodes pacificus, and in Eurasia, where the vectors are Ixodes ricinus and Ixodes persulcatus. Both tick-borne diseases and LB are influenced by climate change. Africa and South America are crossed by the equator and are situated in both the Northern and Southern Hemispheres. In Africa, the LB is present on the Mediterranean and the Indian Ocean coasts. Borrelia lusitaniae is prevalent in countries bordering the Mediterranean Sea, such as Tunisia, Morocco, Algeria, and Egypt. Ticks were detected in the Ixodes Ricinus, which are carried by migratory birds and the Ixodes inopinatus and captured by the Psammodromus algirus lizards. The Borreliae Lyme Group (LG) and, in particular, Borrelia garinii, have been reported in countries bordering the Indian Ocean, such as Kenya, Tanzania, and Mozambique, transported by migratory birds from North African countries, where the vector was identified as Hyalomma rufipes ticks. This review aims to document the presence of Borreliae LG and LB in Africa.
1. Introduction
The Borrelia genus is divided into three groups, namely the Lyme Group (LG), the Echidna-Reptile Group (REPG), and the Relapsing Fever Group (RFG) [1].
Lyme borreliosis (LB) is a form of anthropozoonosis caused by the infection of Borrelia burgdorferi sensu lato (Bbsl) belonging to LG, usually transmitted by a hard tick of the genus Ixodes. Clinically, the first manifestation (present in 75% of cases) is an erythema that spreads around the tick bite (erythema migrans). Other clinical manifestations are migrating arthralgias, neurological manifestations, skin manifestations (with acrodermatitis chronica atrophicans observed in the late stage), and cardiac manifestations, in particular arrhythmia and pericarditis [2].
Currently, 24 species are included in Borreliae LG; to the 23 already reported [1], the most recent addition is Borrelia maritima, which was isolated in California [3]. Nine Borreliae LG are pathogenetic to humans. Of these, B. afzelii is mainly associated with skin manifestations, while B. garinii and B. bavariensis are associated with neuroborreliosis [4]. Lyme arthritis is preferentially related to B. burgdorferi sensu strictu (Bbls). [5]. Also, B. spielmanii, B. valaisiana, B. bissettii, B. lusitaniae, and B. mayonii are pathogenic to humans. Of these, B. mayonii causes spirochetemia [6]. B. lusitaniae was isolated in Portugal from human acrodermatitis chronica atrophicans lesions (strain PoHL1), and from I. ricinus ticks [7], and it has also been identified in lizards. The host-specific infection of these spirochaetes supports that they are specialized in the enzootic cycle, and through the migratory birds, they have crossed the Mediterranean Sea arriving in North Africa [8]. Vectors of Borreliae LG are usually hard ticks of the genus Ixodes, which prefer a cool–humid microclimate in spring and autumn, showing greater activity when the humidity is around 90%. Tick mortality, host-seeking activity, and the height and duration of ticks’ quests above ground are significantly impacted by low humidity exposure [9]. Therefore, the rainy climates favor their diffusion and reproduction whilst Ixodes ticks assume an endophilic life in summer with high temperatures [10]. The distribution of tick-borne pathogens is primarily associated with the density of ticks and with reservoir animals. In Europe, competent host reservoirs of Borreliae LG include common species of small and medium-sized rodents (Apodemus flavicollis, Myodes glareolus), as well as several species of birds (in particular passerines, such as Turdus merula) [11].
Borreliae LG can also be transmitted by cofeeding, which occurs when Borrelia is transferred between infected and naive vectors that feed in close spatiotemporal proximity to a host that has not yet developed a systemic infection [12].
Lyme borreliosis (LB) is mainly present in the Northern Hemisphere, mostly in North America [13] and in Europe [14], with a distribution that is closely related to Ixodes species, notably Ixodes ricinus in Europe, I. persulcatus in Asia and Eastern Europe, and I. scapularis and I. pacificus in the US. In 2022, 62,551 cases of Lyme disease were reported to the US Centers for Disease Control and Prevention (CDC) [15], representing 1.7 times the annual average (37,118 cases) for the period 2017–2019 [16]. However, according to the health insurance claims database, the annual incidence of Lyme disease diagnoses per 100,000 enrollees ranged from 49 to 88 in the period ranging from 2010 to 2018 in the US, which is about 6–8 times higher than that observed for cases reported through notifiable disease surveillance [17], which was about 476,00 cases/year [18]. In Europe, the population-weighted incidence rate of LB was estimated to be 22.04/100,000 person-years, with 200,000 new cases of LB per year in Western Europe [19]. In Italy, LB is widespread throughout the territory, but it is endemic in the Northern Alpine regions [20].
The spread of LB is closely related to the distribution of hard ticks, mainly of the Ixodes genus. These ticks are highly plastic and can adapt even to suboptimal environmental conditions. They prefer a humid habitat, which allows them to spread and develop rapidly. Consequently, these ticks are mainly present in the Northern Hemisphere, decreasing approaching the equator and in the Southern Hemisphere [21].
Ixodes ricinus is present in Europe and North Africa. Its great adaptability increases the risk area for infection by Borreliae LG and other tick-borne pathogens. The sheep tick, I. ricinus, has a low specificity that enables it to infest various host species, such as birds and mammals (including humans) [22]. LB is less frequent in the Southern Hemisphere [23], where the vector ticks can be of the genus Ixodes but also of the Hyalomma, Amblyomma, and Riphicephalus genera [24].
Africa and South America, crossed by the equator line, are represented in both hemispheres and are in contiguity with Europe and North America, respectively, where LB has a high incidence. The increase in cases of Lyme disease has been associated with the increase in reservoirs and the long summer seasons caused by climate change. In addition, the warming of temperatures has caused an expansion of Ixodes spp. ticks [25].
This study aims to highlight the different clinical, epidemiological, and management peculiarities of LB in the African continent.
2. Africa
In Africa, hard ticks belonging to the genera Hyalomma, Ixodes, Amblyomma, Dermacentor, Haemaphysalis, and Rhipicephalus can parasitize livestock, camels, horses, giraffes, and domestic animals (dogs). Some of these ticks damage livestock by transmitting infections, which can cause anemia and abortions, leading to a consequent reduction in food products (milk and meat) and significant economic losses [26]. Tick-borne diseases affecting livestock are particularly relevant for the livelihood of resource-poor farm communities, such as in sub-Saharan Africa [27]. Ticks can damage livestock merely by injecting the neurotoxins contained in their saliva. This has been reported for Ixodes rubicundus, which is responsible for the Karoo paralysis in sheep, goats, and young calves in South Africa [28], and for Ixodes holocyclus in Australia [29]. Of note, Ixodes rubicundus can survive in mountain and desertic environments [28].
