Biobank Digitalization: From Data Acquisition to Efficient Use
Simple Summary
Abstract
1. Introduction
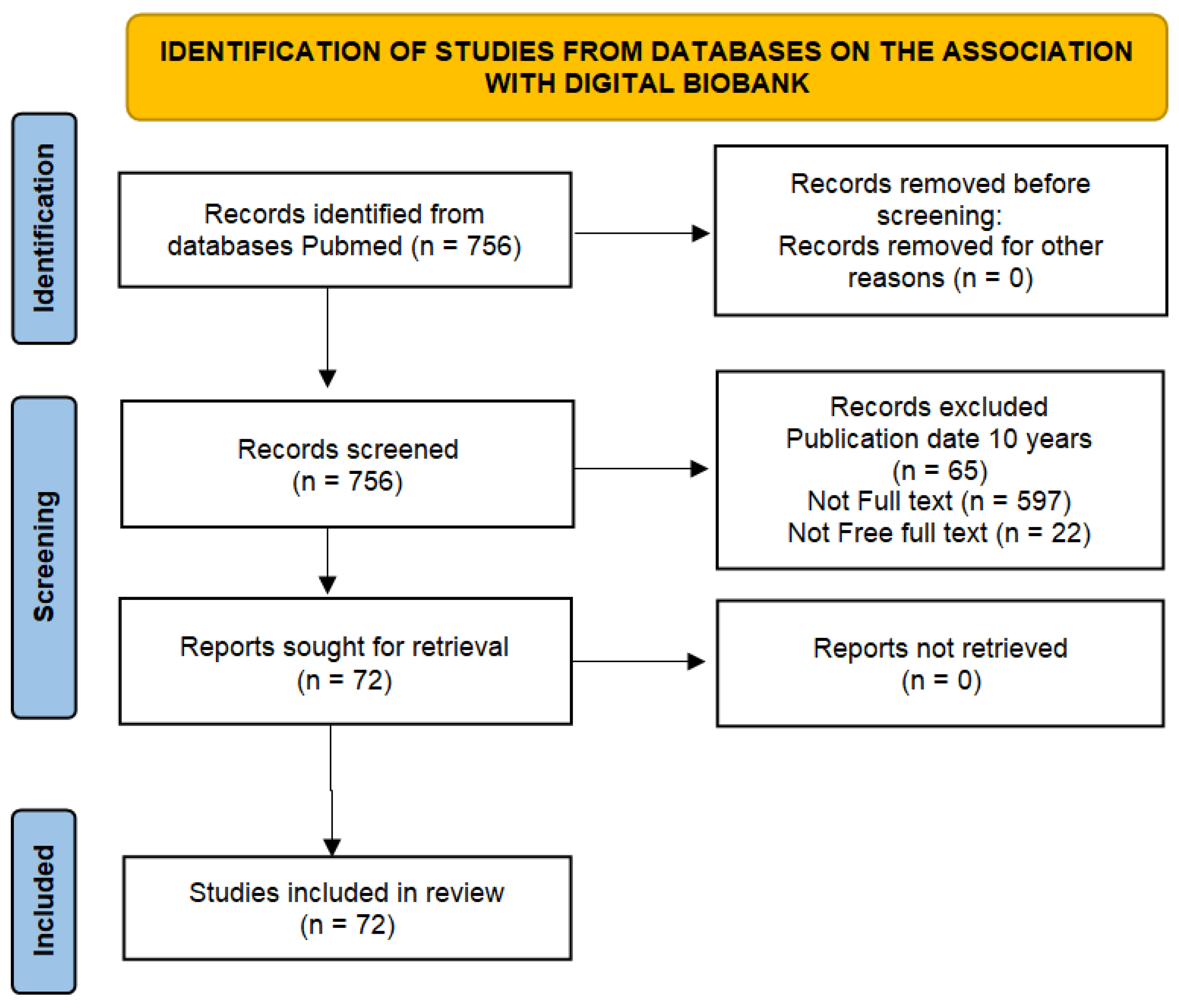
2. Materials and Methods
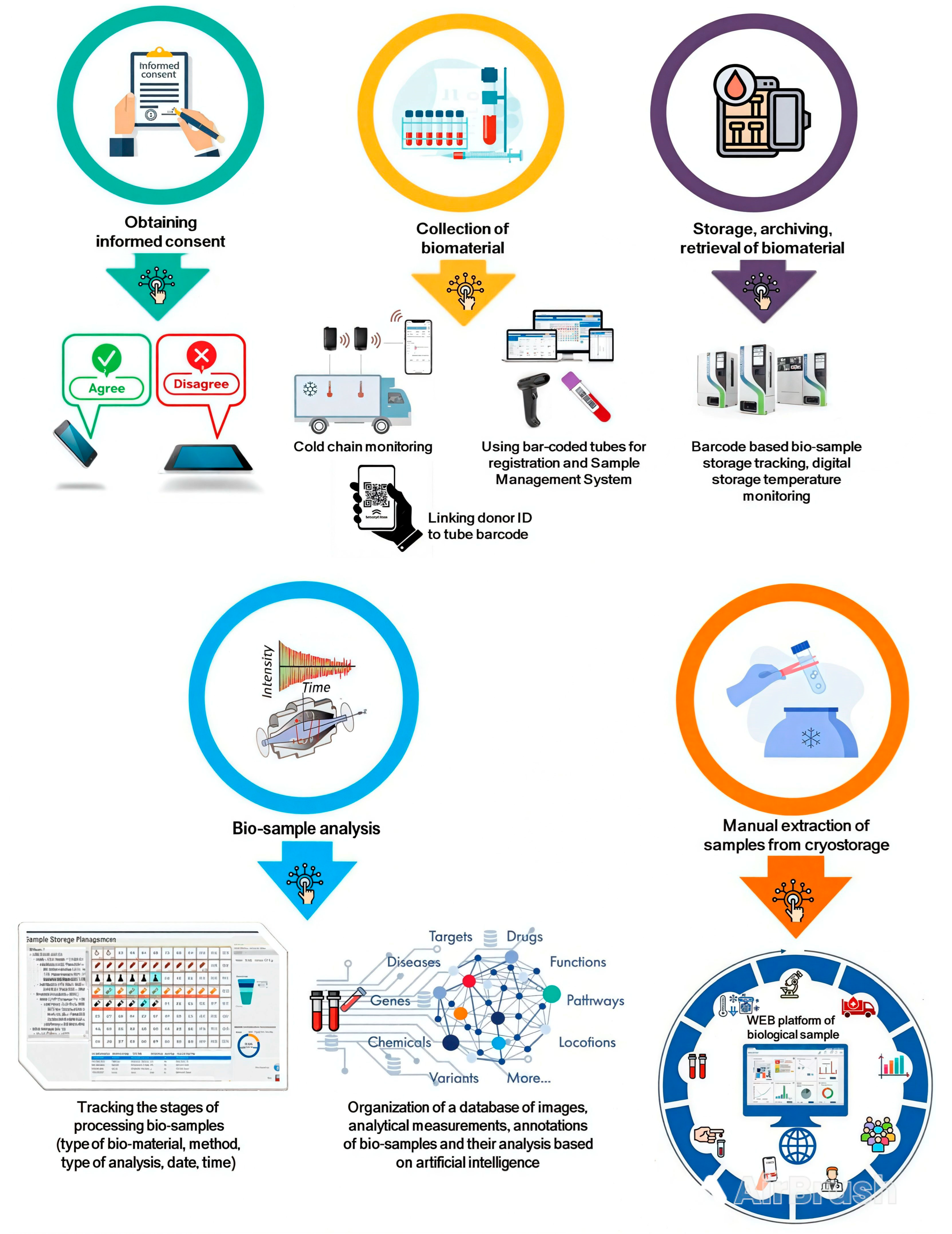
3. Biobanking and Digitalization
- Biomaterial collection;
- Shelf life;
- Pre-analytical processing of a biospecimen;
- Freeze–thaw cycles;
- Biospecimen analysis (analytical stage);
- Disposal [10].
- -
- Significantly simplify biobank management by increasing the speed and reliability of data processing;
- -
- Digitization of information will allow researchers to quickly find and access specific biospecimens;
- -
- Digitization of information will reduce errors that are inevitable when using manual data entry and processing;
- -
- Improve data security (advanced security features and resource access control reduce the risk of unauthorized access to data and adequately protect confidentiality) [12].
3.1. Operating Procedures
- -
- They contain more information and do not require visualization for reading;
- -
- They can be placed inside a transport box or a cryotube as well as inside liquid or tissue samples, thus enabling monitoring of temperature during storage, transportation or during the pre-analytical stage [16].
3.2. Comprehensive Biobank Information Management System
- -
- The Laboratory Information Management System, which is utilized for processing data related to the lifecycle of a biospecimen;
- -
- Hospital information systems for monitoring patient data;
- -
- The system that monitors and controls the temperature and levels of liquid nitrogen. Biobank information management systems can also interact with these software systems and databases. Thus, biobank information management systems are often the subsets of the Laboratory Information Management System repurposed and customized to meet the needs of a biobank [12].
