Forecasting Suitable Habitats of the Clouded Leopard (Neofelis nebulosa) in Asia: Insights into the Present and Future Climate Projections Within and Beyond Extant Boundaries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
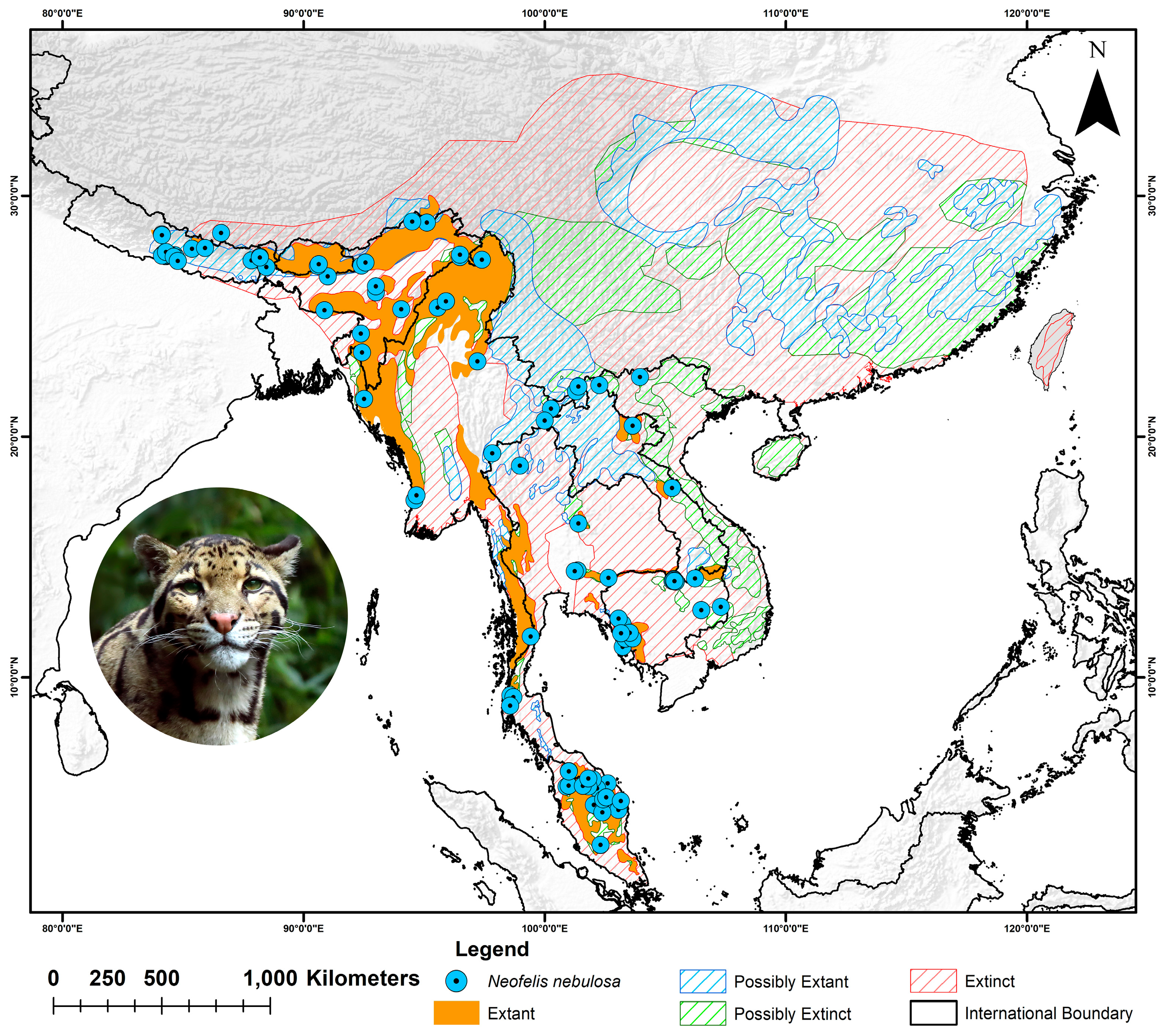
2.1. Study Area and Species Occurrence Records
2.2. Distribution Predictors for the Clouded Leopard
2.3. Assessment of Future Climate Change Projections
2.4. Species Distribution Model for Clouded Leopard
2.5. Assessment of Habitat Quality and Shape Geometry
2.6. Assessment of Biological Corridor Connectivity
3. Results
3.1. Ecological Niche Modelling and Predictor Importance
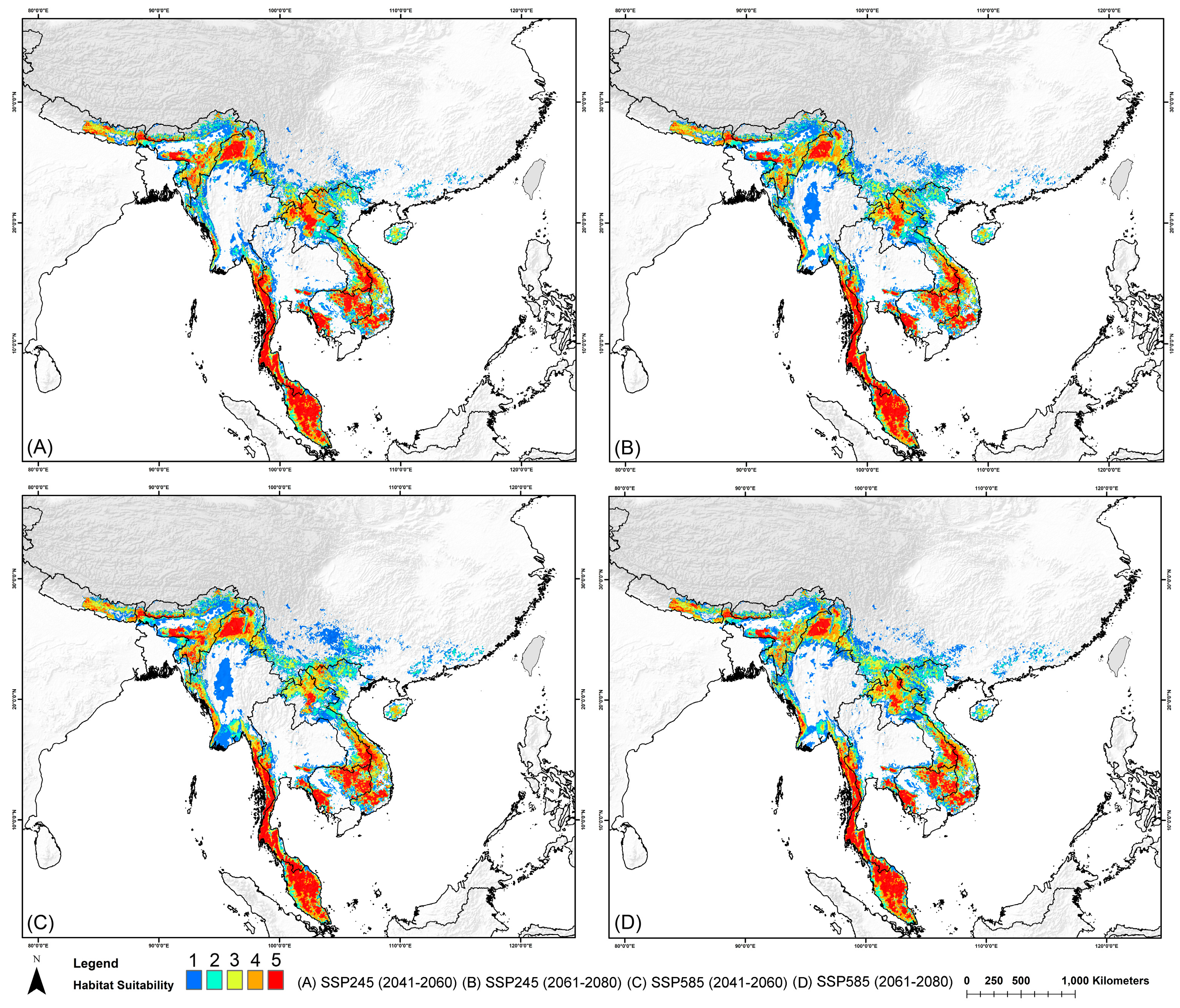
3.2. Habitat Suitability in Present and Historical Range
3.3. Country-Level Mean Habitat Suitability
3.4. Habitat Quality and Shape Geometry
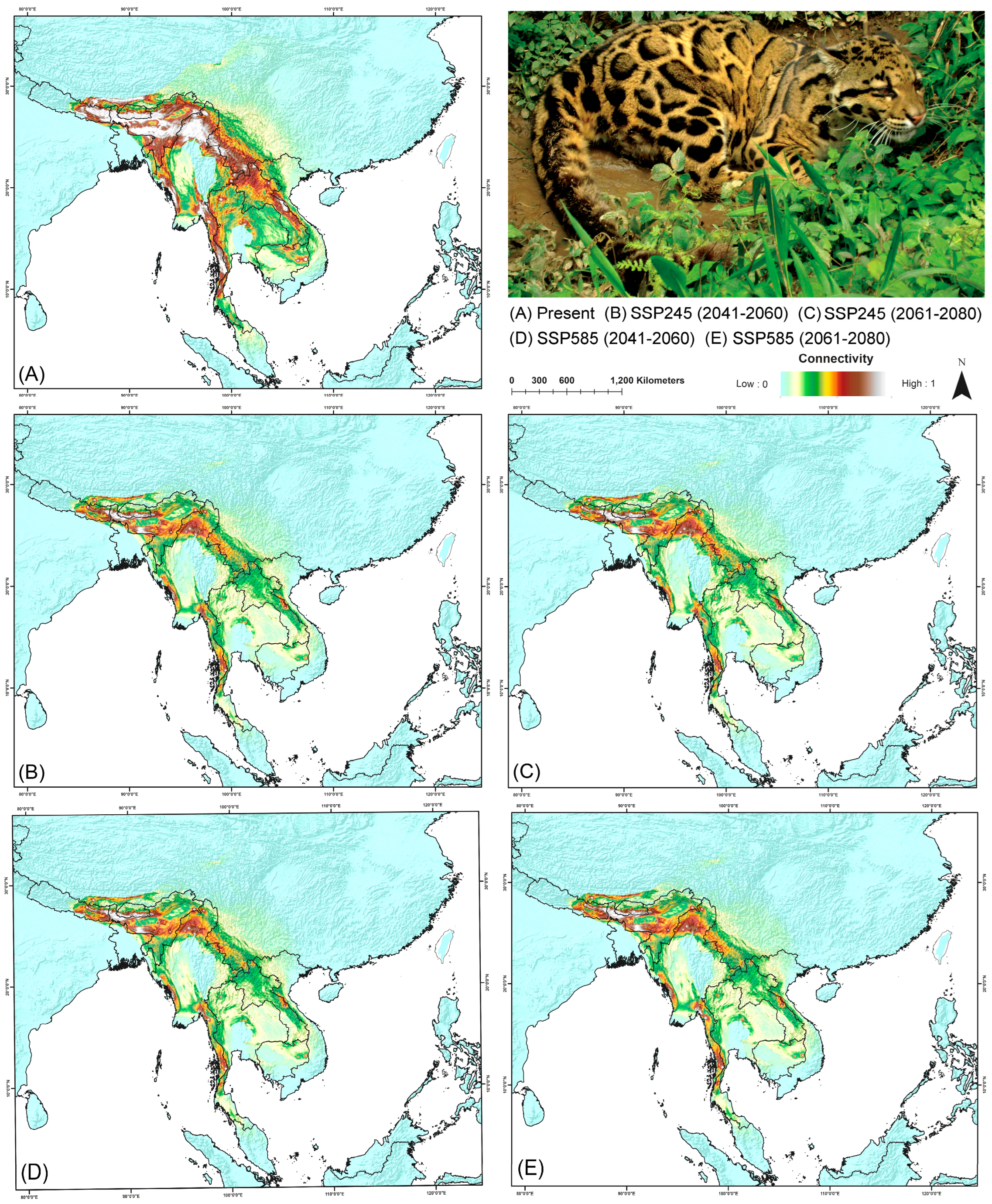
3.5. Biological Corridor Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, A.; Kim, M.; Lee, S. Analysis of priority conservation areas using habitat quality models and MaxEnt models. Animals 2024, 14, 1680. [Google Scholar] [CrossRef]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J.; et al. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 2022, 8, 9982. [Google Scholar] [CrossRef]
- Mitchell, C.; Bolam, J.; Bertola, L.D.; Naude, V.N.; Gonçalves da Silva, L.; Razgour, O. Leopard subspecies conservation under climate and land-use change. Ecol. Evol. 2024, 14, e11391. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Preston, K.L.; Rotenberry, J.T.; Redak, R.A.; Allen, M.F. Habitat shifts of endangered species under altered climate conditions: Importance of biotic interactions. Glob. Chang. Biol. 2008, 14, 2501–2515. [Google Scholar] [CrossRef]
- Petersen, W.J.; Savini, T.; Ngoprasert, D. Strongholds under siege: Range-wide deforestation and poaching threaten mainland clouded leopards (Neofelis nebulosa). Glob. Ecol. Conserv. 2020, 24, e01354. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Bennett, A.F.; Saunders, D.A. Habitat fragmentation and landscape change. In Conservation Biology for All; Sodhi, N.S., Ehrlich, P.R., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 88–106. [Google Scholar]
- Kaszta, Z.; Cushman, S.A.; Macdonald, D.W. Prioritizing habitat core areas and corridors for a large carnivore across its range. Anim. Conserv. 2020, 23, 607–616. [Google Scholar] [CrossRef]
- Cushman, S.A.; McRae, B.; Adriansen, F.; Beier, P.; Shirley, M.; Zeller, K. Biological corridors and connectivity. In Key Topics in Conservation Biology 2; Willis, K.J., Ed.; Wiley: New York, NY, USA, 2013; pp. 384–404. [Google Scholar]
- Kaszta, Z.; Cushman, S.A.; Hearn, A.J.; Burnham, D.; Macdonald, E.A.; Goossens, B.; Nathan, S.K.S.S.; Macdonald, D.W. Integrating Sunda clouded leopard (Neofelis diardi) conservation into development and restoration planning in Sabah (Borneo). Biol. Conserv. 2019, 235, 63–76. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and ecological effects of the world’s largest carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef]
