Red Blood Cell Morphology Is Associated with Altered Hemorheological Properties and Fatigue in Patients with Long COVID
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Blood Sampling
2.3. Measurement of Hematological Parameters
2.4. Measurement of RBC Rheological Parameters of Deformability and Aggregation
2.4.1. RBC Deformability
2.4.2. RBC Aggregation
2.4.3. Measurement of Mechanical Sensitivity Index
- con: unsheared first baseline measurement
2.5. Morphological Analyses
2.6. Statistics
3. Results
3.1. Hematological Parameters
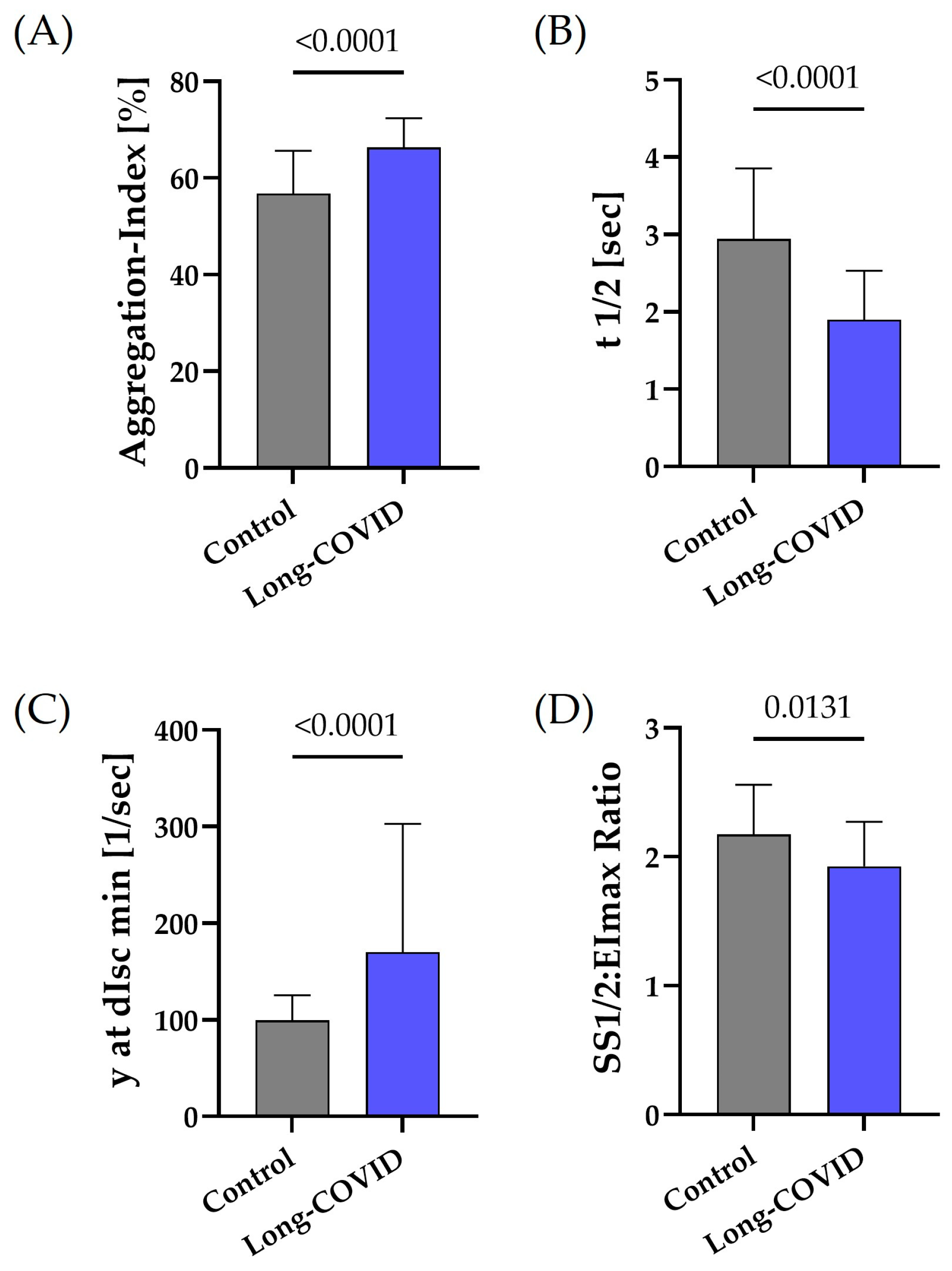
3.2. RBC Aggregation and Deformability
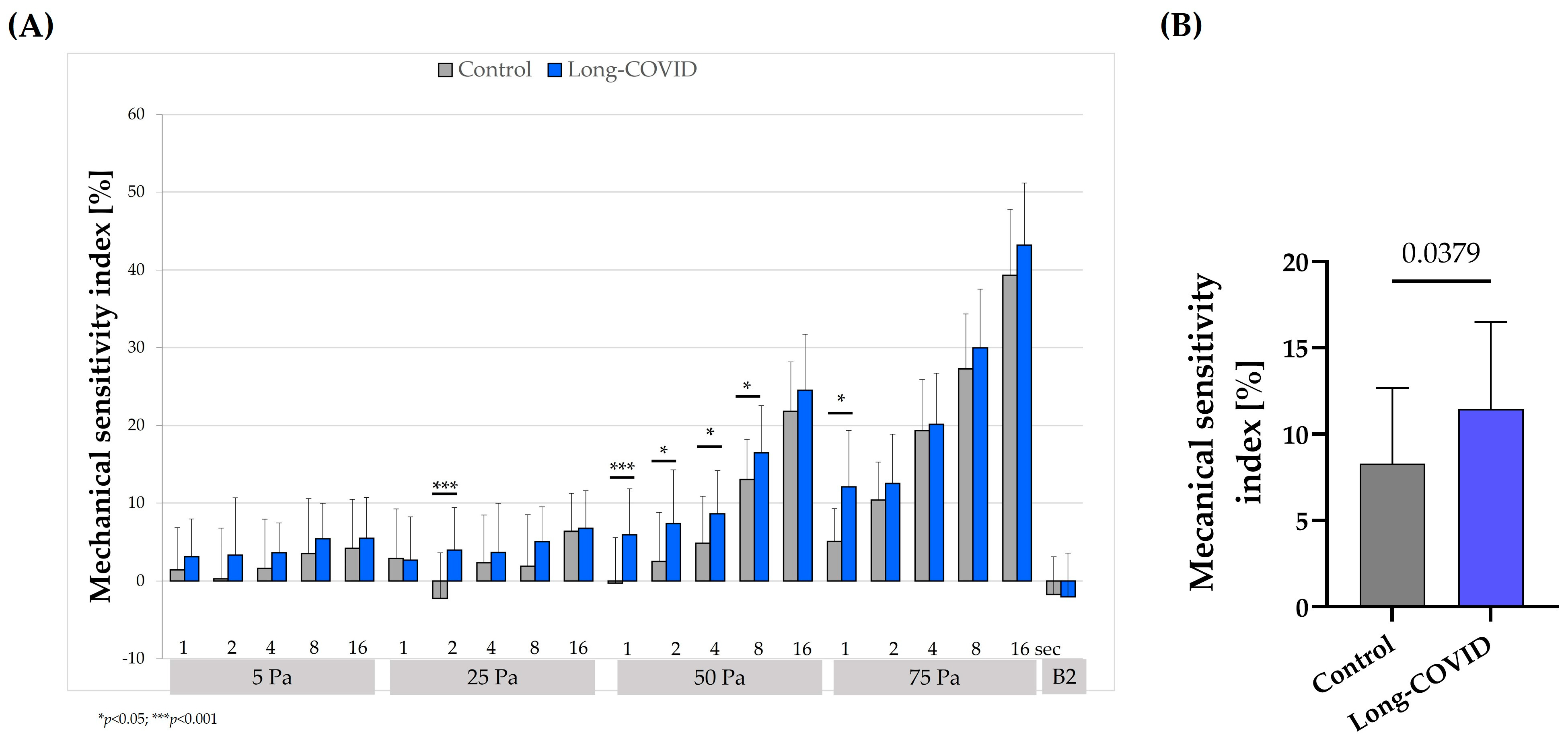
3.3. Mechanical Sensitivity Index
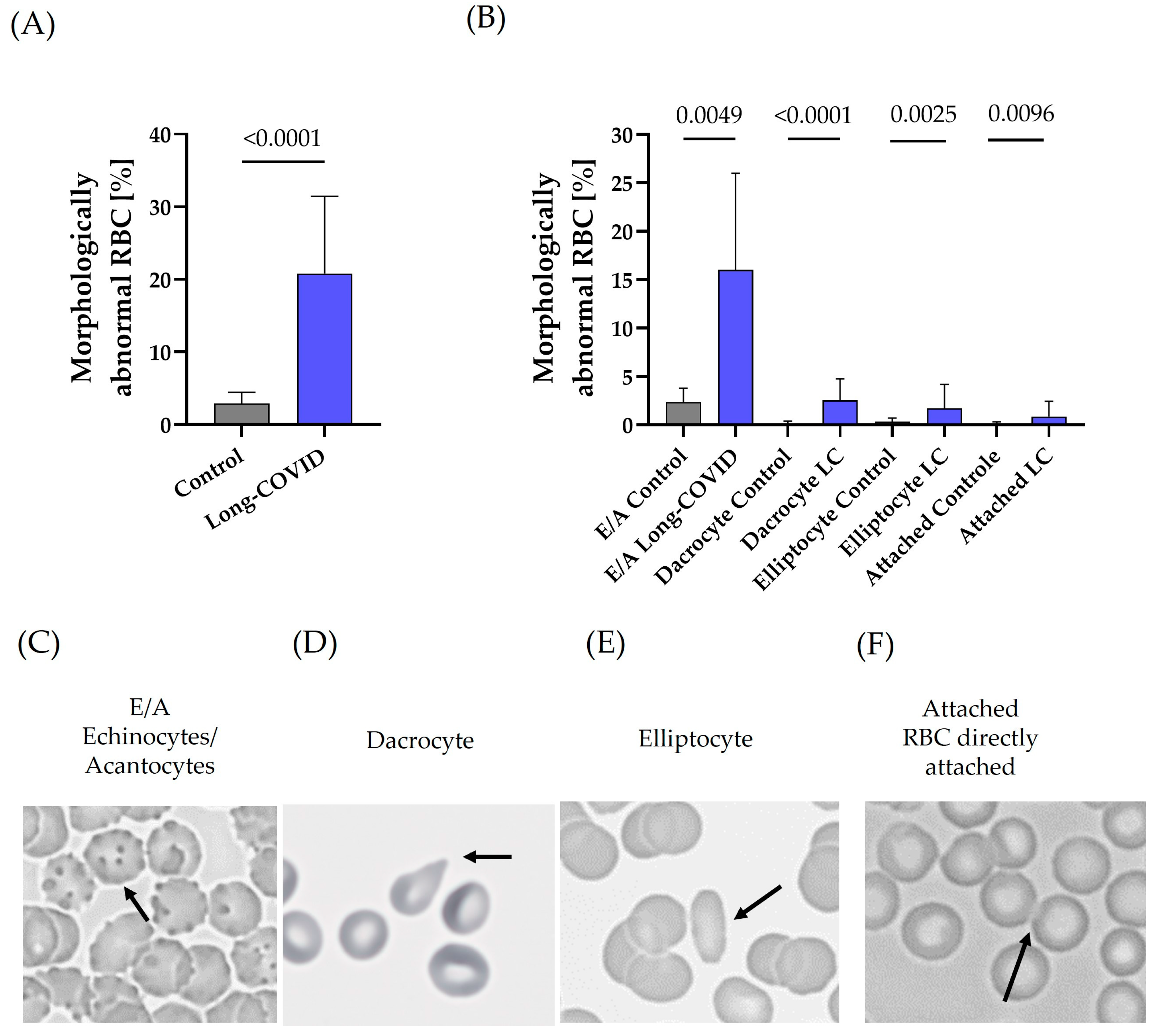
3.4. RBC Morphology
3.5. Contextual Analyses Between FACIT-Fatigue Score and RBC Shape Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Number of COVID-19 Cases Reported to WHO (Cumulative Total). Available online: https://data.who.int/dashboards/covid19/cases (accessed on 14 November 2024).
- RKI. COVID-19 (Coronavirus SARS-CoV-2). Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/nCoV_node.html (accessed on 14 November 2024).
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 14 November 2024).