4. Digital Biobanks as a Part of the Global Biobanking Market
5. Problems and Limitations
5.1. Data Confidentiality and Security
5.2. Standardization of Specimen Collection and Storage
5.3. Ethical and Legal Aspects
- Obtaining informed consent from donors and providing them with comprehensive information and a right to withdraw their biological material at any time;
- Ensuring the security of the data stored in digital biobanks to prevent risks of jeopardizing privacy, personal data security, and potential discrimination of donors;
- Ensuring transparency of biobank management and explicit rules for involvement of commercial subjects in research;
- Using the biospecimens exclusively to conduct ethical experiments that aim to benefit humans and society.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Sinitsyna, A.; Izotov, A.; Kaysheva, A. Biobanks—A Platform for Scientific and Biomedical Research. Diagnostics 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Meir, K.; Cohen, Y.; Mee, B.; Gaffney, E. Biobank Networking for Dissemination of Data and Resources: An Overview. J. Biorepository Sci. Appl. Med. 2014, 2, 29–42. [Google Scholar] [CrossRef]
- Capocasa, M.; Anagnostou, P.; D’Abramo, F.; Matteucci, G.; Dominici, V.; Destro Bisol, G.; Rufo, F. Samples and Data Accessibility in Research Biobanks: An Explorative Survey. PeerJ 2016, 4, e1613. [Google Scholar] [CrossRef] [PubMed]
- de Santiago, I.; Polanski, L. Data-Driven Medicine in the Diagnosis and Treatment of Infertility. J. Clin. Med. 2022, 11, 6426. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.; Romagnoli, S. Registers and Biobanks in ICU and Anesthesia. Minerva Anestesiol. 2022, 88, 864–869. [Google Scholar] [CrossRef]
- Eldjarn, G.H.; Ferkingstad, E.; Lund, S.H.; Helgason, H.; Magnusson, O.T.; Gunnarsdottir, K.; Olafsdottir, T.A.; Halldorsson, B.V.; Olason, P.I.; Zink, F.; et al. Large-Scale Plasma Proteomics Comparisons through Genetics and Disease Associations. Nature 2023, 622, 348–358. [Google Scholar] [CrossRef]
- Jacobs, G.; Wolf, A.; Krawczak, M.; Lieb, W. Biobanks in the Era of Digital Medicine. Clin. Pharmacol. Ther. 2018, 103, 761–762. [Google Scholar] [CrossRef]
- Tozzo, P.; Delicati, A.; Marcante, B.; Caenazzo, L. Digital Biobanking and Big Data as a New Research Tool: A Position Paper. Healthcare 2023, 11, 1825. [Google Scholar] [CrossRef]
- Henny, J.; Nadif, R.; Got, S.L.; Lemonnier, S.; Ozguler, A.; Ruiz, F.; Beaumont, K.; Brault, D.; Sandt, E.; Goldberg, M.; et al. The CONSTANCES Cohort Biobank: An Open Tool for Research in Epidemiology and Prevention of Diseases. Front. Public Health 2020, 8, 605133. [Google Scholar] [CrossRef]
- Baber, R.; Kiehntopf, M. Automation in Biobanking from a Laboratory Medicine Perspective. J. Lab. Med. 2019, 43, 329–338. [Google Scholar] [CrossRef]
- Lippi, G.; Betsou, F.; Cadamuro, J.; Cornes, M.; Fleischhacker, M.; Fruekilde, P.; Neumaier, M.; Nybo, M.; Padoan, A.; Plebani, M.; et al. Preanalytical Challenges—Time for Solutions. Clin. Chem. Lab. Med. 2019, 57, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Elkhwsky, F.; Kazem, A.; Arafat, W.; Diaa, N.; Nabil, A.; El-Tahan, R.; Maher, A.; Saied, S. Biobank Digitization in Low-Middle Income Countries (LMICs): Current and Future Technological Developments. In Digitalization of Medicine in Low- and Middle-Income Countries; Kozlakidis, Z., Muradyan, A., Sargsyan, K., Eds.; Sustainable Development Goals Series; Springer International Publishing: Cham, Switzerland, 2024; pp. 195–205. ISBN 978-3-031-62331-8. [Google Scholar]
- Niedermair, T. Digitization in Biobanking: Where to Find It and What Can We Expect? Trillium Pathol. 2024, 3, 8–11. [Google Scholar] [CrossRef]
- Appenzeller, A.; Hornung, M.; Kadow, T.; Krempel, E.; Beyerer, J. Sovereign Digital Consent through Privacy Impact Quantification and Dynamic Consent. Technologies 2022, 10, 35. [Google Scholar] [CrossRef]
- Haas, M.A.; Teare, H.; Prictor, M.; Ceregra, G.; Vidgen, M.E.; Bunker, D.; Kaye, J.; Boughtwood, T. “CTRL”: An Online, Dynamic Consent and Participant Engagement Platform Working towards Solving the Complexities of Consent in Genomic Research. Eur. J. Hum. Genet. 2021, 29, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.J.; Andrechak, G.; Riben, M.; Yong, W.H. A Review of Radio Frequency Identification Technology for the Anatomic Pathology or Biorepository Laboratory: Much Promise, Some Progress, and More Work Needed. J. Pathol. Inform. 2011, 2, 34. [Google Scholar] [CrossRef]
- Powell, S.; Molinolo, A.; Masmila, E.; Kaushal, S. Real-Time Temperature Mapping in Ultra-Low Freezers as a Standard Quality Assessment. Biopreserv. Biobank. 2019, 17, 139–142. [Google Scholar] [CrossRef]
- Babel, M.; Mamilos, A.; Seitz, S.; Niedermair, T.; Weber, F.; Anzeneder, T.; Ortmann, O.; Dietmaier, W.; Brochhausen, C. Compared DNA and RNA Quality of Breast Cancer Biobanking Samples after Long-Term Storage Protocols in −80 °C and Liquid Nitrogen. Sci. Rep. 2020, 10, 14404. [Google Scholar] [CrossRef]