- Penjor, U.; MacDonald, D.W.; Wangchuk, S.; Tandin, T.; Tan, C.K.W. Identifying important conservation areas for the clouded leopard (Neofelis nebulosa) in a mountainous landscape: Inference from spatial modelling techniques. Ecol. Evol. 2018, 8, 4278–4291. [Google Scholar] [CrossRef]
- Mohammadi, A.; Almasieh, K.; Nayeri, D.; Ataei, F.; Khani, A.; López-Bao, J.V.; Penteriani, V.; Cushman, S.A. Identifying priority core habitats and corridors for effective conservation of brown bears in Iran. Sci. Rep. 2021, 11, 1044. [Google Scholar] [CrossRef]
- Noss, R.F.; Quigley, H.B.; Hornocker, M.G.; Merrill, T.; Paquet, P.C. Conservation biology and carnivore conservation in the Rocky Mountains. Conserv. Biol. 1996, 10, 949–963. [Google Scholar] [CrossRef]
- Cardillo, M.; Purvis, A.; Sechrest, W.; Gittleman, J.L.; Bielby, J.; Mace, G.M. Human Population Density and Extinction Risk in the World’s Carnivores. PLoS Biol. 2004, 2, e197. [Google Scholar] [CrossRef]
- Wolf, C.; Ripple, W.J. Prey depletion as a threat to the world’s large carnivores. R. Soc. Open Sci. 2016, 3, 160252. [Google Scholar] [CrossRef]
- Atkins, J.L.; Long, R.A.; Pansu, J.; Daskin, J.H.; Potter, A.B.; Stalmans, M.E.; Tarnita, C.E.; Pringle, R.M. Cascading impacts of large-carnivore extirpation in an African ecosystem. Science 2019, 364, 173–177. [Google Scholar] [CrossRef]
- Tshabalala, T.; McManus, J.; Treves, A.; Masocha, V.; Faulconbridge, S.; Schurch, M.; Goets, S.; Smuts, B. Leopards and mesopredators as indicators of mammalian species richness across diverse landscapes of South Africa. Ecol. Indic. 2021, 121, 107201. [Google Scholar] [CrossRef]
- Abade, L.; Macdonald, D.W.; Dickman, A.J. Using Landscape and Bioclimatic Features to Predict the Distribution of Lions, Leopards and Spotted Hyaenas in Tanzania’s Ruaha Landscape. PLoS ONE 2014, 9, e96261. [Google Scholar] [CrossRef]
- Hollings, T.; Jones, M.; Mooney, N.; McCallum, H. Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. Conserv. Biol. 2014, 28, 63–75. [Google Scholar] [CrossRef]
- Beier, P.; Majka, D.R.; Spencer, W.D. Forks in the Road: Choices in Procedures for Designing Wildland Linkages. Conserv. Biol. 2008, 22, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Kaboodvandpour, S.; Almasieh, K.; Zamani, N. Habitat suitability and connectivity implications for the conservation of the Persian leopard along the Iran–Iraq border. Ecol. Evol. 2021, 11, 13464–13474. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.W.; Bothwell, H.M.; Kaszta, Ż.; Ash, E.; Bolongon, G.; Burnham, D.; Can, O.E.; Campos-Arceiz, A.; Phan, C.; Clements, G.R.; et al. Multi-scale habitat modelling identifies spatial conservation priorities for mainland clouded leopards (Neofelis nebulosa). Divers. Distrib. 2019, 25, 1639–1654. [Google Scholar] [CrossRef]