In Africa, ticks transmit several pathogens, including Borreliae, which most often belong to the Relapsing Fever Group (RFG), where the vector is often a soft tick, usually of the genus Ornithodoros spp. or similar. The RFG Borreliae are transmitted vertically into the ticks, which can act as a reservoirs (O. moubata) and contribute to the maintenance and spread of the infection [30]. In LG Borreliae, the vertical transmission is negligible, and ticks should be infected by a reservoir host (rodents or lizards) to transmit LB [31]. RFG Borreliae can also be transmitted by hard ticks (Hard-Tick-Borne Relapsing Fever—HTBRF). Both Borrelia miyamotoi and Borrelia theileri are present in Africa [32].
Borrelia miyamotoi is transmitted by Ixodes spp. ticks, the same as LB, and can infect humans [33,34] and wildlife [35]. Borrelia theileri, transmitted by Boophilus (Rhipicephalus) microplus [36], infects domestic animals and livestock [37].
The distribution of Bbsl in Africa is not fully known and is closely related to migratory birds, ticks of Ixodes and Hyalomma genera, lizards as possible reservoirs, and climate change.
The migratory birds, infested by Ixodes frontalis and Ixodes ricinus, have probably led to the settlement of Borrelia lusitaniae from Portugal to North Africa, notably to Tunisia, Morocco, and Algeria [38] (see Table 1). Ixodes ricinus and I. inopinatus have been detected and characterized in the humid area of Northern Tunisia [39]. I. Inopinatus, in addition, seems to extend also to the sub-humid area [39] and is usually infesting the lizards [40]. Lizards are one of the least studied taxa, and in Africa, they play an important role in the enzootic cycle of the Bbsl, acting as reservoirs and amplifiers of these spirochetes [41].

Table 1.
Lyme group Borreliae in Africa.
Both I. ricinus and I. inopinatus can transmit Borrelia burgdorferi s.l.; however, B. lusitaniae is prevalent in North Africa, with genotypes supporting a population division separating samples from southern Portugal and Algeria from samples from northern Portugal and other European countries [42].
The other tick species that have been recovered and identified infesting small ruminants and wild animals (giraffes and buffaloes) [43] throughout Africa belong to the genera Amblyomma, Rhipicephalus, and Hyalomma [24]. Of these, Hyalomma ticks, including also H. rufipes, which tested the highest positivity for tick-borne pathogens, have been collected from sheep, camels, horses, goats, and dogs in Sudan [44]. Hyalomma marginatum infests mainly sheep and goats in North Africa and Ivory Coast [45], while Hyalomma dromedarii, parasitizing mainly camels, sheep, and goats, is found in Algeria, Ethiopia, Nigeria, and Sudan [46].
The presence of Bbls has been documented in ticks collected in North African countries, along the Mediterranean Sea (Algeria, Morocco, Tunisia, and Egypt), and in the countries bordering the Indian Ocean (Tanzania, Kenya, and Mozambique) (Figure 1), but infection in humans appears currently negligible [47].
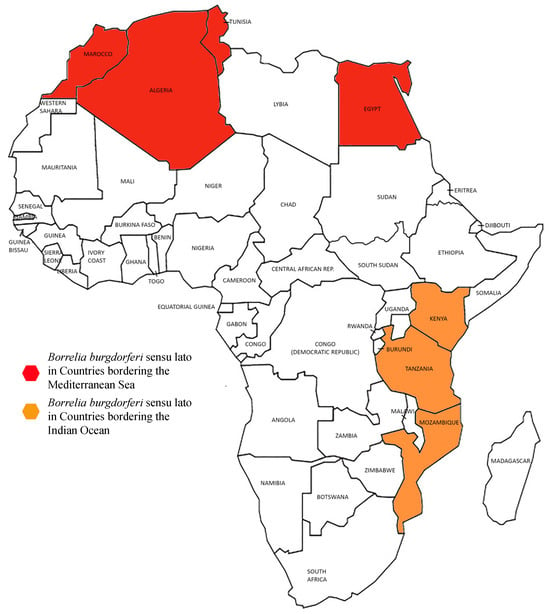
Figure 1.
Borreliae LG distribution in Africa.
The following paragraphs describe the documentation of LG Borreliae in detail for each of the abovementioned countries.
2.1. African Countries Along the Mediterranean Sea
In North African countries, a Mediterranean climate predominates in the coastal belt (Maghreb) from Morocco to Tunisia, with four seasons characterized by hot and dry summers and cool and wet winters, which is more suitable for the settlement of Ixodes spp. ticks and Borreliae LG. The climate becomes arid in the south (Sahara desert), extending from the Atlantic Ocean to the Red Sea. At the eastern edge of the Sahara flows the Nile River, which starts from the tributaries of Sudan Azzurro and Atbara and opens into the Mediterranean. In this area, the climate is tropical.
These climatic conditions and the unique range of geographic features support a wide diversity of tick species and tick-borne diseases in North Africa [48].
2.1.1. Algeria
Borrelia lusitaniae (strain ID 3117) has been documented in Ixodes inopinatus ticks in Kabylie [42]. LB was detected in two horses (Equus caballus) by indirect immunofluorescence antibody and ELISA tests [49].
2.1.2. Egypt
Egypt is positioned on a migratory route, the African–Eurasian way, for birds migrating between breeding sites in Eurasia and wintering sites in Africa, crossing the Mediterranean Sea. Migratory birds crossing Egypt are infested by several species and subspecies of ticks, including Ixodes spp. and Hyalomma spp.; this infection can occur during the migratory flight. Accordingly, Bbsl has been identified from Hyalomma dromedarii ticks infesting camels, and from Rhipicephalus annulatus from cattle [50]. Molecular analyses carried out in Hyalomma dromedarii and H. marginatus ticks and in the blood of infested camels in Cairo and Giza revealed Borrelia afzelii (LG) and Borrelia miyamotoi (RFG) [51]. Bbls were also detected in dogs, although at a relatively low rate both by molecular methods [52] and by serology [53].
Regarding human infection in Egypt, LB was documented in two farmers living in the Nile Delta [54]. Earlier, in 1995, four guys in El-Shatby reported skin lesions suggestive of erythema migrans and myo-arthralgia, and all of them tested positive for antibodies against Borrelia burgdorferi by ELISA [55].
2.1.3. Mali
LB has not been documented in Mali [56]; however, its geographical location, bordering Algeria, suggests that its presence cannot be ruled out.
2.1.4. Morocco
In 2002, Bbls was documented for the first time in Morocco with 26 pure culture isolates, of which 25 were B. lusitaniae and one was B. burdorferi ss. In ticks, B. garinii was also isolated [57]. Infection with LG Borreliae, confirmed by Western blot analysis and causing neuroborreliosis, was documented in a 23-year-old woman in Morocco [58].