- Germann, A.; Oh, Y.-J.; Schmidt, T.; Schön, U.; Zimmermann, H.; Von Briesen, H. Temperature Fluctuations during Deep Temperature Cryopreservation Reduce PBMC Recovery, Viability and T-Cell Function. Cryobiology 2013, 67, 193–200. [Google Scholar] [CrossRef]
- Ackerman, E.; Strickland, E. Medical Delivery Drones Take Flight in East Africa. IEEE Spectr. 2018, 55, 34–35. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Schönmehl, R.; Artinger, A.; Winter, L.; Böck, H.; Schreml, S.; Gürtler, F.; Daza, J.; Schmitt, V.H.; Mamilos, A.; et al. 3D Visualization, Skeletonization and Branching Analysis of Blood Vessels in Angiogenesis. Int. J. Mol. Sci. 2023, 24, 7714. [Google Scholar] [CrossRef]
- Aeffner, F.; Zarella, M.D.; Buchbinder, N.; Bui, M.M.; Goodman, M.R.; Hartman, D.J.; Lujan, G.M.; Molani, M.A.; Parwani, A.V.; Lillard, K.; et al. Introduction to Digital Image Analysis in Whole-Slide Imaging: A White Paper from the Digital Pathology Association. J. Pathol. Inform. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Zattoni, L.; Fusco, N. Biobanking in the Digital Pathology Era. Oncol. Res. 2021, 29, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.H.; Wilson-McManus, J.E.; Barnes, R.O.; Giesz, S.C.; Png, A.; Hegele, R.G.; Brinkman, J.N.; Mackenzie, I.R.; Huntsman, D.G.; Junker, A.; et al. Evolutionary Concepts in Biobanking—The BC BioLibrary. J. Transl. Med. 2009, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Grimson, J. Delivering the Electronic Healthcare Record for the 21st Century. Int. J. Med. Inform. 2001, 64, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Yuille, M.; Dixon, K.; Platt, A.; Pullum, S.; Lewis, D.; Hall, A.; Ollier, W. The UK DNA Banking Network: A “Fair Access” Biobank. Cell Tissue Bank. 2010, 11, 241–251. [Google Scholar] [CrossRef]
- Tassé, A.M.; Budin-Ljøsne, I.; Knoppers, B.M.; Harris, J.R. Retrospective Access to Data: The ENGAGE Consent Experience. Eur. J. Hum. Genet. 2010, 18, 741–745. [Google Scholar] [CrossRef]
- Im, K.; Gui, D.; Yong, W.H. An Introduction to Hardware, Software, and Other Information Technology Needs of Biomedical Biobanks. In Biobanking; Yong, W.H., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1897, pp. 17–29. ISBN 978-1-4939-8933-1. [Google Scholar]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef]
- Alkhatib, R.; Gaede, K.I. Data Management in Biobanking: Strategies, Challenges, and Future Directions. BioTech 2024, 13, 34. [Google Scholar] [CrossRef]
- Heyder, R.; NUM Coordination Office; Kroemer, H.K.; Wiedmann, S.; Pley, C.; Heyer, C.; Heuschmann, P.; Vehreschild, J.J.; Krefting, D.; Illig, T.; et al. Das Netzwerk Universitätsmedizin: Technisch-organisatorische Ansätze für Forschungsdatenplattformen. Bundesgesundheitsbl 2023, 66, 114–125. [Google Scholar] [CrossRef]
- Anisimov, S.V.; Meshkov, A.N.; Glotov, A.S.; Borisova, A.L.; Balanovsky, O.P.; Belyaev, V.E.; Granstrem, O.K.; Grivtsova, L.Y.; Efimenko, A.Y.; Pokrovskaya, M.S.; et al. National Association of Biobanks and Biobanking Specialists: New Community for Promoting Biobanking Ideas and Projects in Russia. Biopreserv. Biobank. 2021, 19, 73–82. [Google Scholar] [CrossRef]
- Seidler, D.; Karlíková, M.; Topolčan, O.; Snítilá, M.; Niedermair, T.; Brochhausen, C. Establishing Biobanking in Medical Curricula—The Education Program “Precision Medicine International” (eduBRoTHER). Biopreserv. Biobank. 2023, 21, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.; Catchpoole, D.R.; Reaiche-Miller, G.; Gilbert, T.; Ng, W.; Watson, P.H.; Byrne, J.A. What Do Biomedical Researchers Want from Biobanks? Results of an Online Survey. Biopreserv. Biobank. 2022, 20, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Courtot, M.; Gupta, D.; Liyanage, I.; Xu, F.; Burdett, T. BioSamples Database: FAIRer Samples Metadata to Accelerate Research Data Management. Nucleic Acids Res. 2022, 50, D1500–D1507. [Google Scholar] [CrossRef] [PubMed]
- Asiimwe, R.; Lam, S.; Leung, S.; Wang, S.; Wan, R.; Tinker, A.; McAlpine, J.N.; Woo, M.M.M.; Huntsman, D.G.; Talhouk, A. From Biobank and Data Silos into a Data Commons: Convergence to Support Translational Medicine. J. Transl. Med. 2021, 19, 493. [Google Scholar] [CrossRef] [PubMed]
- Eklund, N.; Andrianarisoa, N.H.; van Enckevort, E.; Anton, G.; Debucquoy, A.; Müller, H.; Zaharenko, L.; Engels, C.; Ebert, L.; Neumann, M.; et al. Extending the Minimum Information About BIobank Data Sharing Terminology to Describe Samples, Sample Donors, and Events. Biopreserv. Biobank. 2020, 18, 155–164. [Google Scholar] [CrossRef]
- Mallappallil, M.; Sabu, J.; Gruessner, A.; Salifu, M. A Review of Big Data and Medical Research. SAGE Open Med. 2020, 8, 2050312120934839. [Google Scholar] [CrossRef]
- UK Prostate Cancer Sample Collection Database—Virtual Biobank. Available online: https://prostatedatabase.org.uk/ (accessed on 3 July 2024).