- Tian, T.; Chen, X.; Pan, H.; Jin, Y.; Zhang, X.; Xiang, Y.; Song, D.; Yang, B.; Zhang, L. Habitat Selection Differences of Two Sympatric Large Carnivores in the Southwestern Mountains of China. Diversity 2023, 15, 968. [Google Scholar] [CrossRef]
- Gordon, C.H.; Stewart, E.A.M. The use of logging roads by a solitary felid, the clouded leopard. Cat News 2007, 47, 12–13. [Google Scholar]
- Pocock, R.I. Genus Neofelis Gray. The Clouded Leopard. In The Fauna of British India, Including Ceylon and Burma; Taylor and Francis, Ltd.: London, UK, 1939; Volume Mammalia—Volume 1, pp. 247–253. [Google Scholar]
- Gray, T.; Borah, J.; Coudrat, C.N.Z.; Ghimirey, Y.; Giordano, A.; Greenspan, E.; Petersen, W.; Rostro-García, S.; Shariff, M.; Wong, W. Neofelis nebulosa. The IUCN Red List of Threatened Species 2021: E.T14519A198843258; IUCN: Gland, Switzerland, 2021. [Google Scholar] [CrossRef]
- Can, Ö.E.; Yadav, B.P.; Johnson, P.J.; Ross, J.; D’Cruze, N.; Macdonald, D.W. Factors affecting the occurrence and activity of clouded leopards, common leopards and leopard cats in the Himalayas. Biodivers. Conserv. 2020, 29, 839–851. [Google Scholar] [CrossRef]
- Austin, S.C.; Tewes, M.E.; Grassman, L.I., Jr.; Silvy, N.J. Ecology and conservation of the leopard cat Prionailurus bengalensis and clouded leopard Neofelis nebulosa in Khao Yai National Park, Thailand. Acta Zool. Sin. 2007, 53, 1–14. [Google Scholar]
- Wilting, A.; Buckley-Beason, V.A.; Feldhaar, H.; Gadau, J.; O’Brien, S.J.; Linsenmair, S.E. Clouded leopard phylogeny revisited: Support for species and subspecies recognition. Front. Zool. 2007, 4, 15. [Google Scholar] [CrossRef]
- Chiang, P.J.; Pei, K.J.C.; Vaughan, M.R.; Li, C.F.; Chen, M.T.; Liu, J.N.; Lin, C.Y.; Lin, L.K.; Lai, Y.C. Is the clouded leopard Neofelis nebulosa extinct in Taiwan, and could it be reintroduced? An assessment of prey and habitat. Oryx 2015, 49, 261–269. [Google Scholar] [CrossRef]
- Petersen, W.J.; Steinmetz, R.; Sribuarod, K.; Ngoprasert, D. Density and movements of mainland clouded leopards (Neofelis nebulosa) under conditions of high and low poaching pressure. Glob. Ecol. Conserv. 2020, 23, e01117. [Google Scholar] [CrossRef]
- Singh, P.; Macdonald, D.W. Populations and activity patterns of clouded leopards and marbled cats in Dampa Tiger Reserve, India. J. Mammal. 2017, 98, 1453–1462. [Google Scholar] [CrossRef]
- Borah, J.; Sharma, T.; Das, D.; Rabha, N.; Kakati, N.; Basumatary, A.; Ahmed, M.F.; Vattakaven, J. Abundance and density estimates for common leopard (Panthera pardus) and clouded leopard (Neofelis nebulosa) in Manas National Park, Assam, India. Oryx 2014, 48, 149–155. [Google Scholar] [CrossRef]
- Namkhan, M.; Sukumal, N.; Savini, T. Impact of climate change on Southeast Asian natural habitats, with focus on protected areas. Glob. Ecol. Conserv. 2022, 39, e02293. [Google Scholar] [CrossRef]
- Radchuk, V.; Kramer-Schadt, S.; Fickel, J.; Wilting, A. Distributions of mammals in Southeast Asia: The role of the legacy of climate and species body mass. J. Biogeogr. 2019, 46, 2350–2362. [Google Scholar] [CrossRef]