2.1.5. Tunisia
Lyme borreliosis is quite well documented in Tunisia. From 1988 to 1992, 23 cases of erythema migrans were detected in Sfax [59], and from 1992 to 1996, 29 cases of Lyme disease were identified by ELISA, each involving neurological and articular manifestations [60]. Bbsl infection was also investigated in ruminants, notably goats, sheep, cattle, and camels. Animals were infested by ticks only of the Rhipicephalus and Hyalomma genera. The prevalence of B. burgdorferi s.l. in those animals varied significantly according to the humidity of the localities and revealed a significantly higher rate of infection in goats that were located in humid areas than those located in sub-humid areas [61].
In 2014, the Ixodes inopinatus tick was identified in North Africa [40]. This tick is the main vector of Borrelia lusitaniae and parasitizes mostly lizards (Psammodromus algirus) and foxes, but also occasionally humans and other animals, including horses [62]. Isolates of B. lusitaniae (15/16) and B. garinii (1/16) were obtained from ticks, namely Ixodes ricinus and I. inopinatus [63], sharing the same geographic areas in Northern Tunisia [64].
It is more likely that Psammodromus algirus lizards could act as reservoirs for B. lusitaniae; 17% of ticks feeding on P. algirus indeed acquired B. lusitaniae, demonstrating the ability of the lizards to sustain Borrelia infection [65]. Also, the sand lizard, Lacerta agilis, is thought to be a reservoir of B. lusitaniae. Collar scale samples of the lizards tested PCR-positive for B. lusitaniae, and, in addition, some I. ricinus collected from the lizards were PCR-positive for Bbls [66].
2.2. African Countries Bordering the Indian Ocean
African countries bordering the Indian Ocean have tropical climates, which can facilitate the survival and spread of Borreliae LG.
2.2.1. Kenya
Kenya has three different climates, with large regional climatic variations influenced by several factors, including altitude. There are two dry seasons: the first one runs from December to March and the second from July to October. The two rainy seasons come in between. The heaviest rains usually fall from mid-March to May.
Most studies carried out in Kenya have focused on the veterinary cases of tick-borne pathogens. Ticks collected from livestock from the major abattoirs in Nairobi and Mombasa revealed a 5% prevalence of infection from B. burgdorferi s.l. [67]. In addition, this study also showed that ticks of the genus Rhipicephalus and Amblyomma were the dominant carriers of zoonotic pathogens and that LG Borreliae were almost detected in Rhipicephalus pulchellus [67].
From 2009 to 2017, ticks were collected in various locations in Kenya from sympatric wild and domestic animals, and B. garinii DNA was detected in a H. Rufipes tick collected from a giraffe [68]. B. garinii is usually vectored by Ixodes ticks in Europe and Asia, but it has also been documented in North Africa, vectored by I. ricinus and I. inopinatus [40,47].
The picture of LB in human cases is incomplete in Kenya. In 2005, two men from the Rift Valley developed neuroborreliosis after a tick bite [69]. In a study investigating seroprevalence for different tick-borne pathogens in 1033 patients presenting with acute febrile illness in nine healthcare facilities from different ecological zones of Kenya, 21% tested positive for LB. In addition, exposure to LB was highest in the younger age group [70].
The presence of Borreliae LG in these countries is probably linked to the Europe–Africa–Europe two-way transport mechanism of the African ticks Hyalomma rufipes, which parasitizes migratory birds, such as Acrocephalus schoenobaenus, traveling from Africa to Southern Norway and Finland. This is not the first case where Ixodes spp., a vector of Borrelia LG, is replaced by Hyalomma spp.; previously, indeed, B. lusitaniae (strain PotiB1) was found in H. marginatum [71]. Long-distance exchange of tick-borne pathogens due to migratory birds is clearly the basis of the introduction of Bbls in Africa. It is possible that tick exchanges occur between birds migrating to Northern Europe and birds migrating to Africa [72]. This is also supported by the geographic distribution of the ticks: Hyalomma spp. is more represented in Africa, while Ixodes spp. is more represented in Europe.
2.2.2. Mozambique
Mozambique has a tropical to subtropical climate. Rainfall distribution follows a north–south gradient, with more rainfall along the coast. The rainy season lasts from January to March. The south of Mozambique is generally drier.
The first autochthonous case of LB was documented in Mozambique in 1993 [73]. Some years later, a cross-sectional serological survey was carried out in Maputo for LB and leptospirosis, which revealed only some cases of seropositivity for LB, which was more likely attributed to cross-reactivity. Since no molecular characterizations were carried out, LB has been considered an unlikely occurrence in this country [74].
2.2.3. Tanzania
Tanzania has a generally comfortable climate year-round, although there are significant regional variations. The tropical coast is quite hot and humid with heavy and reliable rainfall, especially during the rainy seasons, from mid-March to May and from November to mid-January.
Two studies in Tanzania investigated the presence of tick-borne pathogens in ticks that were collected from cattle and wild animals. In each tick, there was evidence of Borreliae LG [75,76].
Despite those findings, in Dar es Salaam, a pregnant woman developed erythema migrans after a tick bite. She also had arthralgia and tested positive for Borrelia on an ELISA test [77]. The same author reported other patients with clinical symptoms suggestive of LB and positive serology [77].
2.2.4. Other African Countries
There are no reports on LB in the following African countries: Angola, Benin, Botswana, Burkina-Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Congo Republic, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Ivory Coast, Lesotho Kingdom, Liberia, Libya, Madagascar, Malawi, Mauritania, Mauritius, Namibia, Niger, Nigeria, Rwanda, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, Sudan, and Togo.
3. Discussion
Tick-borne diseases are one of the risks associated with global warming and climate change. Many studies predicted and already noted the expansion of tick habitats, both northern and southern [78,79]. This can determine the diffusion of tick-borne pathogens in areas that were previously not endemic. Climate variables, such as temperature and humidity, influence ticks’ survival and define their habitat areas [80]. The presence and spread of Bbls in Africa are closely related to:
- migratory birds;
- climate change;
- Hyalomma spp. ticks becoming Bbsl vectors;
- reservoirs other than small rodents, such as lizards;
- infection in humans, domestic animals, livestock, and wildlife.