- FIND VBD—Index. Available online: https://vbd.finddx.org/ (accessed on 25 June 2024).
- APPRISE Virtual Biobank—BioGrid. Available online: https://www.biogrid.org.au/apprise (accessed on 5 October 2024).
- BioIVT. Available online: https://bioivt.com/ (accessed on 3 July 2024).
- Web Repository “BioVitrina”. Available online: https://enbb.ibmc.msk.ru/Main/Home (accessed on 11 November 2024).
- Home—BBMRI-ERIC. Available online: https://www.bbmri-eric.eu/ (accessed on 3 July 2024).
- UK Biobank—UK Biobank. Available online: https://www.ukbiobank.ac.uk/ (accessed on 3 July 2024).
- iSpecimen Marketplace. Available online: https://www.ispecimen.com/ispecimen-marketplace/ (accessed on 11 November 2024).
- German Biobank Node. Available online: https://www.bbmri.de/ (accessed on 11 November 2024).
- Engels, C.; Kern, J.; Dudová, Z.; Deppenwiese, N.; Kiel, A.; Kroll, B.; Kussel, T.; Schüttler, C.; Tomášik, R.; Hummel, M.; et al. The Sample Locator: A Federated Search Tool for Biosamples and Associated Data in Europe Using HL7 FHIR. Comput. Biol. Med. 2024, 180, 108941. [Google Scholar] [CrossRef]
- van Draanen, J.; Davidson, P.; Bour-Jordan, H.; Bowman-Carpio, L.; Boyle, D.; Dubinett, S.; Gardner, B.; Gardner, J.; McFall, C.; Mercola, D.; et al. Assessing Researcher Needs for a Virtual Biobank. Biopreserv. Biobank. 2017, 15, 203–210. [Google Scholar] [CrossRef]
- Snapes, E.; Astrin, J.J.; Bertheussen Krüger, N.; Grossman, G.H.; Hendrickson, E.; Miller, N.; Seiler, C. Updating International Society for Biological and Environmental Repositories Best Practices, Fifth Edition: A New Process for Relevance in an Evolving Landscape. Biopreserv. Biobank. 2023, 21, 537–546. [Google Scholar] [CrossRef]
- GDPR in Biobanking for Precision Medicine Research: The Challenges. Available online: https://www.openaccessgovernment.org/gdpr-in-biobanking-for-precision-medicine/54468/ (accessed on 5 October 2024).