- Ye, X.; Wu, Q.; Li, X.; Zhao, X. Incorporating interspecific relationships into species distribution models can better assess the response of species to climate change, a case study of two Chinese primates. Ecol. Indic. 2022, 142, 109255. [Google Scholar] [CrossRef]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the distributions of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef]
- Bai, D.F.; Chen, P.J.; Atzeni, L.; Cering, L.; Li, Q.; Shi, K. Assessment of habitat suitability of the snow leopard (Panthera uncia) in Qomolangma National Nature Reserve based on MaxEnt modeling. Zool. Res. 2018, 39, 373–386. [Google Scholar]
- Forrest, J.L.; Wikramanayake, E.; Shrestha, R.; Areendran, G.; Gyeltshen, K.; Maheshwari, A.; Mazumdar, S.; Naidoo, R.; Thapa, G.J.; Thapa, K. Conservation and climate change: Assessing the vulnerability of snow leopard habitat to treeline shift in the Himalaya. Biol. Conserv. 2012, 150, 129–135. [Google Scholar] [CrossRef]
- Watts, S.M.; McCarthy, T.M.; Namgail, T. Modelling potential habitat for snow leopards (Panthera uncia) in Ladakh, India. PLoS ONE 2019, 14, e0211509. [Google Scholar] [CrossRef]
- Morovati, M.; Panahandeh, M.; Rousta, Z.; Shorakaei, M.J. Habitat desirability modelling of cheetah (Acinonyx jubatus venaticus) using maximum entropy model in central Iran (a case study: Yazd province-Dareh Anjir wildlife refuge). Appl. Ecol. Environ. Res. 2015, 13, 725–739. [Google Scholar]
- Mohammadi, A.; Almasieh, K.; Nayeri, D.; Adibi, M.A.; Wan, H.Y. Comparison of habitat suitability and connectivity modelling for three carnivores of conservation concern in an Iranian montane landscape. Landsc. Ecol. 2022, 37, 411–430. [Google Scholar] [CrossRef]
- Rather, T.A.; Kumar, S.; Khan, J.A. Multi-scale habitat modelling and predicting change in the distribution of tiger and leopard using random forest algorithm. Sci. Rep. 2020, 10, 11473. [Google Scholar] [CrossRef]
- Giovanelli, J.G.R.; de Siqueira, M.F.; Haddad, C.F.B.; Alexandrino, J. Modeling a spatially restricted distribution in the Neotropics: How the size of calibration area affects the performance of five presence-only methods. Ecol. Model. 2010, 221, 215–224. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Jamali, F.; Amininasab, S.M.; Taleshi, H.; Madadi, H. Using an ensemble modeling to predict the potential distribution and habitat suitability of caracal (Caracal caracal) in southwestern Iran. Glob. Ecol. Conserv. 2024, 52, e02968. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Meller, L.; Cabeza, M.; Pironon, S.; Barbet-Massin, M.; Maiorano, L.; Georges, D.; Thuiller, W. Ensemble distribution models in conservation prioritization: From consensus predictions to consensus reserve networks. Diversity Distrib. 2014, 20, 309–321. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Brito, J.C.; Crespo, E.G.; Watts, M.E.; Possingham, H.P. Conservation planning under climate change: Toward accounting for uncertainty in predicted species distributions to increase confidence in conservation investments in space and time. Biol. Conserv. 2011, 144, 2020–2030. [Google Scholar] [CrossRef]