3.1. Migratory Birds
The presence of Borreliae LG in North African countries is due to migratory birds infested by Ixodes ricinus and I. frontalis, acting as vectors for Borreliae LG, most commonly Borrelia lusitaniae, which, according to phylogenetic analysis, most likely originated in Serbia and spread later throughout Europe and North Africa. Many bird species travel annually across the Mediterranean Sea from their European breeding sites to their wintering sites in Africa, and then back again. Ticks infesting these birds are often transported in a two-way flow from Europe to North Africa via the Mediterranean Sea [48]. The analyses of the routes confirmed that the birds fall under the families Sylviidae, Muscicapidae, Turdidae, and Acrocephaliidae [81]. This trans-Mediterranean flow of ticks and their pathogens creates a dual threat to health in both Europe and North Africa, enabling some of these species to settle in new areas [82]. Scenarios regarding the future spatial distribution of I. ricinus already show overlaps between the North African region and Europe [83].
3.2. Hyalomma spp. Ticks as Vectors of Bbls
In spring and autumn, migratory birds transport Ixodes ricinus with Bbsl from Europe to North Africa and Acrocephalus schoenobaenus birds transport Hyalomma rufipes from Africa to North European countries. The latter ticks can transport new infections in Europe, such as the Crimean-Congo hemorrhagic fever (CCHF), isolated from H. marginatum ticks carried by migratory birds Iduna opaca, Phylloscopus trochilus, and Sylvia communis from Morocco [79,84]. At the same time, those ticks can become infected by Bbsl, which they can serve as vectors for in African countries, as shown for di B. lusitaniae infecting H. marginatum and B. garinii infecting H. rufipes [8,71]. The mechanism allowing the transmission of Borreliae is the cofeeding of ticks on birds with ticks infected by Bbls [85]. Nevertheless, further observations are needed to confirm the vector competence of these ticks for Borreliae LG, focusing on the acquisition, maintenance, and subsequent transmission in a vertebrate host during the blood feeding [68].
3.3. Climate Change
One of the consequences of global climate change, affecting habitat suitability and tick demographics, is its effect on infectious diseases and human health [86]. Global surface temperature has increased significantly over the past 50 years. Future climate scenarios include increased frequency and intensity of heat extremes, heavy precipitation, and agricultural and ecological drought. Pathogens, vectors, hosts, and disease transmission may be sensitive to these changing conditions. The reproduction and extrinsic incubation period of pathogens within a vector are temperature-dependent, and higher temperatures accelerate pathogen maturation. Environmental temperature influences the spatial and temporal distribution of disease vectors that transport and transmit pathogens to humans. Disease transmission may, in turn, be influenced by meteorological factors and by changes in contact between humans, competent animals, vectors, and pathogens [21].
Tick-Borne Relapsing Fever (TBRF), which is more common in central and southern Africa [87], can be vectored by Ornithodors spp. ticks, which thrive in low-humidity environments and desert areas (Ornithodoros savignyi vectors Borrelia kalaharica) [88]. Ixodes ticks, which carry Bbls, have a preference for humid environments. A humidity level of 84–98% is ideal for the survival of larvae, nymphs, adult ticks, and eggs [89]. Ixodes spp. ticks in northern endemic areas (Europe, North America) climb on the upper surface of leaves, grasses, and twigs waiting to attack hosts, whereas ticks in southern areas and Africa tend to remain under the surface of leaves [90]. Therefore, a summer walk in the woods can be related to direct exposure to Ixodes spp. in Europe and, to a lesser extent, in Africa. This is well correlated with the incidence of Lyme disease in humans [91].
3.4. Lizards as Reservoirs
Differences in the spread of LB in Europe (North) and Africa (South) could be related to a dilution of the pathogen in Africa because of the different reservoirs (mammals and lizards). In Europe, Ixodes ticks mainly have rodents as reservoirs, while in Africa, they abound on lizards, which are known to be poor reservoirs of Bbsl [92]. Mice, voles, and shrews are usually excellent reservoirs for Bbsl, while larger mammals and lizards are not. Nevertheless, there are certain exceptions. It has been reported that the competence of lizards as reservoirs differs in Africa and America [41]. Ixodes inopinatus, indeed, infests the lizards Psammodromus algirus, Lacerta agilis, which are efficient reservoirs of Bbss in North Africa, amplifying its spread [65,93]. The host competence to transmit Borreliae LG was reported to vary between old-world and new-world lizards [41]. The old-world lizards (North Africa) seem to serve as amplifying hosts for B. lusitaniae and to be competent reservoirs in their maintenance and diffusion cycle [93]. New-world lizards (North America) seem to be poor hosts of Bbss, reducing the prevalence of this spirochete in nature. Studies along the West Coast of the United States have shown a consistent infestation of the fence lizards Sceloporus occidentalis by Ixodes pacificus ticks, which were minimally infected by Bbss [94,95].
3.5. LB in Humans, Domestic Animals, Livestock, and Wildlife
The diagnosis of Lyme disease should be considered in patients in endemic areas who have had a tick bite and have clinical symptoms of LB. When erythema migrans is present, the diagnosis is clinical, without the need for laboratory tests of confirmation. Anti-Borrelia antibodies with a two-tiered test (ELISA and Western blot) are recommended when symptoms or clinical manifestations suggestive of LB are present [2].
In African countries, tick-borne zoonotic pathogens (TBDs) circulate widely. Their infections can affect humans, such as LB and Relapsing Fever (RF), and livestock, causing significant economic damage. Domestic livestock is, indeed, an integral part of many families in African countries. Therefore, for the prevention of these diseases, it is important to carry out surveillance and epidemiological studies of TBDs and LB in ticks, humans, animals, and reservoirs, combined with adequate laboratory diagnostics. Furthermore, it is necessary to increase border inspections of imported animals to stop the cross-movement of ticks and TBDs [54].
Personal protective measures, such as protective clothing and tick repellents, are recommended to prevent infection and transmission. During and immediately after exposure to a tick habitat, patients should be advised to examine their bodies and promptly remove attached ticks (statement of good practice). In African countries, these measures are often difficult to apply.
Regarding the therapy, the effectiveness of a short course of doxycycline (10 days) in the early phase of LB is welcome in African countries due to the low cost of the drug and the applicability of an effective treatment [96]. Nevertheless, doxycycline can cause photodermatitis, so amoxicillin is preferred during pregnancy [97]. In neuroborreliosis and in more severe forms, ceftriaxone iv can be used for 14–21 days [4].
Other factors, such as international travels, the transfer of pathogens between wild and domestic animals, and climate–environmental changes, can alter the spread and distribution of LB and its vectors [98]. Also, human activities, such as deforestation and urbanization, can impact the diffusion of tick-borne pathogens by damaging the vector habitat and increasing the possibilities of interaction between humans and animals [99].