- Watson, P.H.; Nussbeck, S.Y.; Carter, C.; O’Donoghue, S.; Cheah, S.; Matzke, L.A.M.; Barnes, R.O.; Bartlett, J.; Carpenter, J.; Grizzle, W.E.; et al. A Framework for Biobank Sustainability. Biopreserv. Biobank. 2014, 12, 60–68. [Google Scholar] [CrossRef]
- Zhou, G.; Xia, J. OmicsNet: A Web-Based Tool for Creation and Visual Analysis of Biological Networks in 3D Space. Nucleic Acids Res. 2018, 46, W514–W522. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chatterjee, M.K. Data Sharing Solutions for Biobanks for the COVID-19 Pandemic. Biopreserv. Biobank. 2020, 18, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Chassang, G.; Marelli, L. The Impact of the GDPR on the Governance of Biobank Research. In GDPR and Biobanking: Individual Rights, Public Interest and Research Regulation Across Europe; Slokenberga, S., Tzortzatou, O., Reichel, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 45–60. ISBN 978-3-030-49388-2. [Google Scholar]
- Rychnovská, D. Anticipatory Governance in Biobanking: Security and Risk Management in Digital Health. Sci. Eng. Ethics 2021, 27, 30. [Google Scholar] [CrossRef]
- Mamo, N.; Martin, G.M.; Desira, M.; Ellul, B.; Ebejer, J.-P. Dwarna: A Blockchain Solution for Dynamic Consent in Biobanking. Eur. J. Hum. Genet. 2020, 28, 609–626. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Pham, Q.-V.; Pathirana, P.N.; Ding, M.; Seneviratne, A.; Lin, Z.; Dobre, O.; Hwang, W.-J. Federated Learning for Smart Healthcare: A Survey. ACM Comput. Surv. 2023, 55, 60. [Google Scholar] [CrossRef]
- Pietrzykowski, T.; Smilowska, K. The Reality of Informed Consent: Empirical Studies on Patient Comprehension-Systematic Review. Trials 2021, 22, 57. [Google Scholar] [CrossRef]
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic Principles of Biobanking: From Biological Samples to Precision Medicine for Patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef]
- De Blasio, P.; Biunno, I. New Challenges for Biobanks: Accreditation to the New ISO 20387:2018 Standard Specific for Biobanks. BioTech 2021, 10, 13. [Google Scholar] [CrossRef]
- Gao, F.; Tao, L.; Ma, X.; Lewandowski, D.; Shu, Z. A Study of Policies and Guidelines for Collecting, Processing, and Storing Coronavirus Disease 2019 Patient Biospecimens for Biobanking and Research. Biopreserv. Biobank. 2020, 18, 511–516. [Google Scholar] [CrossRef]
- Bendou, H.; Sizani, L.; Reid, T.; Swanepoel, C.; Ademuyiwa, T.; Merino-Martinez, R.; Meuller, H.; Abayomi, A.; Christoffels, A. Baobab Laboratory Information Management System: Development of an Open-Source Laboratory Information Management System for Biobanking. Biopreserv. Biobank. 2017, 15, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Kinkorová, J.; Topolčan, O. Biobanks in the Era of Big Data: Objectives, Challenges, Perspectives, and Innovations for Predictive, Preventive, and Personalised Medicine. EPMA J. 2020, 11, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.X.; Wolf, S.M.; Bhavnani, S.; Deoni, S.; Elison, J.T.; Fair, D.; Garwood, M.; Gee, M.S.; Geethanath, S.; Kay, K.; et al. Emerging Ethical Issues Raised by Highly Portable MRI Research in Remote and Resource-Limited International Settings. Neuroimage 2021, 238, 118210. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.; Sims, J.; Gander, A.; Garibaldi, J.M.; Fuller, B.; Davidson, B.; Quinlan, P.R. The Barriers and Motivators to Using Human Tissues for Research: The Views of UK-Based Biomedical Researchers. Biopreserv. Biobank. 2020, 18, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, S. Human Biobanking in Developed and Developing Countries: An Ethico-Legal Comparative Analysis of the Frameworks in the United Kingdom, Australia, Uganda, and South Africa. Camb. Q. Healthc. Ethics 2021, 30, 146–160. [Google Scholar] [CrossRef]
- Tzortzatou-Nanopoulou, O.; Akyüz, K.; Goisauf, M.; Kozera, Ł.; Mežinska, S.; Th Mayrhofer, M.; Slokenberga, S.; Reichel, J.; Croxton, T.; Ziaka, A.; et al. Ethical, Legal, and Social Implications in Research Biobanking: A Checklist for Navigating Complexity. Dev. World Bioeth. 2023, 24, 139–150. [Google Scholar] [CrossRef]
- Gille, F.; Brall, C. Can We Know If Donor Trust Expires? About Trust Relationships and Time in the Context of Open Consent for Future Data Use. J. Med. Ethics 2022, 48, 184–188. [Google Scholar] [CrossRef]
- Nordberg, A. Biobank and Biomedical Research: Responsibilities of Controllers and Processors Under the EU General Data Protection Regulation. In GDPR and Biobanking: Individual Rights, Public Interest and Research Regulation Across Europe; Slokenberga, S., Tzortzatou, O., Reichel, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 61–89. ISBN 978-3-030-49388-2. [Google Scholar]
- Hoofnagle, C.J.; van der Sloot, B.; Borgesius, F.Z. The European Union General Data Protection Regulation: What It Is and What It Means. Inf. Commun. Technol. Law 2019, 28, 65–98. [Google Scholar] [CrossRef]
- Veit, R.-D. Safeguarding Regional Data Protection Rights on the Global Internet—The European Approach Under the GDPR. In Personality and Data Protection Rights on the Internet: Brazilian and German Approaches; Albers, M., Sarlet, I.W., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 445–484. ISBN 978-3-030-90331-2. [Google Scholar]
- Lenartz, A.; Scherer, A.M.; Uhlmann, W.R.; Suter, S.M.; Anderson Hartley, C.; Prince, A.E.R. The Persistent Lack of Knowledge and Misunderstanding of the Genetic Information Nondiscrimination Act (GINA) More than a Decade after Passage. Genet. Med. 2021, 23, 2324–2334. [Google Scholar] [CrossRef]