- Nowell, K.; Jackson, P. Wild Cats. In Status Survey and Conservation Action Plan; IUCN/SSC Cat Specialist Group: Gland, Switzerland; Cambridge, UK, 1996. [Google Scholar]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting red list threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Lee, S.R.; Kim, H.W.; Kundu, S. Fragile futures: Evaluating habitat and climate change response of hog badgers (Mustelidae: Arctonyx) in the conservation landscape of mainland Asia. Ecol. Evol. 2024, 14, e70160. [Google Scholar] [CrossRef]
- UNEP-WCMC and IUCN. Protected Planet: The World Database on Protected Areas (WDPA) and World Database on Other Effective Area-Based Conservation Measures (WD OECM); UNEP-WCMC and IUCN: Cambridge, UK, 2024; Available online: www.protectedplanet.net (accessed on 22 August 2024).
- Salas, E.A.L.; Valdez, R.; Michel, S.; Boykin, K.G. Habitat assessment of Marco Polo sheep (Ovis ammon polii) in Eastern Tajikistan: Modeling the effects of climate change. Ecol. Evol. 2018, 8, 5124–5138. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Abedin, J.; Kim, H.-W.; Kundu, S. Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia. Biology 2024, 13, 667. [Google Scholar] [CrossRef]
- Buchhorn, M.; Bertels, L.; Smets, B.; De Roo, B.; Lesiv, M.; Tsendbazar, N.E.; Masiliunas, D.; Li, L. Copernicus Global Land Operations “Vegetation and Energy”: Algorithm Theoretical Basis Document—Moderate Dynamic Land Cover 100 m, Version 3; Copernicus Global Land Service: Barcelona, Spain, 2020. [Google Scholar]
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and workflow management for species habitat modeling. Ecography 2013, 36, 129–135. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A new scenario framework for climate change research: The concept of shared socioeconomic pathways. Clim. Chang. 2014, 122, 387–400. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Andrews, M.B.; Ridley, J.K.; Wood, R.A.; Andrews, T.; Blockley, E.W.; Booth, B.; Burke, E.; Dittus, A.J.; Florek, P.; Gray, L.J.; et al. Historical Simulations with HadGEM3-GC3.1 for CMIP6. J. Adv. Model. Earth Syst. 2020, 12, e2019MS001995. [Google Scholar] [CrossRef]
- Desmet, Q.; Ngo-Duc, T. A novel method for ranking CMIP6 global climate models over the southeast Asian region. Int. J. Climatol. 2022, 42, 97–117. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.-W.; Kang, H.-E.; Kundu, S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology 2024, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Hill, D.J.; Burke, A.M.; Clark, M.; Marchant, R.; Stringer, L.C.; Williams, D.R.; Lyon, C. Projected future climatic forcing on the global distribution of vegetation types. Philos. Trans. R. Soc. B 2024, 379, 20230011. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails. 2012. Available online: https://pubs.usgs.gov/publication/70118102 (accessed on 2 September 2024).
