Dynamics of Marenzelleria spp. Biomass and Environmental Variability: A Case Study in the Neva Estuary (The Easternmost Baltic Sea)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
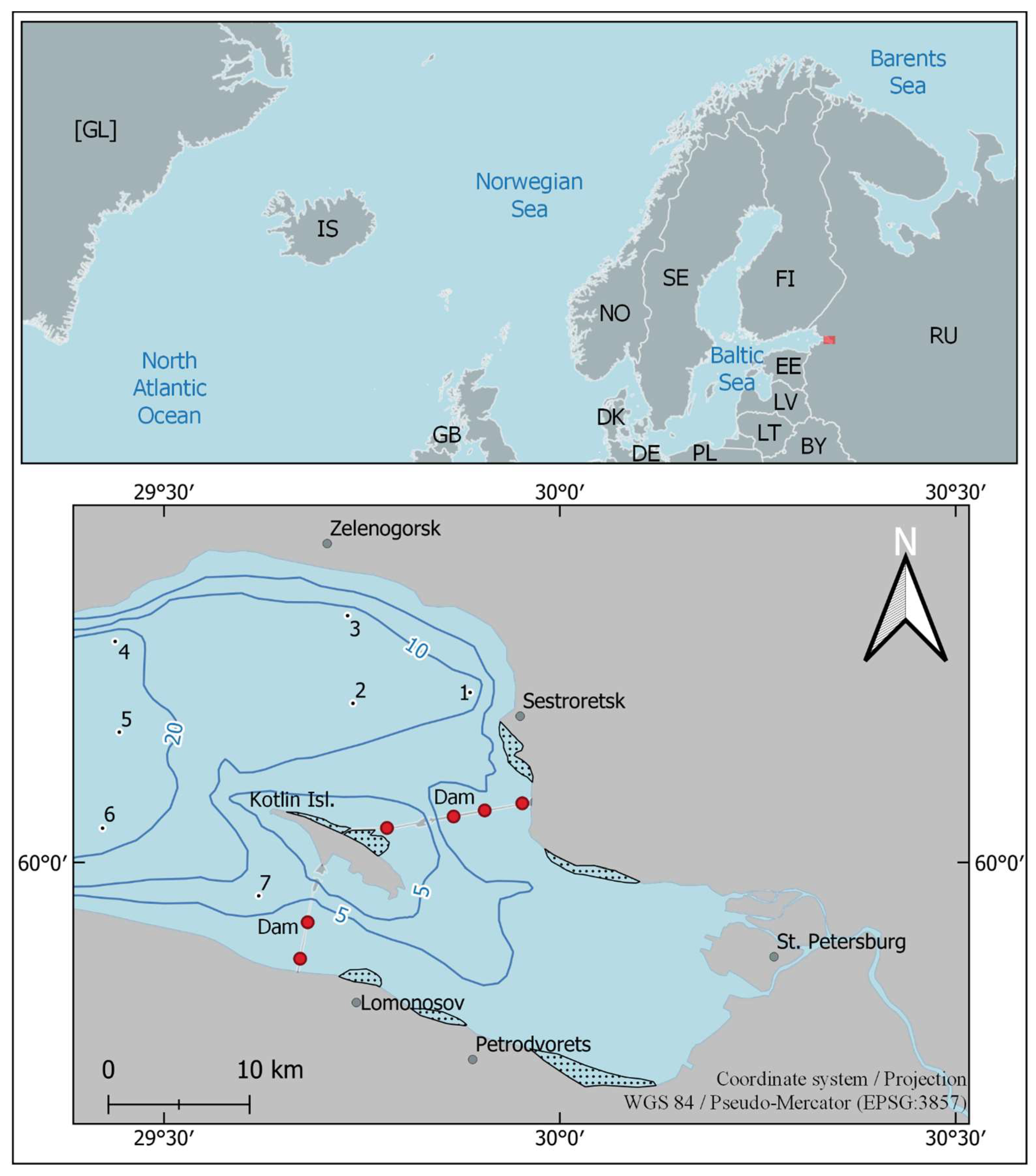
2.1. Study Site and Sampling
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Rousou, M.; Langeneck, J.; Apserou, C.; Arvanitidis, C.; Charalambous, S.; Chrysanthou, K.; Constantinides, G.; Dimitriou, P.D.; García Gómez, S.C.; Hadjieftychiou, S.I.; et al. Polychaetes (Annelida) of Cyprus (Eastern Mediterranean Sea): An Updated and Annotated Checklist including New Distribution Records. Diversity 2023, 15, 941. [Google Scholar] [CrossRef]
- Kotta, J.; Ojaveer, H.; Kaasik, A.; Szava-Kovats, R.; Nurkse, K.; Outinen, O.; Einberg, H.; Zaiko, A.; Lehtiniemi, M.; Orav-Kotta, H.; et al. Universal framework for assessing the environmental impact of marine non-indigenous species in different situations of data availability. Hydrobiologia 2024. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Sanders, D.; Frago, E. Ecosystem engineers shape ecological network structure and stability: A framework and literature review. Funct. Ecol. 2024, 38, 1683–1696. [Google Scholar] [CrossRef]
- Barney, J.N.; Whitlow, T.H. A unifying framework for biological invasions: The state factor model. Biol. Invasions 2008, 10, 259–272. [Google Scholar] [CrossRef]
- Zaiko, A.; Cardeccia, A.; Carlton, J.T.; Clark, G.F.; Creed, J.C.; Davidson, I.; Floerl, O.; Galil, B.; Grosholz, E.; Hopkins, G.A.; et al. Structural and functional effects of global invasion pressure on benthic marine communities—Patterns, challenges and priorities. Divers. Distrib. 2024, 30, e13838. [Google Scholar] [CrossRef]
- Cardeccia, A.; Marchini, A.; Occhipinti-Ambrogi, A.; Galil, B.; Gollasch, S.; Minchin, D.; Narščius, A.; Olenin, S.; Ojaveer, H. Assessing biological invasions in European Seas: Biological traits of the most widespread non-indigenous species. Estuar. Coast. Shelf Sci. 2018, 201, 17–28. [Google Scholar] [CrossRef]
- Radashevsky, V.I.; Pankova, V.V.; Neretina, T.V.; Tzetlin, A.B. Canals and invasions: A review of the distribution of Marenzelleria (Annelida: Spionidae) in Eurasia, with a key to Marenzelleria species and insights on their relationships. Aquat. Invasions 2022, 17, 186–206. [Google Scholar] [CrossRef]