- Callard, F.; Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence: London, UK, 2020. [Google Scholar]
- Peter, R.S.; Nieters, A.; Kräusslich, H.-G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V. Post-acute sequelae of covid-19 six to 12 months after infection: Population based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef] [PubMed]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef] [PubMed]
- Jangnin, R.; Ritruangroj, W.; Kittisupkajorn, S.; Sukeiam, P.; Inchai, J.; Maneeton, B.; Maneetorn, N.; Chaiard, J.; Theerakittikul, T. Long-COVID Prevalence and Its Association with Health Outcomes in the Post-Vaccine and Antiviral-Availability Era. J. Clin. Med. 2024, 13, 1208. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Espín, E.; Tebbutt, S.J. Association of SARS-CoV-2 infection and persistence with long COVID. Lancet Respir. Med. 2023, 11, 504–506. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Cagnin, S.; Bondi, D.; Santangelo, C.; Marramiero, L.; Purcaro, C.; Bonadio, R.S.; Di Filippo, E.S.; Mancinelli, R.; Fulle, S.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome from current evidence to new diagnostic perspectives through skeletal muscle and metabolic disturbances. Acta Physiol. 2024, 240, e14122. [Google Scholar] [CrossRef]
- Kronstein-Wiedemann, R.; Tausche, K.; Kolditz, M.; Teichert, M.; Thiel, J.; Koschel, D.; Tonn, T.; Künzel, S.R. Long-COVID is Associated with Impaired Red Blood Cell Function. Horm. Metab. Res. 2024, 56, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Böning, D.; Kuebler, W.M.; Vogel, D.; Bloch, W. The oxygen dissociation curve of blood in COVID-19-An update. Front. Med. 2023, 10, 1098547. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.C.; Brummet, M.; Safari, Z.; Wang, Q.; Rowden, T.; Boyer, T.; Doctor, A. COVID-19 impairs oxygen delivery by altering red blood cell hematological, hemorheological, and oxygen transport properties. Front. Physiol. 2023, 14, 1320697. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Ibershoff, L.; Zacher, J.; Bros, J.; Tomschi, F.; Diebold, K.F.; Predel, H.-G.; Bloch, W. Even patients with mild COVID-19 symptoms after SARS-CoV-2 infection show prolonged altered red blood cell morphology and rheological parameters. J. Cell. Mol. Med. 2022, 26, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Marchi, G.; Bozzini, C.; Bertolone, L.; Dima, F.; Busti, F.; Castagna, A.; Stranieri, C.; Fratta Pasini, A.M.; Friso, S.; Lippi, G.; et al. Red Blood Cell Morphologic Abnormalities in Patients Hospitalized for COVID-19. Front. Physiol. 2022, 13, 932013. [Google Scholar] [CrossRef]
- Elahi, S. Hematopoietic responses to SARS-CoV-2 infection. Cell. Mol. Life Sci. 2022, 79, 187. [Google Scholar] [CrossRef]
- Bizjak, D.A.; John, L.; Matits, L.; Uhl, A.; Schulz, S.V.W.; Schellenberg, J.; Peifer, J.; Bloch, W.; Weiß, M.; Grüner, B.; et al. SARS-CoV-2 Altered Hemorheological and Hematological Parameters during One-Month Observation Period in Critically Ill COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 15332. [Google Scholar] [CrossRef]
- Bros, J.; Ibershoff, L.; Zollmann, E.; Zacher, J.; Tomschi, F.; Predel, H.-G.; Bloch, W.; Grau, M. Changes in Hematological and Hemorheological Parameters Following Mild COVID-19: A 4-Month Follow-Up Study. Hematol. Rep. 2023, 15, 543–554. [Google Scholar] [CrossRef]
- Recktenwald, S.M.; Simionato, G.; Lopes, M.G.M.; Gamboni, F.; Dzieciatkowska, M.; Meybohm, P.; Zacharowski, K.; von Knethen, A.; Wagner, C.; Kaestner, L.; et al. Cross-talk between red blood cells and plasma influences blood flow and omics phenotypes in severe COVID-19. Elife 2022, 11, e81316. [Google Scholar] [CrossRef]