- Lavazza, L.; Morasca, S.; Rotoloni, G. On the reliability of the area under the ROC curve in empirical software engineering. In Proceedings of the International Conference on Evaluation and Assessment in Software Engineering (EASE’23), Oulu, Finland, 14–16 June 2023; ACM: New York, NY, USA, 2023; pp. 93–100. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination capacity in species distribution models depends on the representativeness of the environmental domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC plots: Calibrating species distribution models with presence-only data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Gen. Tech. Rep. PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; Volume 351, 122p. [Google Scholar] [CrossRef]
- Raines, G.L. Description and comparison of geologic maps with FRAGSTATS—A spatial statistics program. Comput. Geosci. 2002, 28, 169–177. [Google Scholar] [CrossRef]
- Midha, N.; Mathur, P.K. Assessment of forest fragmentation in the conservation priority Dudhwa landscape, India using FRAGSTATS computed class level metrics. J. Indian Soc. Remote Sens. 2010, 38, 487–500. [Google Scholar] [CrossRef]
- Hedges, L.; Clements, G.R.; Aziz, S.A.; Yap, W.; Laurance, S.; Goosem, M.; Laurance, W.F. Small carnivore records from a threatened habitat linkage in Terengganu, Peninsula Malaysia. Small Carniv. Conserv. 2013, 49, 9–14. [Google Scholar]
- Dickson, B.G.; Albano, C.M.; Anantharaman, R.; Beier, P.; Fargione, J.; Graves, T.A.; Gray, M.E.; Hall, K.R.; Lawler, J.J.; Leonard, P.B.; et al. Circuit-theory applications to connectivity science and conservation. Conserv. Biol. 2019, 33, 239–249. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Wang, F.; McShea, W.J.; Wang, D.; Li, S.; Zhao, Q.; Wang, H.; Lu, Z. Evaluating Landscape Options for Corridor Restoration between Giant Panda Reserves. PLoS ONE 2014, 9, e105086. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Brook, B.W.; Ng, P.K.L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 2004, 19, 654–660. [Google Scholar] [CrossRef]
- Clements, G.R. The Environmental and Social Impacts of Roads in Southeast Asia. Ph.D. Thesis, James Cook University, Douglas, Australia, 2013. [Google Scholar]
- Haidir, I.A.; Dinata, Y.; Linkie, M.; Macdonald, D.W. Asiatic Golden Cat and Sunda Clouded Leopard Occupancy in the Kerinci Seblat Landscape, West-Central Sumatra. Cat News 2013, 59, 7–10. [Google Scholar]
- Hutajulu, M.B.; Sunarto; Klenzendorf, S.; Supriatna, J.; Budiman, A.; Yahya, A. Study on the Ecological Characteristics of Clouded Leopard in Riau, Sumatra; Hughes, J., Mercer, R., Eds.; Felid Biology and Conservation; WildCRU: Oxford, UK, 2007; p. 122. [Google Scholar]
- Sunarto, S.; Kelly, M.J.; Parakkasi, K.; Klenzendorf, S.; Septayuda, E.; Kurniawan, H. Tigers Need Cover: Multi-Scale Occupancy Study of the Big Cat in Sumatran Forest and Plantation Landscapes. PLoS ONE 2012, 7, e30859. [Google Scholar] [CrossRef]
- Conde, D.A.; Staerk, J.; Colchero, F.; da Silva, R.; Schöley, J.; Baden, H.M.; Jouvet, L.; Fa, J.E.; Syed, H.; Jongejans, E.; et al. Data gaps and opportunities for comparative and conservation biology. Proc. Natl. Acad. Sci. USA 2019, 116, 9658–9664. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, A.; Lo, Y.T.E.; Valdes, P.J.; Buzan, J.R.; Mills, B.J.W.; Merdith, A.S.; Scotese, C.R.; Wakeford, H.R. Climate extremes likely to drive land mammal extinction during next supercontinent assembly. Nat. Geosci. 2023, 16, 901–908. [Google Scholar] [CrossRef]
- Kaszta, Ż.; Cushman, S.A.; Htun, S.; Naing, H.; Burnham, D.; Macdonald, D.W. Simulating the impact of Belt and Road Initiative and other major developments in Myanmar on an ambassador felid, the clouded leopard (Neofelis nebulosa). Landsc. Ecol. 2020, 35, 727–746. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Wu, W.; Liu, J. Global Forest fragmentation change from 2000 to 2020. Nat. Commun. 2023, 14, 3752. [Google Scholar] [CrossRef]