- Kauppi, L.; Norkko, A.; Norkko, J. Large-scale species invasion into a low-diversity system: Spatial and temporal distribution of the invasive polychaetes Marenzelleria spp. in the Baltic Sea. Biol. Invasions 2015, 17, 2055–2074. [Google Scholar] [CrossRef]
- Maximov, A.; Bonsdorff, E.; Eremina, T.; Kauppi, L.; Norkko, A.; Norkko, J. Context-dependent consequences of Marenzelleria spp. (Spionidae: Polychaeta) invasion for nutrient cycling in the northern Baltic Sea. Oceanologia 2015, 57, 342–348. [Google Scholar] [CrossRef]
- Berezina, N.A.; Maximov, A.A.; Vladimirova, O.M. Influence of benthic invertebrates on phosphorus flux at the sediment-water interface in the easternmost Baltic Sea. Mar. Ecol. Prog. Ser. 2019, 608, 33–43. [Google Scholar] [CrossRef]
- Vivó-Pons, A.; Blomqvist, M.; Törnroos, A.; Lindegren, M. A trait-based approach to assess niche overlap and functional distinctiveness between non-indigenous and native species. Ecol. Lett. 2023, 26, 1911–1925. [Google Scholar] [CrossRef] [PubMed]
- Golubkov, S.; Tiunov, A.; Golubkov, M. Food-web modification in the eastern Gulf of Finland after invasion of Marenzelleria arctia (Spionidae, Polychaeta). NeoBiota 2021, 66, 75–94. [Google Scholar] [CrossRef]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Chapter One–Impacts of invasive species on food webs: A review of empirical data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar] [CrossRef]
- Moyle, P.B.; Light, T. Fish invasions in California: Do abiotic factors determine success? Ecology 1996, 77, 1666–1670. [Google Scholar] [CrossRef]
- Marchetti, M.P.; Moyle, P.B.; Levine, R. Alien fishes in California watersheds: Characteristics of successful and failed invaders. Ecol. Appl. 2004, 14, 587–596. [Google Scholar] [CrossRef]
- Lahdes, E.O.; Karjala, L.A. Implications of water ionic composition for invasion of euryhaline species in inland waters—An experimental study with Cercopagis pengoi from the Northern Baltic Sea. Aquat. Invasions 2007, 2, 422–430. [Google Scholar] [CrossRef]
- Golubkov, M.S.; Litvinchuk, L.F.; Golubkov, S.M. Effects of environmental gradients on the biomass of alien Cercopagis pengoi in the Neva Estuary. Front. Mar. Sci. 2020, 7, e573289. [Google Scholar] [CrossRef]