4. Conclusions
In Africa, there is LB, but it is not endemic. At present, LB cases are limited to African countries bordering the Mediterranean Sea and the Indian Ocean, which have the proper climatic conditions and humidity for their survival. Bbls is transported in Africa by migratory birds infested by ticks of the Ixodes genus, which can infect humans, and also savanna animals, dogs, and horses. In addition, there is also the possibility of a tick switch from the Ixodes to the Hyalomma genus, as already shown [100]. Bbls reservoirs can be rodents, birds, and also lizards, which are relevant as possible amplifiers of LB in Africa. As a consequence, North African populations are vulnerable to LB as well as European ones to tick-borne pathogens characteristic of Africa. Climate change and migratory phenomena can further facilitate the mutual exchange of tick-borne pathogens from Europe to Africa and vice versa.
Author Contributions
Conceptualization, G.T.; methodology, S.B. and G.T.; investigation, S.B., P.F., A.M., N.D. and G.T.; writing—original draft preparation, G.T. and S.B.; writing—review and editing, P.F., A.M. and N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for review articles.
Informed Consent Statement
Not applicable for this study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Nausicaa De Rosa for drawing the figures.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Pitrak, D.; Nguyen, C.T.; Cifu, A.S. Diagnosis of Lyme Disease. JAMA 2022, 327, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Fedorova, N.; Becker, N.S.; Kleinjan, J.E.; Marosevic, D.; Krebs, S.; Hui, L.; Fingerle, V.; Lane, R.S. Borrelia maritima sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex, occupying a basal position to North American species. Int. J. Syst. Evol. Microbiol. 2020, 70, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Bonin, S.; Ruscio, M. A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. 2020, 7, 265. [Google Scholar] [CrossRef]
- Trevisan, G.; Cinco, M.; Ruscio, M.; Forgione, P.; Bonoldi, V.L.N.; Falkingham, E.; Trevisini, S.; Tranchini, P.; Bonin, B.; Yoshinari, N.H. Borrelia Lyme Group. J. Dermatol. Res. Rev. Rep. 2022, 3, 1–12. [Google Scholar] [CrossRef]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 66, 4878–4880. [Google Scholar] [CrossRef]
- Collares-Pereira, M.; Couceiro, S.; Franca, I.; Kurtenbach, K.; Schäfer, S.M.; Vitorino, L.; Gonçalves, L.; Baptista, S.; Vieira, M.L.; Cunha, C. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 2004, 42, 1316–1318. [Google Scholar] [CrossRef]
- Cirkovic, V.; Veinovic, G.; Stankovic, D.; Mihaljica, D.; Sukara, R.; Tomanovic, S. Evolutionary dynamics and geographical dispersal of Borrelia lusitaniae. Front. Microbiol. 2024, 15, 1330914. [Google Scholar] [CrossRef]
- Deshpande, G.; Beetch, J.E.; Heller, J.G.; Naqvi, O.H.; Kuhn, K.G. Assessing the Influence of Climate Change and Environmental Factors on the Top Tick-Borne Diseases in the United States: A Systematic Review. Microorganisms 2023, 12, 50. [Google Scholar] [CrossRef]
- Hauser, G.; Rais, O.; Moran Cadenas, F.; Gonseth, Y.; Bouzelboudjen, M.; Gern, L. Influence of climatic factors on Ixodes ricinus nymph abundance and phenology over a long-term monthly observation in Switzerland (2000–2014). Parasit. Vectors 2018, 11, 289. [Google Scholar] [CrossRef]
- Norte, A.C.; Ramos, J.A.; Gern, L.; Núncio, M.S.; Lopes de Carvalho, I. Birds as reservoirs for Borrelia burgdorferi s.l. in Western Europe: Circulation of B. turdi and other genospecies in bird-tick cycles in Portugal. Environ. Microbiol. 2013, 15, 386–397. [Google Scholar] [CrossRef] [PubMed]
- States, S.L.; Huang, C.I.; Davis, S.; Tufts, D.M.; Diuk-Wasser, M.A. Co-feeding transmission facilitates strain coexistence in Borrelia burgdorferi, the Lyme disease agent. Epidemics 2017, 19, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mead, P. Epidemiology of Lyme Disease. Infect. Dis. Clin. N. Am. 2022, 36, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.H.; Pilz, A.; Jodar, L.; Moïsi, J.C. The Epidemiology of Lyme Borreliosis in Europe: An Updated Review on a Growing Public Health Issue. Vector Borne Zoonotic Dis. 2023, 23, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Kugeler, K.J.; Earley, A.; Mead, P.S.; Hinckley, A.F. Surveillance for Lyme Disease After Implementation of a Revised Case Definition—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 118–123. [Google Scholar] [CrossRef]
- Boligarla, S.; Laison, E.K.E.; Li, J.; Mahadevan, R.; Ng, A.; Lin, Y.; Thioub, M.Y.; Huang, B.; Ibrahim, M.H.; Nasri, B. Leveraging machine learning approaches for predicting potential Lyme disease cases and incidence rates in the United States using Twitter. BMC Med. Inform. Decis. Mak. 2023, 23, 217. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Kugeler, K.J.; Nelson, C.A.; Marx, G.E.; Hinckley, A.F. Use of Commercial Claims Data for Evaluating Trends in Lyme Disease Diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 499–507. [Google Scholar] [CrossRef]
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europedagger. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef]
- Trevisan, G.; Ruscio, M.; Cinco, M.; Nan, K.; Forgione, P.; Di Meo, N.; Tranchini, P.; Nacca, M.; Trincone, S.; Rimoldi, S.G.; et al. The history of Lyme disease in Italy and its spread in the Italian territory. Front. Pharmacol. 2023, 14, 1128142. [Google Scholar] [CrossRef]
- Semenza, J.C.; Paz, S. Climate change and infectious disease in Europe: Impact, projection and adaptation. Lancet Reg. Health Eur. 2021, 9, 100230. [Google Scholar] [CrossRef] [PubMed]
- Kahl, O.; Gray, J.S. The biology of Ixodes ricinus with emphasis on its ecology. Ticks Tick Borne Dis. 2023, 14, 102114. [Google Scholar] [CrossRef]