- Tan, C.K.W.; Rocha, D.G.; Clements, G.R.; Brenes-Mora, E.; Hedges, L.; Kawanishi, K.; Mohamad, S.W.; Mark Rayan, D.; Bolongon, G.; Moore, J.; et al. Habitat use and predicted range for the mainland clouded leopard Neofelis nebulosa in Peninsular Malaysia. Biol. Conserv. 2017, 206, 65–74. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J. Species Distribution Modeling and Ecological Niche Modeling: Getting the Concepts Right. Braz. J. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Estoque, R.C.; Ooba, M.; Avitabile, V.; Hijioka, Y.; DasGupta, R.; Togawa, T.; Murayama, Y. The future of Southeast Asia’s forests. Nat. Commun. 2019, 10, 1829. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E. Forest gains and losses in Southeast Asia over 27 years: The slow convergence towards reforestation. For. Policy Econ. 2021, 122, 102332. [Google Scholar] [CrossRef]
- Gray, T.N.E.; Billingsley, A.; Crudge, B.; Prechette, J.; Grosu, R.; Herranz-Muñoz, V.; Holden, H.; Keo, O.; Kong, K.; Macdonald, D.; et al. Status and conservation significance of ground-dwelling mammals in the Cardamom Rainforest Landscape, southwestern Cambodia. Cambodian J. Nat. Hist. 2017, 1, 38–48. [Google Scholar]
- Yuan, J.; Wang, G.; Zhao, L.; Kitchener, A.C.; Sun, T.; Chen, W.; Huang, C.; Wang, C.; Xu, X.; Wang, J.; et al. How genomic insights into the evolutionary history of clouded leopards inform their conservation. Sci. Adv. 2023, 9, eadh9143. [Google Scholar] [CrossRef]


| Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|
| BRT | Train | 0.972 | 0.125 | 92.9 | 0.856 | 0.853 | 0.932 | 0.924 |
| CV | 0.847 | 75.7 | 0.51 | 0.505 | 0.777 | 0.733 | ||
| GLM | Train | 0.925 | 0.093 | 84 | 0.679 | 0.672 | 0.842 | 0.837 |
| CV | 0.832 | 74.2 | 0.477 | 0.474 | 0.775 | 0.702 | ||
| MARS | Train | 0.909 | 0.105 | 83.6 | 0.672 | 0.664 | 0.835 | 0.837 |
| CV | 0.804 | 72.8 | 0.465 | 0.452 | 0.73 | 0.735 | ||
| MaxEnt | Train | 0.95 | 0.109 | 88.8 | 0.778 | 0.771 | 0.886 | 0.891 |
| CV | 0.841 | 78.4 | 0.565 | 0.558 | 0.809 | 0.755 | ||
| RF | Train | 0.866 | 0.016 | 80 | 0.598 | 0.591 | 0.805 | 0.793 |
| CV | 0.882 | 77 | 0.531 | 0.526 | 0.797 | 0.733 |
| Predictor | Abbreviations | BRT | GLM | MARS | MAXENT | RF | Mean | Mean % |
|---|---|---|---|---|---|---|---|---|
| Precipitation Seasonality (Coefficient of Variation) | bio_15 | 0.030 | 0.000 | 0.116 | 0.029 | 0.000 | 0.035 | 8.43 |
| Precipitation of Wettest Quarter | bio_16 | 0.021 | 0.000 | 0.065 | 0.010 | 0.023 | 0.024 | 5.75 |
| Precipitation of Warmest Quarter | bio_18 | 0.000 | 0.110 | 0.037 | 0.005 | 0.000 | 0.030 | 7.33 |
| Precipitation of Coldest Quarter | bio_19 | 0.024 | 0.000 | 0.000 | 0.037 | 0.007 | 0.014 | 3.28 |
| Mean Diurnal Range (Mean of monthly (max temp—min temp)) | bio_2 | 0.029 | 0.113 | 0.023 | 0.058 | 0.000 | 0.045 | 10.70 |
| Temperature Annual Range | bio_7 | 0.145 | 0.267 | 0.085 | 0.258 | 0.023 | 0.155 | 37.37 |
| Built-up | builtup | 0.000 | 0.040 | 0.000 | 0.019 | 0.000 | 0.012 | 2.87 |
| Cropland | cropland | 0.037 | 0.067 | 0.044 | 0.023 | 0.000 | 0.034 | 8.19 |
| Elevation | elevation | 0.000 | 0.000 | 0.000 | 0.039 | 0.000 | 0.008 | 1.87 |
| Evergreen Forests | evergreen_for | 0.000 | 0.092 | 0.000 | 0.070 | 0.040 | 0.041 | 9.74 |
| Mixed/Deciduous Forest | mixed_for | 0.000 | 0.013 | 0.000 | 0.021 | 0.010 | 0.009 | 2.10 |
| Slope | slope | 0.008 | 0.000 | 0.000 | 0.040 | 0.001 | 0.010 | 2.35 |
| Scenario | Overall Range | Extant | Possibly Extant | Extinct | Possibly Extinct | Protected Areas |
|---|---|---|---|---|---|---|
| Present | 93,353 | 44,033 | 20,034 | 14,022 | 15,264 | 25,614 |
| SSP 245 (2041–2060) | 68,171 | 37,706 | 7349 | 11,008 | 12,110 | 24,327 |
| SSP 245 (2061–2080) | 61,146 | 33,804 | 6107 | 10,198 | 11,038 | 22,352 |
| SSP 585 (2041–2060) | 61,666 | 36,200 | 4599 | 10,245 | 10,623 | 23,576 |
| SSP 585 (2061–2080) | 54,968 | 31,830 | 3841 | 9732 | 9565 | 21,217 |
| Country | Present | SSP 245 (2041–2060) | GR of SSP 245 (2041–2060) from Present | SSP 245 (2061–2080) | GR of SSP 245 (2061–2080) from Present | SSP 585 (2041–2060) | GR of SSP 585 (2041–2060) from Present | SSP 585 (2061–2080) | GR of SSP 585 (2061–2080) from Present |
|---|---|---|---|---|---|---|---|---|---|
| Malaysia | +0.743 | +0.672 | −9.54 | +0.662 | −10.88 | +0.659 | −11.25 | +0.649 | −12.59 |
| Laos | +0.493 | +0.352 | −28.62 | +0.342 | −30.68 | +0.323 | −34.47 | +0.315 | −36.10 |
| India | +0.488 | +0.261 | −46.43 | +0.251 | −48.53 | +0.234 | −52.02 | +0.214 | −56.12 |
| Cambodia | +0.410 | +0.330 | −19.44 | +0.321 | −21.70 | +0.291 | −29.03 | +0.276 | −32.69 |