- Ptáčníková, R.; Vanderploeg, H.A.; Cavaletto, J.F. Big versus small: Does Bythotrephes longimanus predation regulate spatial distribution of another invasive predatory cladoceran, Cercopagis pengoi? J. Great Lakes Res. 2015, 41, 143–149. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Pothoven, S.A.; Krueger, D.; Mason, D.M.; Liebig, J.R.; Cavaletto, J.F.; Ruberg, S.A.; Lang, G.A.; Ptáčníková, R. Spatial and predatory interactions of visually preying nonindigenous zooplankton and fish in Lake Michigan during midsummer. J. Great Lakes Res. 2015, 41, 125–142. [Google Scholar] [CrossRef]
- Kornijόw, R.; Pawlikowski, K.; Drgas, A.; Rolbiecki, L.; Rychter, A. Mortality of post-settlement clams Rangia cuneata (Mactridae, Bivalvia) at an early stage of invasion in the Vistula Lagoon (South Baltic) due to biotic and abiotic factors. Hydrobiologia 2018, 811, 207–219. [Google Scholar] [CrossRef]
- Vivó-Pons, A.; van Denderen, P.D.; Flensborg, L.; Jaspers, C.; Lindegren, M. Disentangling the effects of abiotic and biotic processes on non-indigenous species dominance. NeoBiota 2024, 94, 159–177. [Google Scholar] [CrossRef]
- Simberloff, D.; Gibbons, L. Now you See them, Now you don’t!—Population Crashes of Established Introduced Species. Biol. Invasions 2004, 6, 161–172. [Google Scholar] [CrossRef]
- Bochert, R. Marenzelleria viridis (Polychaeta: Spionidae): A review of its reproduction. Aquat. Ecol. 1997, 31, 163–175. [Google Scholar] [CrossRef]
- Daunys, D.; Schiedek, D.; Olenin, S. Species strategy near its boundary: The Marenzelleria cf. viridis (Polychaeta, Spionidae) case in the South-Eastern Baltic Sea. Int. Rev. Hydrobiol. 2000, 85, 639–651. [Google Scholar] [CrossRef]
- Ezhova, E.; Spirido, O. Patterns of spatial and temporal distribution of the Marenzelleria cf. viridis population in the lagoon and marine environment in the Southeastern Baltic Sea. Oceanol. Hydrobiol. Stud. 2005, 34, 209–226. [Google Scholar]
- Maximov, A.A. Large-scale invasion of Marenzelleria spp. (Polychaeta; Spionidae) in the eastern Gulf of Finland, Baltic Sea. Russ. J. Biol. Invasions 2011, 2, 11–19. [Google Scholar] [CrossRef]
- Kotta, J.; Orav, H.; Sandberg-Kilpi, E. Ecological consequence of the introduction of the polychaete Marenzelleria cf. viridis into a shallow-water biotope of the northern Baltic Sea. J. Sea Res. 2001, 46, 273–280. [Google Scholar] [CrossRef]