- Feldman, E.A. The Genetic Information Nondiscrimination Act (GINA): Public Policy and Medical Practice in the Age of Personalized Medicine. J. Gen. Intern. Med. 2012, 27, 743–746. [Google Scholar] [CrossRef]
- Shickle, D.; Griffin, M.; El-Arifi, K. Inter- and Intra-Biobank Networks: Classification of Biobanks. Pathobiology 2010, 77, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bukreeva, A.S.; Malsagova, K.A.; Izotov, A.A.; Lisitsa, A.V.; Kaisheva, A.L. BioVitrina Web Repository—Access to Data on Bioresource Collections. Cardiovasc. Ther. Prev. 2023, 22, 3720. [Google Scholar] [CrossRef]
| Name of Digital Biobank | Description | Types of Biomaterials | Dataset Size | Territory Covered | Research Type | Data Availability | Data Access Format | International Compliance | Specimen Request | Specialty | Access |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UK Post-Cancer Sample Collection Database [39] | The first virtual biobank devoted to prostate cancer research | Tissues, blood, DNA | >10,000 biological specimens | UK | Oncology | On request | Web portal, API | Yes | Yes (available for researchers residing in UK) | Prostate cancer | Free of charge |
| DxConnect Virtual Biobank [40] | A platform providing access to clinical specimens for researchers. It allows one look through collections registered by any institution worldwide | Different types of clinical specimens | N/A | Global | Various | On request | Web portal | N/A | Yes | Multi | Free of charge |
| APPRISE Virtual Biobank [41] | Virtual biobank with information about specimens collected from patients with an infectious pathology | Plasma, serum, peripheral hematopoietic stem cells | 58,000 specimens from 2700 patients | Australia | Infectious diseases | Yes | Web portal | N/A | Yes | Infectious diseases | Free of charge |
| BioIVT [42] | A platform offering access to an extensive repository of biological specimens and clinical database for research purposes | Different types of biological specimens | >1,000,000 | Global | Various | Yes | Web portal | Yes | Yes | Multi | On a fee basis |
| BioVitrina [43] | A web-repository offering access to information about well-annotated biological specimens of various pathologies | Tissues, urine, blood serum, blood Plasma | >1000 | Russia | Various | Yes | Web portal | Yes | Yes | Multi | Free of charge |
| BBMRI-ERIC [44] | The European infrastructure of biobanks offering access to bioresources and services to support biomedical research | Different types of biological specimens | >1520 collections of biological specimens | European Union | Medical research, genomics | Yes | Web platform | Yes | Yes | Biomedical research | Depends on a biobank |
| UK Biobank [45] | One of the largest and most comprehensive biobanks in the world, which provides data and specimens for conducting research in the field of genetics and population health | Genetic data, clinical data, blood samples | >500,000 | UK | Various | Yes | Web platform | Yes | No | Health and diseases | Free of charge |
| iSpecimen Marketplace [46] | iSpecimen serves as a centralized platform for sourcing high-quality human biospecimens from a global network of providers. | Different types of biological specimens | >1,000,000 | Global | Various | Yes | Web platform | Yes | Yes | Multi | |
| German Biobank Node [47] | Serves as a central platform for collaboration among German biobanks and represents their interests within the European BBMRI-ERIC network. It aims to standardize quality management and create an IT infrastructure for accessibility to biomaterials and related data for biomedical research | Different types of biological specimens | N/A * | European Union | Various | Yes | Web platform | Yes | Yes | Precision medicine |
| Problem | Description | Solution |
|---|---|---|
| Big data * | Researchers’ demand for accessibility of “broader” data requires the acquisition and administration of large biospecimen collections and associated data, often from several sources | Paradigm shift both in requirements for storage and in data analysis. The use of computerized mechanisms, implementation of various solutions: from cloud storage to creating secure dedicated repositories |
| High speed of data acquisition | Due to the development of “omics” technologies (proteomics, genomics, etc.), new information about biospecimens appears at a fast pace | |
| Standardization of analytical data | Variations related to collection, processing, and storage of different specimens and associated clinical data significantly hamper extrapolation or pooling data obtained in different studies | The use of unified terminology and international best practices [50] |
| Ethical problems | For collecting specimens and data, personal data that are often identifiable need to be accessed. Sometimes donors cannot fully assess the type or focus of a study conducted due to the lack of knowledge (expertise) or it may be necessary to send study results to the donors, which in turn may cause technical challenges when drafting informed consent | Adaptation to new legal acts (e.g., the EU General Data Protection Regulation (GDPR)) [51] |