| Nepal | +0.404 | +0.340 | −15.83 | +0.330 | −18.31 | +0.311 | −23.07 | +0.299 | −26.04 |
| Vietnam | +0.365 | +0.279 | −23.44 | +0.268 | −26.46 | +0.259 | −29.05 | +0.243 | −33.43 |
| Myanmar | +0.343 | +0.232 | −32.42 | +0.222 | −35.35 | +0.201 | −41.33 | +0.199 | −41.91 |
| Thailand | +0.317 | +0.259 | −18.51 | +0.249 | −21.66 | +0.241 | −24.05 | +0.226 | −28.78 |
| Bhutan | +0.273 | +0.232 | −14.93 | +0.222 | −18.59 | +0.210 | −23.14 | +0.189 | −30.83 |
| Bangladesh | +0.242 | +0.215 | −11.35 | +0.205 | −15.48 | +0.195 | −19.46 | +0.188 | −22.35 |
| China | +0.098 | +0.093 | −5.39 | +0.092 | −6.42 | +0.089 | −9.15 | +0.083 | −15.27 |
| Scenario | NP | PD | LPI | TE | LSI | AI |
|---|---|---|---|---|---|---|
| Present | 468 | 993,511 | 2.767 | 640.794 | 26.4291 | 82.1631 |
| SSP 245 (2041–2060) | 429 | 913,023 | 2.420 | 525.378 | 24.5273 | 70.399 |
| SSP 245 (2061–2080) | 385 | 819,379 | 2.200 | 477.288 | 23.4691 | 66.2725 |
| SSP 585 (2041–2060) | 378 | 804,481 | 2.349 | 506.31 | 23.8346 | 62.7976 |
| Corridors | Present | SSP 245 (2041–2060) | GR of SSP 245 (2041–2060) from Present | SSP 245 (2061–2080) | GR of SSP 245 (2061–2080) from Present | SSP 585 (2041–2060) | GR of SSP 585 (2041–2060) from Present | SSP 585 (2061–2080) | GR of SSP 585 (2061–2080) from Present |
|---|---|---|---|---|---|---|---|---|---|
| BHU_IND | +2.441 | +2.063 | −15.48 | +1.901 | −22.10 | +1.802 | −26.16 | +1.662 | −31.89 |
| NEP_IND | +2.114 | +1.901 | −10.09 | +1.719 | −18.67 | +1.600 | −24.33 | +1.490 | −29.53 |
| MYA_CHI | +1.140 | +0.996 | −12.60 | +0.886 | −22.27 | +0.825 | −27.62 | +0.801 | −29.73 |
| NEP_CHI | +1.044 | +0.767 | −26.48 | +0.657 | −37.02 | +0.644 | −38.30 | +0.621 | −40.51 |
| IND_BAN | +1.041 | +0.909 | −12.76 | +0.709 | −31.96 | +0.689 | −33.88 | +0.656 | −37.01 |
| CHI_LAO | +0.938 | +0.797 | −15.01 | +0.697 | −25.67 | +0.657 | −29.97 | +0.622 | −33.70 |
| MYA_LAO | +0.881 | +0.548 | −37.88 | +0.491 | −44.35 | +0.455 | −48.37 | +0.423 | −52.00 |
| MYA_THA | +0.754 | +0.593 | −21.32 | +0.489 | −35.16 | +0.421 | −44.18 | +0.409 | −45.77 |
| BHU_CHI | +0.614 | +0.375 | −39.00 | +0.289 | −52.93 | +0.269 | −56.19 | +0.235 | −61.73 |
| LAO_VIET | +0.612 | +0.420 | −31.43 | +0.400 | −34.64 | +0.370 | −39.54 | +0.343 | −43.95 |
| BAN_MYA | +0.545 | +0.397 | −27.16 | +0.376 | −31.05 | +0.343 | −37.10 | +0.321 | −41.14 |
| LAO_CAM | +0.446 | +0.356 | −20.15 | +0.333 | −25.31 | +0.314 | −29.57 | +0.301 | −32.49 |
| CHI_VIET | +0.347 | +0.275 | −20.77 | +0.234 | −32.51 | +0.212 | −38.86 | +0.198 | −42.90 |
| THA_LAO | +0.343 | +0.231 | −32.59 | +0.211 | −38.53 | +0.199 | −42.02 | +0.177 | −48.43 |
| IND_CHI | +0.339 | +0.233 | −31.25 | +0.209 | −38.26 | +0.189 | −44.17 | +0.156 | −53.92 |
| LAO_VIET | +0.302 | +0.209 | −30.82 | +0.190 | −37.10 | +0.170 | −43.72 | +0.140 | −53.65 |
| THA_CAM | +0.266 | +0.170 | −36.04 | +0.160 | −39.76 | +0.140 | −47.29 | +0.120 | −54.82 |
| THA_MAL | +0.253 | +0.176 | −30.31 | +0.157 | −37.89 | +0.136 | −46.20 | +0.122 | −51.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Singha, H.; Kang, H.-E.; Kim, H.-W.; Kundu, S. Forecasting Suitable Habitats of the Clouded Leopard (Neofelis nebulosa) in Asia: Insights into the Present and Future Climate Projections Within and Beyond Extant Boundaries. Biology 2024, 13, 902. https://doi.org/10.3390/biology13110902
Abedin I, Singha H, Kang H-E, Kim H-W, Kundu S. Forecasting Suitable Habitats of the Clouded Leopard (Neofelis nebulosa) in Asia: Insights into the Present and Future Climate Projections Within and Beyond Extant Boundaries. Biology. 2024; 13(11):902. https://doi.org/10.3390/biology13110902
Chicago/Turabian StyleAbedin, Imon, Hilloljyoti Singha, Hye-Eun Kang, Hyun-Woo Kim, and Shantanu Kundu. 2024. "Forecasting Suitable Habitats of the Clouded Leopard (Neofelis nebulosa) in Asia: Insights into the Present and Future Climate Projections Within and Beyond Extant Boundaries" Biology 13, no. 11: 902. https://doi.org/10.3390/biology13110902
APA StyleAbedin, I., Singha, H., Kang, H.-E., Kim, H.-W., & Kundu, S. (2024). Forecasting Suitable Habitats of the Clouded Leopard (Neofelis nebulosa) in Asia: Insights into the Present and Future Climate Projections Within and Beyond Extant Boundaries. Biology, 13(11), 902. https://doi.org/10.3390/biology13110902