- Neideman, R.; Wenngren, J.; Ólafsson, E. Competition between the introduced polychaete Marenzelleria sp. and the native amphipod Monoporeia affinis in Baltic soft bottoms. Mar. Ecol. Prog. Ser. 2003, 264, 49–55. [Google Scholar] [CrossRef]
- Kauppi, L.; Norkko, A.; Norkko, J. Seasonal population dynamics of the invasive polychaete genus Marenzelleria spp. in contrasting soft-sediment habitats. J. Sea Res. 2018, 131, 46–60. [Google Scholar] [CrossRef]
- Golubkov, M.; Golubkov, S. Eutrophication in the Neva Estuary (Baltic Sea): Response to temperature and precipitation patterns. Mar. Freshw. Res. 2020, 71, 583–595. [Google Scholar] [CrossRef]
- Golubkov, M.; Golubkov, S. Relationships between northern hemisphere teleconnection patterns and phytoplankton productivity in the Neva Estuary (Northeastern Baltic Sea). Front. Mar. Sci. 2021, 8, 735790. [Google Scholar] [CrossRef]
- Telesh, I.V.; Golubkov, S.M.; Alimov, A.F. The Neva Estuary ecosystem. In Ecology of Baltic Coastal Waters, Ecological Studies; Schiewer., U., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 197, pp. 259–284. [Google Scholar] [CrossRef]
- Golubkov, S.; Alimov, A. Ecosystem changes in the Neva Estuary (Baltic Sea): Natural dynamics or response to anthropogenic impacts? Mar. Pollut. Bull. 2010, 61, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Golubkov, S.; Golubkov, M.; Tiunov, A.; Nikulina, L. Long-term changes in primary production and mineralization of organic matter in the Neva Estuary (Baltic Sea). J. Mar. Syst. 2017, 171, 73–80. [Google Scholar] [CrossRef]
- Golubkov, S.M.; Berezina, N.A.; Gubelit, Y.I.; Demchuk, A.S.; Golubkov, M.S.; Tiunov, A.V. A relative contribution of carbon from green tide algae Cladophora glomerata and Ulva intestinalis in the coastal food webs in the Neva Estuary (Baltic Sea). Mar. Pollut. Bull. 2018, 126, 43–50. [Google Scholar] [CrossRef]
- Golubkov, S.M.; Golubkov, M.S.; Tiunov, A.V. Anthropogenic carbon as a basal resource in the benthic food webs in the Neva Estuary (Baltic Sea). Mar. Pollut. Bull. 2019, 146, 190–200. [Google Scholar] [CrossRef]
- Orlova, M.; Golubkov, S.; Kalinina, L.; Ignatieva, N. Dreissena polymorpha (Bivalvia: Dreissenidae) in the Neva Estuary (eastern Gulf of Finland, Baltic Sea): Is it a biofilter or source for pollution? Mar. Pollut. Bull. 2004, 49, 196–205. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Country Codes–ISO 3166. Available online: https://www.iso.org/iso-3166-country-codes.html (accessed on 3 October 2024).