- Barbieri, A.M.; Venzal, J.M.; Marcili, A.; Almeida, A.P.; Gonzalez, E.M.; Labruna, M.B. Borrelia burgdorferi sensu lato infecting ticks of the Ixodes ricinus complex in Uruguay: First report for the Southern Hemisphere. Vector Borne Zoonotic Dis. 2013, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; MacLeod, E.T. Hard ticks (Acari: Ixodidae) and tick-borne diseases of sheep and goats in Africa: A review. Ticks Tick. Borne Dis. 2023, 14, 102232. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: Climate Change and the Spread of Vector-Borne Diseases, Including Dengue, Malaria, Lyme Disease, and West Nile Virus Infection. Med. Sci. Monit. 2024, 29, e943546. [Google Scholar] [CrossRef] [PubMed]
- Kasaija, P.D.; Estrada-Pena, A.; Contreras, M.; Kirunda, H.; de la Fuente, J. Cattle ticks and tick-borne diseases: A review of Uganda’s situation. Ticks Tick Borne Dis. 2021, 12, 101756. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. S1), S3–S14. [Google Scholar] [CrossRef]
- Fourie, L.J.; Belozerov, V.N.; Needham, G.R. Ixodes rubicundus nymphs are short-day diapause-induced ticks with thermolabile sensitivity and desiccation resistance. Med. Vet. Entomol. 2001, 15, 335–341. [Google Scholar] [CrossRef]
- Roeber, F.; Webster, M. Protecting dogs and cats against the Australian paralysis tick, Ixodes holocyclus (Acari: Ixodidae): A review of the Australian acaricide registration process. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100054. [Google Scholar] [CrossRef]
- Morel, P.-C. Les tiques d’Afrique et du bassin méditerranéen; CIRAD-EMVT: Montpellier, France, 2003; p. 1 Cd-Rom. [Google Scholar]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Tang, T.; Zhu, Y.; Zhang, Y.-Y.; Chen, J.-J.; Tian, J.-B.; Xu, Q.; Jiang, B.-G.; Wang, G.-L.; Golding, N.; Mehlman, M.L.; et al. The global distribution and the risk prediction of relapsing fever group Borrelia: A data review with modelling analysis. Lancet Microbe 2024, 5, e442–e451. [Google Scholar] [CrossRef] [PubMed]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R., 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.E.; Breuner, N.E.; Hojgaard, A.; Oliver, J.; Eisen, L.; Eisen, R.J. A comparison of horizontal and transovarial transmission efficiency of Borrelia miyamotoi by Ixodes scapularis. Ticks Tick Borne Dis. 2022, 13, 102003. [Google Scholar] [CrossRef] [PubMed]
- Zinck, C.B.; Lloyd, V.K. Borrelia burgdorferi and Borrelia miyamotoi in Atlantic Canadian wildlife. PLoS ONE 2022, 17, e0262229. [Google Scholar] [CrossRef]
- Khan, M.; Almutairi, M.M.; Alouffi, A.; Tanaka, T.; Chang, S.C.; Chen, C.C.; Ali, A. Molecular evidence of Borrelia theileri and closely related Borrelia spp. in hard ticks infesting domestic animals. Front. Vet. Sci. 2023, 10, 1297928. [Google Scholar] [CrossRef]
- Abanda, B.; Paguem, A.; Abdoulmoumini, M.; Kingsley, M.T.; Renz, A.; Eisenbarth, A. Molecular identification and prevalence of tick-borne pathogens in zebu and taurine cattle in North Cameroon. Parasites Vectors 2019, 12, 448. [Google Scholar] [CrossRef]
- Younsi, H.; Sarih, M.; Jouda, F.; Godfroid, E.; Gern, L.; Bouattour, A.; Baranton, G.; Postic, D. Characterization of Borrelia lusitaniae isolates collected in Tunisia and Morocco. J. Clin. Microbiol. 2005, 43, 1587–1593. [Google Scholar] [CrossRef]
- Younsi, H.; Fares, W.; Cherni, S.; Dachraoui, K.; Barhoumi, W.; Najjar, C.; Zhioua, E. Ixodes inopinatus and Ixodes ricinus (Acari: Ixodidae) Are Sympatric Ticks in North Africa. J. Med. Entomol. 2020, 57, 952–956. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; Nava, S.; Petney, T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick Borne Dis. 2014, 5, 734–743. [Google Scholar] [CrossRef]
- Nowak, T.A.; Burke, R.L.; Diuk-Wasser, M.A.; Lin, Y.P. Lizards and the enzootic cycle of Borrelia burgdorferi sensu lato. Mol. Microbiol. 2024, 121, 1262–1272. [Google Scholar] [CrossRef]
- Norte, A.C.; Boyer, P.H.; Castillo-Ramirez, S.; Chvostac, M.; Brahami, M.O.; Rollins, R.E.; Woudenberg, T.; Didyk, Y.M.; Derdakova, M.; Nuncio, M.S.; et al. The Population Structure of Borrelia lusitaniae Is Reflected by a Population Division of Its Ixodes Vector. Microorganisms 2021, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Horak, I.G.; Golezardy, H.; Uys, A.C. Ticks associated with the three largest wild ruminant species in southern Africa. Onderstepoort J. Vet. Res. 2007, 74, 231–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shuaib, Y.A.; Elhag, A.M.W.; Brima, Y.A.; Abdalla, M.A.; Bakiet, A.O.; Mohmed-Noor, S.E.; Lemhofer, G.; Bestehorn, M.; Poppert, S.; Schaper, S.; et al. Ixodid tick species and two tick-borne pathogens in three areas in the Sudan. Parasitol. Res. 2020, 119, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick Borne Dis. 2020, 11, 101330. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Shuaib, Y.A.; Isaa, M.H.; Ezz-Eldin, M.I.; Osman, A.Y.; Yagoub, I.A.; Abdalla, M.A.; Bakiet, A.O.; Mohmed-Noor, S.E.; Schaper, S.; et al. Tick Fauna and Associated Rickettsia, Theileria, and Babesia spp. in Domestic Animals in Sudan (North Kordofan and Kassala States). Microorganisms 2020, 8, 1969. [Google Scholar] [CrossRef]
- Bouattour, A.; Ghorbel, A.; Chabchoub, A.; Postic, D. Lyme borreliosis situation in North Africa. Arch. Inst. Pasteur Tunis. 2004, 81, 13–20. [Google Scholar]
- Abdelbaset, A.E.; Kwak, M.L.; Nonaka, N.; Nakao, R. Human-biting ticks and zoonotic tick-borne pathogens in North Africa: Diversity, distribution, and trans-Mediterranean public health challenges. One Health 2023, 16, 100547. [Google Scholar] [CrossRef]
- Laamari, A.; Azzag, N.; Tennah, S.; Derdour, S.Y.; China, B.; Bouabdallah, R.; Ghalmi, F. Seroprevalence of Antibodies Against Anaplasma Phagocytophilum and Borrelia Burgdorferi in Horses (Equus caballus) from Northern Algeria. J. Vet. Res. 2020, 64, 413–419. [Google Scholar] [CrossRef]