- Renoux, C.; Fort, R.; Nader, E.; Boisson, C.; Joly, P.; Stauffer, E.; Robert, M.; Girard, S.; Cibiel, A.; Gauthier, A.; et al. Impact of COVID-19 on red blood cell rheology. Br. J. Haematol. 2021, 192, e108–e111. [Google Scholar] [CrossRef]
- Nader, E.; Nougier, C.; Boisson, C.; Poutrel, S.; Catella, J.; Martin, F.; Charvet, J.; Girard, S.; Havard-Guibert, S.; Martin, M.; et al. Increased blood viscosity and red blood cell aggregation in patients with COVID-19. Am. J. Hematol. 2022, 97, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Connes, P. Morphology and Function of Red Blood Cells in COVID-19 Patients: Current Overview 2023. Life 2024, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Horobin, J.T.; Sabapathy, S.; Simmonds, M.J. Red blood cell tolerance to shear stress above and below the subhemolytic threshold. Biomech. Model. Mechanobiol. 2020, 19, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Montan, I.; Löwe, B.; Cella, D.; Mehnert, A.; Hinz, A. General Population Norms for the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale. Value Health 2018, 21, 1313–1321. [Google Scholar] [CrossRef]
- Hardeman, M.R.; Dobbe, J.C.; Ince, C. The Laser-assisted Optical Rotational Cell Analyzer (LORCA) as red blood cell aggregometer. Clin. Hemorheol. Microcirc. 2001, 25, 1–11. [Google Scholar]
- Baskurt, O.K.; Meiselman, H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin. Hemorheol. Microcirc. 2013, 54, 99–107. [Google Scholar] [CrossRef]
- Tripette, J.; Alexy, T.; Hardy-Dessources, M.-D.; Mougenel, D.; Beltan, E.; Chalabi, T.; Chout, R.; Etienne-Julan, M.; Hue, O.; Meiselman, H.J.; et al. Red blood cell aggregation, aggregate strength and oxygen transport potential of blood are abnormal in both homozygous sickle cell anemia and sickle-hemoglobin C disease. Haematologica 2009, 94, 1060–1065. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol. Transfus. Cell Ther. 2020, 42, 116–117. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B.; Aleman, S.; Bach, K.; Boribong, B.P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. 2023, 24, 1616–1627. [Google Scholar] [CrossRef]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86002. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Lipowsky, H.H. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am. J. Physiol. 1999, 277, H2145-57. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Bosek, M.; Wybranowski, T.; Napiórkowska-Mastalerz, M.; Pyskir, J.; Cyrankiewicz, M.; Pyskir, M.; Pilaczyńska-Cemel, M.; Szołna-Chodór, A.; Wrembel, M.; Kruszewski, S.; et al. The Impact of COVID-19 on Cellular Factors Influencing Red Blood Cell Aggregation Examined in Dextran: Possible Causes and Consequences. Int. J. Mol. Sci. 2023, 24, 4952. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Alexy, T.; Shin, S.; Hardeman, M.R.; Meiselman, H.J. Comparison of three instruments for measuring red blood cell aggregation. Clin. Hemorheol. Microcirc. 2009, 43, 283–298. [Google Scholar] [CrossRef]
- Lazari, D.; Freitas Leal, J.K.; Brock, R.; Bosman, G. The Relationship Between Aggregation and Deformability of Red Blood Cells in Health and Disease. Front. Physiol. 2020, 11, 288. [Google Scholar] [CrossRef]
- Marton, Z.; Kesmarky, G.; Vekasi, J.; Cser, A.; Russai, R.; Horvath, B.; Toth, K. Red blood cell aggregation measurements in whole blood and in fibrinogen solutions by different methods. Clin. Hemorheol. Microcirc. 2001, 24, 75–83. [Google Scholar]
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef]
- Eder, J.; Schumm, L.; Armann, J.P.; Puhan, M.A.; Beuschlein, F.; Kirschbaum, C.; Berner, R.; Toepfner, N. Increased red blood cell deformation in children and adolescents after SARS-CoV-2 infection. Sci. Rep. 2023, 13, 9823. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef]