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis, 3rd ed.; Wiley-VCH: New York, NY, USA, 1999. [Google Scholar]
- Hall, R.O., Jr.; Thomas, S.; Gaiser, E.E. Measuring freshwater primary production and respiration. In Principles and Standards for Measuring Primary Production; Fahey, T.J., Knapp, A.K., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 175–203. [Google Scholar] [CrossRef]
- Vernet, M.; Smith, R.C. Measuring and modeling primary production in marine pelagic ecosystems. In Principles and Standards for Measuring Primary Production; Fahey, T.J., Knapp, A.K., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 142–174. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing (Version 4.4.0). Available online: https://www.r-project.org (accessed on 3 October 2024).
- Vegan: Community Ecology Package (2.6–8). [Software]. Available online: https://cran.r-project.org/web/packages/vegan/ (accessed on 3 October 2024).
- Factoextra: Extract and Visualize the Results of Multivariate Data Analyses (1.0.7). [Software]. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 3 October 2024).
- ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics (3.5.1). [Software]. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 3 October 2024).
- Karatayev, A.Y.; Burlakova, L.E.; Padilla, D.K. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 433–446. [Google Scholar] [CrossRef]
- Norkko, J.; Reed, D.C.; Timmermann, K.; Norkko, A.; Gustafsson, B.G.; Bondsdorff, E.; Slomp, C.P.; Carstensen, J.; Conley, D.J. A welcome can of worms? Hypoxia mitigation by an invasive species. Glob. Change Biol. 2012, 18, 422–434. [Google Scholar] [CrossRef]
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.A.; Bakiu, R.; et al. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci. Total Environ. 2021, 88, 147868. [Google Scholar] [CrossRef]
- Davis, M.A. Biotic globalization: Does competition from introduced species threaten biodiversity? BioScience 2003, 53, 481–489. [Google Scholar] [CrossRef]
- Maximov, A. The long-term dynamics and current distribution of macrozoobenthos communities in the eastern Gulf of Finland, Baltic Sea. Russ. J. Mar. Biol. 2015, 41, 300–310. [Google Scholar] [CrossRef]