| Separation of biobanks | Organizations at which biobanks have been founded and that fund their infrastructure often assume that biobanks must become “independent” at some point. However, most often it cannot be attained, especially for the biobanks integrated into research or medical institutions [52] | New, more flexible funding models are needed, which will empower the development of biobanking, thus unlocking the potential of opportunities emerging along with advances in precision medical research |
| Data integration | Integrating data from multiple studies becomes challenging due to differences in formats, data structures, and methodologies used across studies and platforms | Network-based data integration tools (OmicsNet [53], etc.) allow combining data from different studies. MetaboAnalyst [54] can perform cross-platform analyses by aligning metabolomic data with proteomic or transcriptomic data |
| Data Protection Technique | Protocols Used | Description |
|---|---|---|
| Data encryption | SSL/TLS, AES, RSA, SSH, PGP/GPG | Data encryption is used to protect the confidentiality of data by transforming them to a format that is incomprehensible to third parties. Examples of encryption algorithms include SSL/TLS for secure data transmission over the network; AES and RSA for protecting data at rest; and SSH for providing secure remote access to systems. |
| Authentication and authorization | LDAP, Kerberos, OAuth, OpenID Connect | Authentication is the process of verifying the identity of a user or a device. Authorization is determining whether a user has rights to access resources after successful authentication. Examples of protocols include LDAP for centralized account management; Kerberos for secure authentication; and OAuth and OpenID Connect for authorization and authentication in web applications. |
| Access control | RBAC, ABAC, ACL | Access control determines which users have access to which resources. Examples include RBAC (Role-Based Access Control), where access depends on the user’s role; ABAC (Attribute-Based Access Control), where access depends on the user’s attributes; and ACL (Access Control Lists), where access to resources is set up manually. |
| Monitoring and audit | SIEM, SNMP, Syslog | Monitoring and audit are used to track user activity and detect potential security threats. Examples include SIEM (Security Information and Event Management) for analyzing and aggregating event logs, SNMP (Simple Network Management Protocol) for monitoring network devices, and Syslog for centralized logging of events. |
| Country | Law | Description | Ethical Issue to Be Solved |
|---|---|---|---|
| European Union | GDPR (General Data Protection Regulation) | Controls personal data processing and storage; requires consent from data subjects for data processing; confers rights to data subjects. | Personal data protection and enforcement of rights for maintaining data confidentiality. |
| USA | GINA (Genetic Information Discrimination Act) | Prohibits discrimination based on their genetic information in health coverage and in employment. | Prevention of discrimination based on one’s genetic information, protection of individuals’ rights. |
| Japan | The Law on Human Genome and Biobanks | Regulates the management and use of genetic data; measures to protect personal data of donors. | Regulation of using genetic data, protection of donors’ personal data, management of the access to biological specimens. |
| China | National Ethical Guidelines for Biobanks | Sets standards for collection, storage, and use of specimens, as well as requirements for obtaining consent and confidentiality. | Setting standards for protecting donor confidentiality, regulation of obtaining consent for specimen use. |
| Germany | Law on Data Protection and Bioethics | Regulates handling personal data in medical research, including obtaining consent for the use of biological specimens. | Protection of personal data in medical research, enforcement of confidentiality rights. |
| UK | Human Tissue Act | Regulates the use of human tissues and cells by setting requirements for obtaining consent and using specimens for research purposes. | Properly obtaining consent for using human tissues and cells; regulating access to specimens for research purposes. |
| Russia | Federal Law “On Personal Data” | Regulates handling personal data, including the data collected during medical studies and biobanking, ensuring personal data protection. | Protection of personal data, including the medical and biological data; prevention of data misuse. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukreeva, A.S.; Malsagova, K.A.; Petrovskiy, D.V.; Butkova, T.V.; Nakhod, V.I.; Rudnev, V.R.; Izotov, A.A.; Kaysheva, A.L. Biobank Digitalization: From Data Acquisition to Efficient Use. Biology 2024, 13, 957. https://doi.org/10.3390/biology13120957
Bukreeva AS, Malsagova KA, Petrovskiy DV, Butkova TV, Nakhod VI, Rudnev VR, Izotov AA, Kaysheva AL. Biobank Digitalization: From Data Acquisition to Efficient Use. Biology. 2024; 13(12):957. https://doi.org/10.3390/biology13120957
Chicago/Turabian StyleBukreeva, Anastasiia S., Kristina A. Malsagova, Denis V. Petrovskiy, Tatiana V. Butkova, Valeriya I. Nakhod, Vladimir R. Rudnev, Alexander A. Izotov, and Anna L. Kaysheva. 2024. "Biobank Digitalization: From Data Acquisition to Efficient Use" Biology 13, no. 12: 957. https://doi.org/10.3390/biology13120957
APA StyleBukreeva, A. S., Malsagova, K. A., Petrovskiy, D. V., Butkova, T. V., Nakhod, V. I., Rudnev, V. R., Izotov, A. A., & Kaysheva, A. L. (2024). Biobank Digitalization: From Data Acquisition to Efficient Use. Biology, 13(12), 957. https://doi.org/10.3390/biology13120957