- Renoux, C.; Faivre, M.; Bessaa, A.; Da Costa, L.; Joly, P.; Gauthier, A.; Connes, P. Impact of surface-area-to-volume ratio, internal viscosity and membrane viscoelasticity on red blood cell deformability measured in isotonic condition. Sci. Rep. 2019, 9, 6771. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Morel, C.M.; De-Simone, S.G. Hematological alterations associated with long COVID-19. Front. Physiol. 2023, 14, 1203472. [Google Scholar] [CrossRef] [PubMed]
- Bazdar, S.; Bloemsma, L.D.; Baalbaki, N.; Blankestijn, J.M.; Cornelissen, M.E.B.; Beijers, R.J.H.C.G.; Sondermeijer, B.M.; van Wijck, Y.; Downward, G.S.; Maitland-van der Zee, A.H. Hemoglobin and Its Relationship with Fatigue in Long-COVID Patients Three to Six Months after SARS-CoV-2 Infection. Biomedicines 2024, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Cervia-Hasler, C.; Brüningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef]
- Kondratov, K.A.; Artamonov, A.A.; Mikhailovskii, V.Y.; Velmiskina, A.A.; Mosenko, S.V.; Grigoryev, E.A.; Anisenkova, A.Y.; Nikitin, Y.V.; Apalko, S.V.; Sushentseva, N.N.; et al. SARS-CoV-2 Impact on Red Blood Cell Morphology. Biomedicines 2023, 11, 2902. [Google Scholar] [CrossRef]
- Meiselman, H.J. Morphological determinants of red cell deformability. Scand. J. Clin. Lab. Investig. Suppl. 1981, 156, 27–34. [Google Scholar] [CrossRef]
- McCully, K.K.; Natelson, B.H. Impaired oxygen delivery to muscle in chronic fatigue syndrome. Clin. Sci. 1999, 97, 611–613. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Ali, N.; El-Sayed Ali, Z. Haemorheology in exercise and training. Sports Med. 2005, 35, 649–670. [Google Scholar] [CrossRef]
- Connes, P.; Simmonds, M.J.; Brun, J.-F.; Baskurt, O.K. Exercise hemorheology: Classical data, recent findings and unresolved issues. Clin. Hemorheol. Microcirc. 2013, 53, 187–199. [Google Scholar] [CrossRef]
| Control | Long-COVID | p-Value | |
|---|---|---|---|
| Number, n | 30 | 30 | |
| Male/female | 18/12 | 19/11 | |
| Age [years] | 55.1 (19.8) | 51.3 (14.9) | 0.20 |
| Height [m] | 1.77 (0.1) | 1.72 (0.1) | 0.02 |
| Weight [kg] | 74.1 (15.1) | 76.2 (16.5) | 0.42 |
| BMI [kg/m2] | 23.4 (3.3) | 25.7 (5.2) | 0.09 |
| Parameter | Control | Long-COVID | p-Value |
|---|---|---|---|
| WBC (×103/μL) | 6.9 (1.9) | 6.5 (1.5) | 0.17 |
| PLT (×103/μL) | 236.1 (63.2) | 254.6 (76.1) | 0.18 |
| RBC (×106/μL) | 4.7 (0.4) | 4.9 (0.6) | 0.44 |
| Hb (g/dL) | 14.2 (1.1) | 14.8 (2.0) | 0.23 |
| Hct (%) | 42.2 (2.9) | 43.5 (5.1) | 0.28 |
| MCV (fL) | 89.3 (3.4) | 89.4 (2.9) | 0.17 |
| MCH (pg) | 30.1 (1.4) | 30.4 (1.5) | 0.08 |
| MCHC (g/dL) | 33.6 (1.1) | 33.9 (0.9) | 0.28 |
| RDW (%) | 12.9 (0.7) | 13.1 (0.7) | 0.14 |
| Ferritin (μg/L) | 63.2 (48.7) | ||
| Fibrinogen (mg/dL) | 305.9 (71.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, M.; Presche, A.; Krüger, A.-L.; Bloch, W.; Haiduk, B. Red Blood Cell Morphology Is Associated with Altered Hemorheological Properties and Fatigue in Patients with Long COVID. Biology 2024, 13, 948. https://doi.org/10.3390/biology13110948
Grau M, Presche A, Krüger A-L, Bloch W, Haiduk B. Red Blood Cell Morphology Is Associated with Altered Hemorheological Properties and Fatigue in Patients with Long COVID. Biology. 2024; 13(11):948. https://doi.org/10.3390/biology13110948
Chicago/Turabian StyleGrau, Marijke, Alena Presche, Anna-Lena Krüger, Wilhelm Bloch, and Björn Haiduk. 2024. "Red Blood Cell Morphology Is Associated with Altered Hemorheological Properties and Fatigue in Patients with Long COVID" Biology 13, no. 11: 948. https://doi.org/10.3390/biology13110948
APA StyleGrau, M., Presche, A., Krüger, A.-L., Bloch, W., & Haiduk, B. (2024). Red Blood Cell Morphology Is Associated with Altered Hemorheological Properties and Fatigue in Patients with Long COVID. Biology, 13(11), 948. https://doi.org/10.3390/biology13110948