- Gebruk, A.; Dgebuadze, P.; Rogozhin, V.; Ermilova, Y.; Shabalin, N.; Mokievsk, V. Macrozoobenthos of the Pechora Bay in 2020–2021 indicates a likely change of common bivalve molluscs in the Arctic estuary. Polar Biol. 2023, 46, 473–487. [Google Scholar] [CrossRef]
- Eriksson Wiklund, A.-K.; Sundelin, B. Impaired reproduction in the amphipods Monoporeia affinis and Pontoporeia femorata as a result of moderate hypoxia and increased temperature. Mar. Ecol. Progr. Ser. 2001, 222, 131–141. [Google Scholar] [CrossRef]
- Kotta, J.; Ólafsson, E. Competition for food between the introduced polychaete Marenzelleria viridis (Verrill) and the native amphipod Monoporeia affinis Lindström in the Baltic Sea. J. Sea Res. 2003, 50, 27–35. [Google Scholar] [CrossRef]
- Schiedek, D. Marenzelleria cf. viridis (Polychaeta: Spionidae)—Ecophysiological adaptations to a life in the coastal waters of the Baltic Sea. Aquat. Ecol. 1997, 31, 199–210. [Google Scholar] [CrossRef]
- Schiedek, D. Marenzelleria viridis (Verrill, 1873) (Polychaeta), a new benthic species within European coastal waters. Some metabolic features. J. Exp. Mar. Biol. Ecol. 1997, 211, 85–101. [Google Scholar] [CrossRef]
- Rousi, H.; Fält-Nardmann, J.; Niemelä, P.; Hänninen, J. Changes in Atlantic climatic regulation mechanisms that underlie mesozooplankton biomass loss in the northern Baltic Sea. Heliyon 2024, 10, e31268. [Google Scholar] [CrossRef]
- Bochert, R.; Fritzsche, D.; Burckhardt, R. Influence of salinity and temperature on growth and survival of the planktonic larvae of Marenzelleria viridis (Polychaeta: Spionidae). J. Plankton Res. 1996, 18, 1239–1251. [Google Scholar] [CrossRef]
- Moyle, P.B.; Marchetti, M.P. Predicting Invasion Success: Freshwater Fishes in California as a Model. BioScience 2006, 56, 515–524. [Google Scholar] [CrossRef]
- Golubkov, S.M.; Balushkina, E.V. Long-term changes in water quality and structure of benthic animal communities in the Neva estuary under anthropogenic stress. Proc. Zool. Inst. RAS 2024, 328, 46–65. [Google Scholar] [CrossRef]
- Outinen, Q.; Katajisto, T.; Nygård, H.; Puntila-Dodd, R.; Lehtiniemi, M. National assessment on the status, trends and impacts of marine non-indigenous species for the European Union marine strategy framework directive. Ecol. Indic. 2024, 158, 111593. [Google Scholar] [CrossRef]