- Senbill, H.; Karawia, D.; Zeb, J.; Alyami, N.M.; Almeer, R.; Rahman, S.; Sparagano, O.; Baruah, A. Molecular screening and genetic diversity of tick-borne pathogens associated with dogs and livestock ticks in Egypt. PLoS Negl. Trop. Dis. 2024, 18, e0012185. [Google Scholar] [CrossRef]
- Ashour, R.; Hamza, D.; Kadry, M.; Sabry, M.A. The Surveillance of Borrelia Species in Camelus dromedarius and Associated Ticks: The First Detection of Borrelia miyamotoi in Egypt. Vet. Sci. 2023, 10, 141. [Google Scholar] [CrossRef]
- Elhelw, R.; Elhariri, M.; Hamza, D.; Abuowarda, M.; Ismael, E.; Farag, H. Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet. Res. 2021, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Alanazi, A.D.; Sazmand, A.; Otranto, D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasites Vectors 2021, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset, A.E.; Nonaka, N.; Nakao, R. Tick-borne diseases in Egypt: A one health perspective. One Health 2022, 15, 100443. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, N.A.; Hegazy, I.H.; el-Sawy, E.H. ELISA screening for Lyme disease in children with chronic arthritis. J. Egypt. Soc. Parasitol. 1995, 25, 525–533. [Google Scholar] [PubMed]
- Marjolet, M.; Gueglio, B.; Traore, M. Does Lyme disease (or an analogous disease) exist in Mali, West Africa? Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 387. [Google Scholar] [CrossRef]
- Sarih, M.; Jouda, F.; Gern, L.; Postic, D. First isolation of Borrelia burgdorferi sensu lato from Ixodes ricinus ticks in Morocco. Vector Borne Zoonotic Dis. 2003, 3, 133–139. [Google Scholar] [CrossRef]
- Rochd, S.; Benhoummad, O.; Salhi, S.; Lakhdar, Y.; Rochdi, Y.; Raji, A.; Oualhadj, H.; Kamouni, Y.E.; Zouhair, S. Isolated Sudden Bilateral Neurosensory Hearing Loss as a Presentation of Lyme Neuroborreliosis: A Case Study. J. Audiol. Otol. 2024, 28, 72–75. [Google Scholar] [CrossRef]
- Zahaf, A.; Bouassida, S.; Boudaya, S.; Turki, H. Lyme disease in Sfax. Ann. Dermatol. Venereol. 1994, 121, 177–179. [Google Scholar]
- Aoun, K.; Kechrid, A.; Lagha, N.; Zarrouk, A.; Bouzouaia, N. Lyme disease in Tunisia, results of a clinical and serological study (1992–1996). Sante 1998, 8, 98–100. [Google Scholar]
- Ben Said, M.; Belkahia, H.; Alberti, A.; Abdi, K.; Zhioua, M.; Daaloul-Jedidi, M.; Messadi, L. First molecular evidence of [i]Borrelia burgdorferi[/i] sensu lato in goats, sheep, cattle and camels in Tunisia. Ann. Agric. Environ. Med. 2016, 23, 442–447. [Google Scholar] [CrossRef]
- Veronesi, F.; Laus, F.; Passamonti, F.; Tesei, B.; Piergili Fioretti, D.; Genchi, C. Occurrence of Borrelia lusitaniae infection in horses. Vet. Microbiol. 2012, 160, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Younsi, H.; Postic, D.; Baranton, G.; Bouattour, A. High prevalence of Borrelia lusitaniae in Ixodes ricinus ticks in Tunisia. Eur. J. Epidemiol. 2001, 17, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Selmi, R.; Abdi, K.; Belkahia, H.; Abdallah, M.B.; Mamlouk, A.; Kratou, M.; Said, M.B.; Messadi, L. Detection and genetic identification of Borrelia lusitaniae in questing Ixodes inopinatus tick from Tunisia. Infect. Med. 2024, 3, 100093. [Google Scholar] [CrossRef] [PubMed]
- Dsouli, N.; Younsi-Kabachii, H.; Postic, D.; Nouira, S.; Gern, L.; Bouattour, A. Reservoir role of lizard Psammodromus algirus in transmission cycle of Borrelia burgdorferi sensu lato (Spirochaetaceae) in Tunisia. J. Med. Entomol. 2006, 43, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Ekner, A.; Dudek, K.; Sajkowska, Z.; Majlathova, V.; Majlath, I.; Tryjanowski, P. Anaplasmataceae and Borrelia burgdorferi sensu lato in the sand lizard Lacerta agilis and co-infection of these bacteria in hosted Ixodes ricinus ticks. Parasit. Vectors 2011, 4, 182. [Google Scholar] [CrossRef]
- Oswe, M.; Odhiambo, R.; Mutai, B.; Nyakoe, N.; Awinda, G.; Waitumbi, J.N. Zoonotic Pathogens in Ticks Collected from Livestock in Kenya. Open J. Prev. Med. 2018, 8, 248–259. [Google Scholar] [CrossRef]
- Olivieri, E.; Kariuki, E.; Floriano, A.M.; Castelli, M.; Tafesse, Y.M.; Magoga, G.; Kumsa, B.; Montagna, M.; Sassera, D. Multi-country investigation of the diversity and associated microorganisms isolated from tick species from domestic animals, wildlife and vegetation in selected african countries. Exp. Appl. Acarol. 2021, 83, 427–448. [Google Scholar] [CrossRef]
- Jowi, J.O.; Gathua, S.N. Lyme disease: Report of two cases. East. Afr. Med. J. 2005, 82, 267–269. [Google Scholar] [CrossRef]
- Nyataya, J.; Maraka, M.; Lemtudo, A.; Masakhwe, C.; Mutai, B.; Njaanake, K.; Estambale, B.B.; Nyakoe, N.; Siangla, J.; Waitumbi, J.N. Serological Evidence of Yersiniosis, Tick-Borne Encephalitis, West Nile, Hepatitis E, Crimean-Congo Hemorrhagic Fever, Lyme Borreliosis, and Brucellosis in Febrile Patients Presenting at Diverse Hospitals in Kenya. Vector Borne Zoonotic Dis. 2020, 20, 348–357. [Google Scholar] [CrossRef]
- De Michelis, S.; Sewell, H.S.; Collares-Pereira, M.; Santos-Reis, M.; Schouls, L.M.; Benes, V.; Holmes, E.C.; Kurtenbach, K. Genetic diversity of Borrelia burgdorferi sensu lato in ticks from mainland Portugal. J. Clin. Microbiol. 2000, 38, 2128–2133. [Google Scholar] [CrossRef]
- England, M.E.; Phipps, P.; Medlock, J.M.; Atkinson, P.M.; Atkinson, B.; Hewson, R.; Gale, P. Hyalomma ticks on northward migrating birds in southern Spain: Implications for the risk of entry of Crimean-Congo haemorrhagic fever virus to Great Britain. J. Vector Ecol. 2016, 41, 128–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nozais, J.P.; Assous, M.; Cordier, F.; Gentilini, M. A probable case of Lyme Disease contracted in Mozambique. Bull. Soc. Pathol. Exot. 1993, 86, 345–346. [Google Scholar] [PubMed]