| Years | Shallow Stations | Deep Stations | ||||
|---|---|---|---|---|---|---|
| AB, (g m−2) | MaxB, (g m−2) | MinB, (g m−2) | AB, (g m−2) | MaxB, (g m−2) | MinB, (g m−2) | |
| 2014 | 1.61 | 4.08 | 0.20 | 16.67 | 20.06 | 12.72 |
| 2015 | 4.83 | 12.28 | 1.19 | 28.83 | 34.02 | 23.66 |
| 2016 | 0.25 | 0.50 | 0.04 | 12.82 | 26.24 | 2.06 |
| 2017 | 0.25 | 0.55 | 0.00 | 10.80 | 21.16 | 5.12 |
| 2018 | 0.11 | 0.36 | 0.02 | 5.68 | 7.98 | 1.94 |
| 2019 | 0.90 | 1.94 | 0.36 | 7.84 | 9.42 | 6.26 |
| 2020 | 0.46 | 1.09 | 0.06 | 5.51 | 11.40 | 0.06 |
| 2021 | 0.08 | 0.24 | 0.00 | 0.36 | 0.62 | 0.03 |
| 2022 | 0.16 | 0.41 | 0.02 | 0.14 | 0.41 | 0.02 |
| 2023 | 0.20 | 0.40 | 0.00 | 0.33 | 0.75 | 0.00 |
| Years | Shallow Stations | Deep Stations | ||||
|---|---|---|---|---|---|---|
| AN, (ind. m−2) | MaxN, (ind. m−2) | MinN, (ind. m−2) | AN, (ind. m−2) | MaxN, (ind. m−2) | MinN, (ind. m−2) | |
| 2014 | 148 | 400 | 20 | 2527 | 2880 | 2300 |
| 2015 | 360 | 720 | 120 | 8670 | 9080 | 8260 |
| 2016 | 75 | 140 | 20 | 2473 | 4520 | 400 |
| 2017 | 165 | 440 | 0 | 5987 | 8180 | 2740 |
| 2018 | 180 | 520 | 20 | 1720 | 2440 | 420 |
| 2019 | 780 | 2080 | 140 | 4640 | 5560 | 3720 |
| 2020 | 145 | 300 | 60 | 1087 | 1920 | 100 |
| 2021 | 20 | 40 | 0 | 273 | 420 | 60 |
| 2022 | 102 | 180 | 13 | 807 | 1820 | 80 |
| 2023 | 82 | 117 | 26 | 230 | 351 | 0 |
| Source of Variation | Degrees of Freedom | Sum of Squares | R2 | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1 | 0.750 | 0.43 | 49.43 | 0.001 |
| Residuals | 66 | 1.002 | 0.57 | ||
| Total | 67 | 1.752 | 1 |
| Variable | Diff | lwr | upr | p-Value |
|---|---|---|---|---|
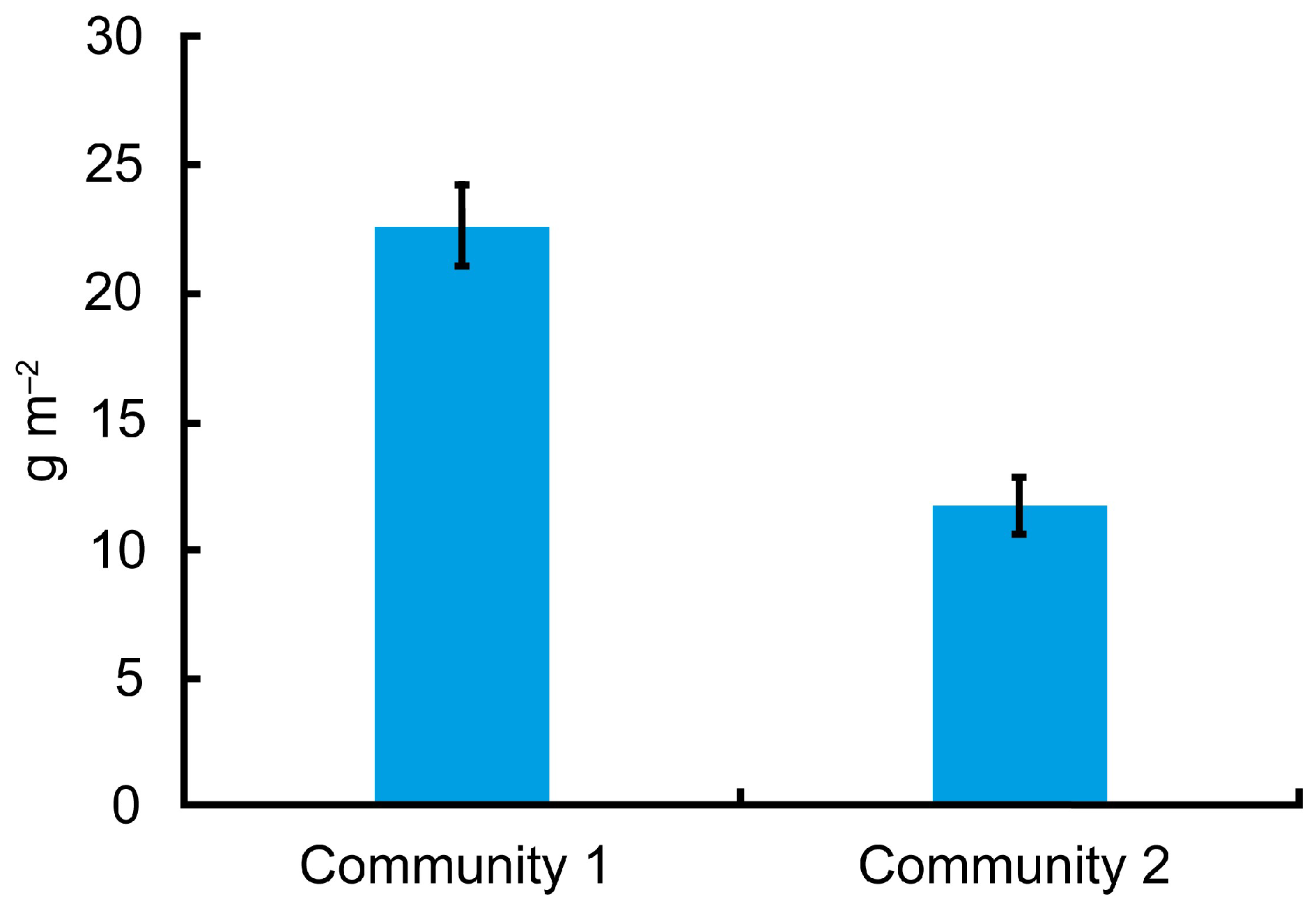
| The mean value of the variable in Community 2 is statistically significantly lower than in Community 1 | ||||
| Pol | −0.746 | −0.374 | −0.627 | <0.0001 |
| Mon | −0.060 | −0.082 | −0.038 | <0.0001 |
| Sad | −0.099 | −0.192 | −0.006 | 0.0376 |
| Dth | −0.193 | −0.262 | −0.125 | <0.0001 |
| Sal | −0.089 | −0.158 | −0.019 | 0.0129 |
| The mean value of the variable in Community 2 is statistically significantly higher than in Community 1 | ||||
| Temp | 0.270 | 0.165 | 0.375 | <0.0001 |
| pH | 0.009 | 0.003 | 0.014 | 0.0011 |
| CHL | 0.149 | 0.064 | 0.234 | 0.0008 |
| PP | 0.061 | 0.015 | 0.107 | 0.0105 |
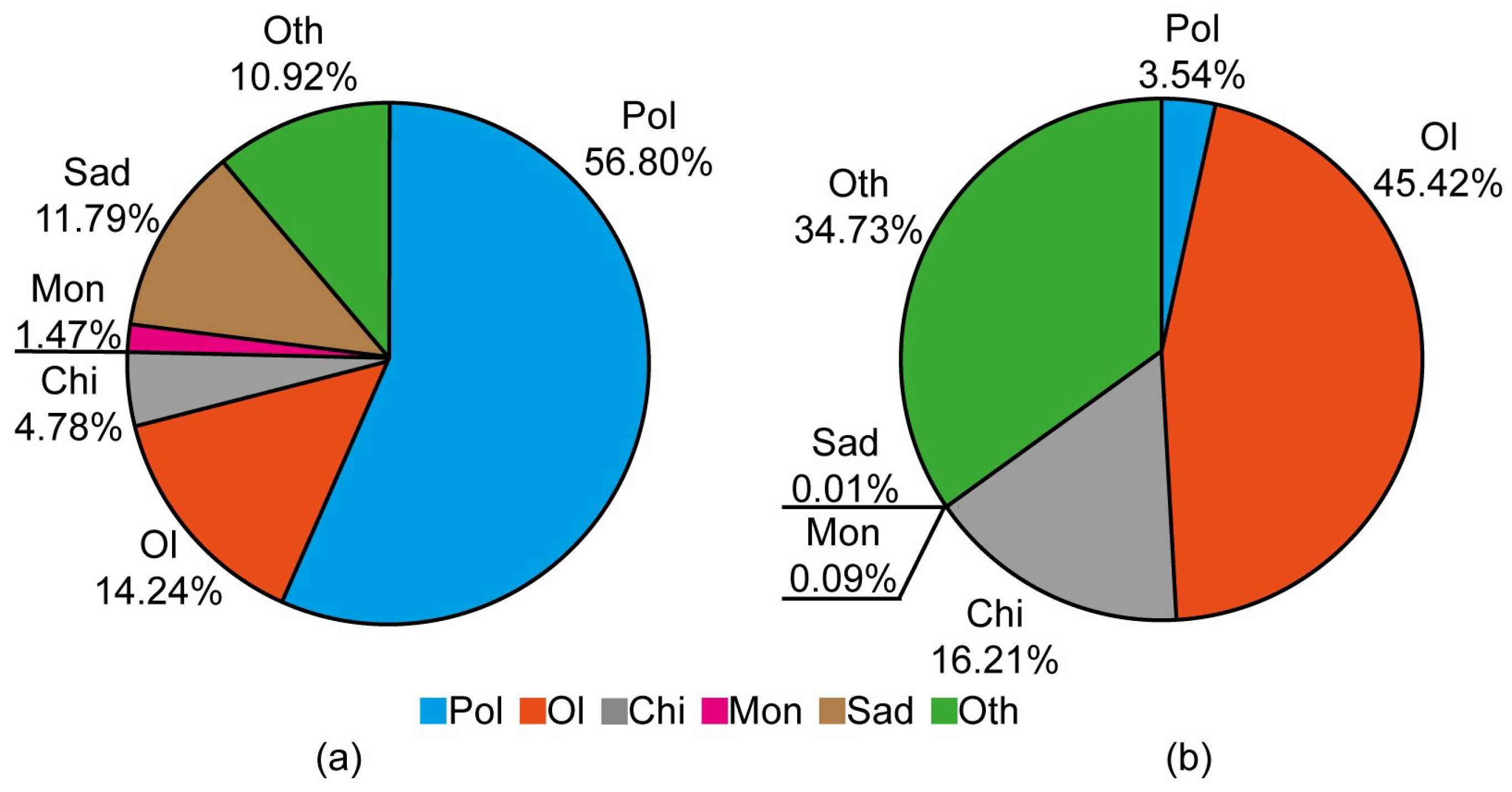
| Comparison of mean values between Community 1 and Community 2, showing no statistically significant differences for the listed variables | ||||
| Chi | 0.119 | −0.008 | 0.247 | 0.0661 |
| Ol | 0.136 | −0.023 | 0.295 | 0.0899 |
| Oth | −0.106 | −0.277 | 0.064 | 0.2176 |
| Eh | −0.008 | −0.077 | 0.060 | 0.8060 |
| Turb | 0.009 | −0.122 | 0.141 | 0.8882 |
| SMe | 0.017 | −0.078 | 0.112 | 0.7230 |
| SMg | −0.009 | −0.096 | 0.078 | 0.8367 |
| MN | 0.053 | −0.025 | 0.130 | 0.1787 |
| PP/MN | 0.019 | −0.023 | 0.062 | 0.3743 |
| Variable | Degrees of Freedom | Sum of Squares | Mean of Squares | F-Value | p-Value | Fraction of Variance |
|---|---|---|---|---|---|---|
| Sal | 1 | 1.013 | 1.013 | 16.93 | 0.00013 | 11.53 |
| Temp | 1 | 1.154 | 1.154 | 19.28 | 0.00005 | 13.13 |
| Turb | 1 | 1.248 | 1.248 | 20.84 | 0.00003 | 14.20 |
| Dth | 1 | 0.597 | 0.597 | 9.97 | 0.00258 | 6.80 |
| SMe | 1 | 0.512 | 0.512 | 8.55 | 0.00501 | 5.82 |
| Eh | 1 | 0.381 | 0.381 | 6.36 | 0.01458 | 4.33 |
| MN | 1 | 0.240 | 0.240 | 4.00 | 0.05030 | 2.73 |
| CHL | 1 | 0.156 | 0.156 | 2.60 | 0.11263 | 1.77 |
| PP | 1 | 0.120 | 0.120 | 2.00 | 0.16325 | 1.36 |
| pH | 1 | 0.061 | 0.061 | 1.02 | 0.31648 | 0.70 |
| PP/MN | 1 | 0.013 | 0.013 | 0.21 | 0.64764 | 0.14 |
| SMg | 1 | 0.000 | 0.000 | 0.000 | 0.98634 | 0.20 |
| Residuals | 55 | 3.293 | 0.0599 | 37.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkov, S.M.; Golubkov, M.S. Dynamics of Marenzelleria spp. Biomass and Environmental Variability: A Case Study in the Neva Estuary (The Easternmost Baltic Sea). Biology 2024, 13, 974. https://doi.org/10.3390/biology13120974
Golubkov SM, Golubkov MS. Dynamics of Marenzelleria spp. Biomass and Environmental Variability: A Case Study in the Neva Estuary (The Easternmost Baltic Sea). Biology. 2024; 13(12):974. https://doi.org/10.3390/biology13120974
Chicago/Turabian StyleGolubkov, Sergey M., and Mikhail S. Golubkov. 2024. "Dynamics of Marenzelleria spp. Biomass and Environmental Variability: A Case Study in the Neva Estuary (The Easternmost Baltic Sea)" Biology 13, no. 12: 974. https://doi.org/10.3390/biology13120974
APA StyleGolubkov, S. M., & Golubkov, M. S. (2024). Dynamics of Marenzelleria spp. Biomass and Environmental Variability: A Case Study in the Neva Estuary (The Easternmost Baltic Sea). Biology, 13(12), 974. https://doi.org/10.3390/biology13120974