- Collares-Pereira, M.; Gomes, A.C.; Prassad, M.; Vaz, R.G.; Ferrinho, P.; Stanek, G.; Rosario, V.E. Preliminary survey of Leptospirosis and Lyme disease amongst febrile patients attending community hospital ambulatory care in Maputo, Mozambique. Cent. Afr. J. Med. 1997, 43, 234–238. [Google Scholar] [PubMed]
- Kim, T.Y.; Kwak, Y.S.; Kim, J.Y.; Nam, S.H.; Lee, I.Y.; Mduma, S.; Keyyu, J.; Fyumagwa, R.; Yong, T.S. Prevalence of Tick-Borne Pathogens from Ticks Collected from Cattle and Wild Animals in Tanzania in 2012. Korean J. Parasitol. 2018, 56, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Haji, I.; Simuunza, M.; Kerario, I.I.; Jiang, N.; Chen, Q. Epidemiology of tick-borne pathogens of cattle and tick control practices among mixed farming and pastoral communities in Gairo and Monduli districts, Tanzania. Vet. Parasitol. Reg. Stud. Rep. 2022, 32, 100738. [Google Scholar] [CrossRef]
- Mhalu, F.S.; Matre, R. Serological evidence of Lyme borreliosis in Africa: Results from studies in Dar es Salaam, Tanzania. East. Afr. Med. J. 1996, 73, 583–585. [Google Scholar]
- Ma, R.; Li, C.; Gao, A.; Jiang, N.; Li, J.; Hu, W.; Feng, X. Tick species diversity and potential distribution alternation of dominant ticks under different climate scenarios in Xinjiang, China. PLoS Negl. Trop. Dis. 2024, 18, e0012108. [Google Scholar] [CrossRef]
- Giesen, C.; Cifo, D.; Gomez-Barroso, D.; Estevez-Reboredo, R.M.; Figuerola, J.; Herrador, Z. The Role of Environmental Factors in Lyme Disease Transmission in the European Union: A Systematic Review. Trop. Med. Infect. Dis. 2024, 9, 113. [Google Scholar] [CrossRef]
- Rollins, R.E.; Sato, K.; Nakao, M.; Tawfeeq, M.T.; Herrera-Mesias, F.; Pereira, R.J.; Kovalev, S.; Margos, G.; Fingerle, V.; Kawabata, H.; et al. Out of Asia? Expansion of Eurasian Lyme borreliosis causing genospecies display unique evolutionary trajectories. Mol. Ecol. 2023, 32, 786–799. [Google Scholar] [CrossRef]
- Burnus, L.; Wynn, J.; Liedvogel, M.; Rollins, R. Beware of hitchhiking ticks? Clarifying the variable roles of bird families in tick movement along migratory routes. TechRxiv 2024. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Kaiser, M.N.; Traylor, M.A.; Guindy, E.; Gaber, S. Ticks (Ixodidae) on birds migrating from Europe and Asia to Africa 1959-61. Bull World Health Organ 1963, 28, 235–262. [Google Scholar] [PubMed]
- Alkishe, A.A.; Peterson, A.T.; Samy, A.M. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE 2017, 12, e0189092. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef] [PubMed]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Brunner, J.L. Climate change and Ixodes tick-borne diseases of humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Elbir, H.; Abi-Rached, L.; Pontarotti, P.; Yoosuf, N.; Drancourt, M. African relapsing Fever borreliae genomospecies revealed by comparative genomics. Front. Public Health 2014, 2, 43. [Google Scholar] [CrossRef]
- Cutler, S.J.; Idris, J.M.; Ahmed, A.O.; Elelu, N. Ornithodoros savignyi, the Tick Vector of “Candidatus Borrelia kalaharica” in Nigeria. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Grigoryeva, L.A. Influence of air humidity on the survival rate, lifetime, and development of Ixodes ricinus (L., 1758) and Ixodes persulcatus Schulze, 1930 (Acari: Ixodidae). Syst. Appl. Acarol. 2022, 27, 2241–2248. [Google Scholar] [CrossRef]
- Arsnoe, I.M.; Hickling, G.J.; Ginsberg, H.S.; McElreath, R.; Tsao, J.I. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human lyme disease risk. PLoS ONE 2015, 10, e0127450. [Google Scholar] [CrossRef]
- Arsnoe, I.; Tsao, J.I.; Hickling, G.J. Nymphal Ixodes scapularis questing behavior explains geographic variation in Lyme borreliosis risk in the eastern United States. Ticks Tick Borne Dis. 2019, 10, 553–563. [Google Scholar] [CrossRef]
- Piesman, J. Ecology of Borrelia burgdorferi sensu lato in North America. In Lyme Borreliosis: Ecology, Epidemiology and Control; Gray, J.S., Kahl, O., Lane, R.S., Stanek, G., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 223–249. [Google Scholar] [CrossRef]
- Richter, D.; Matuschka, F.R. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl. Environ. Microbiol. 2006, 72, 4627–4632. [Google Scholar] [CrossRef] [PubMed]
- Durden, L.A.; Oliver, J.H., Jr.; Banks, C.W.; Vogel, G.N. Parasitism of lizards by immature stages of the blacklegged tick, Ixodes scapularis (Acari, Ixodidae). Exp. Appl. Acarol. 2002, 26, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Mun, J.; Eisen, L.; Eisen, R.J. Refractoriness of the western fence lizard (Sceloporus occidentalis) to the Lyme disease group spirochete Borrelia bissettii. J. Parasitol. 2006, 92, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Cifu, A.S.; Pitrak, D. Prevention and Treatment of Lyme Disease. JAMA 2022, 327, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Ruscio, M.; di Meo, N.; Nan, K.; Cinco, M.; Trevisini, S.; Forgione, P.; Bonin, S. Case Report: Lyme Borreliosis and Pregnancy—Our Experience. Front. Med. 2022, 9, 816868. [Google Scholar] [CrossRef]
- McGarry, J.W. Travel and disease vector ticks. Travel. Med. Infect. Dis. 2011, 9, 49–59. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, A.; Aziz, U.; Song, B.; Zeb, J.; George, D.; Li, J.; Sparagano, O. The Role of Ticks in the Emergence of Borrelia burgdorferi as a Zoonotic Pathogen and Its Vector Control: A Global Systemic Review. Microorganisms 2021, 9, 2412. [Google Scholar] [CrossRef]
- Koutantou, M.; Drancourt, M.; Angelakis, E. Prevalence of Lyme Disease and Relapsing Fever borrelia spp. in Vectors, Animals, and Humans within a One Health Approach in Mediterranean Countries. Pathogens 2024, 13, 512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).