Simple Summary
Recent research and chemical screening work has identified the distribution of two major naphthoquinone regioisomers across a representative selection (including all genera, all sections in the large genus Drosera, and 190 of ca. 260 species recognized to date) in the diverse carnivorous plant family Droseraceae (sundews, Venus’s flytrap and waterwheel plant). A consistent and reliably reproducible foundation and framework for the systematic classification and phylogenetic evaluation of mutual relationships is thus provided. This review gives an updated overview of the naphthoquinone data relevant to the chemotaxonomy of the group under investigation. The essential conclusions from these data are summarized and directions are proposed for future research.
Abstract
Chemotaxonomy is the link between the state of the art in analytical chemistry and the systematic classification and phylogenetic analysis of biota. Although the characteristic secondary metabolites from diverse biotic sources have been used in pharmacology and biological systematics since the dawn of mankind, only comparatively recently established reproducible methods have allowed the precise identification and distinction of structurally similar compounds. Reliable, rapid screening methods like TLC (Thin Layer Chromatography) can be used to investigate sufficiently large numbers of samples for chemotaxonomic purposes. Using distribution patterns of mutually exclusive naphthoquinones, it is demonstrated in this review how a simple set of chemical data from a representative sample of closely related species in the sundew family (Droseraceae, Nepenthales) provides taxonomically and phylogenetically informative signal within the investigated group and beyond.
1. Introduction
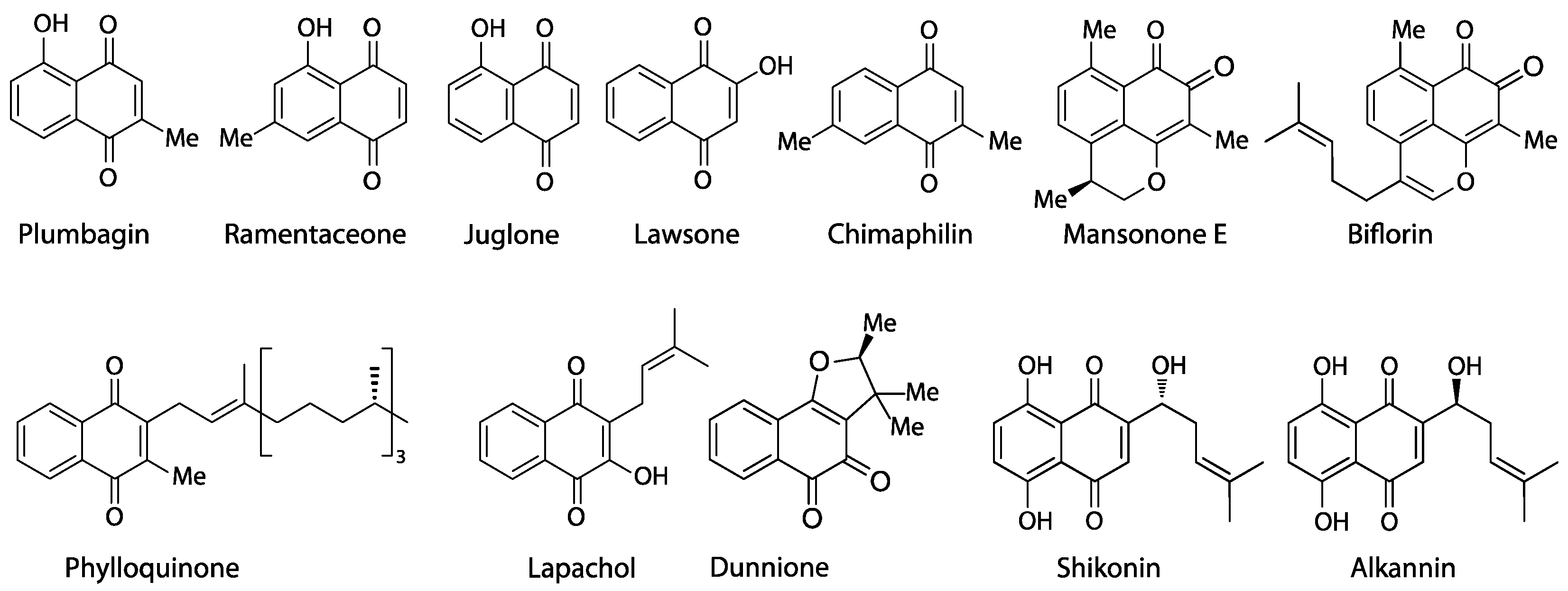
Naphthoquinones are an important class of natural products (Figure 1) containing pigments (e.g., alkannin, shikonin, lawsone), allelochemicals (juglone), cytostatics (lapachol, dunnione, plumbagin, chimaphilin), phytoalexins (mansonones), and vitamins (phylloquinone) [1,2]. At least five distinct biosynthetic routes (several of which established several times independently) are known to yield these bicyclic metabolites [3]:
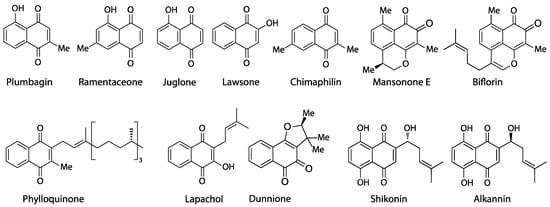
Figure 1.
Representative natural naphthoquinones.
- The acetate-polymalonate or polyketide pathway (plumbagin, ramentaceone), a versatile and widespread route commonly used to produce aromatic compounds characterized by oxygen substitution at every second atom of the carbon backbone that is even retained in metabolites of mixed biosynthetic origin (like, e.g., flavonoids and stilbenoids);
- The o-succinylbenzoate pathway (juglone, lawsone, lapachol, phylloquinone, dunnione), a route that is almost ubiquitous in plants, as it leads to the essential phylloquinone (vitamin K group);
- The homogentisate/mevalonate pathway (chimaphilin) so far known only from Ericaceae-Pyroloideae to produce chimaphilin and its immediate derivatives;
- The 4-hydroxybenzoate/geranyl-pyrophosphate pathway (shikonin, alkannin), known with certainty only from Boraginaceae;
- The farnesyl-pyrophosphate pathway (mansonones) common to the huge and widespread class of sesquiterpenoids, of which only a comparatively limited number become quinones by oxidation.
Nevertheless, there are only a few cases in which a specific naphthoquinone structure is formed convergently via different routes.
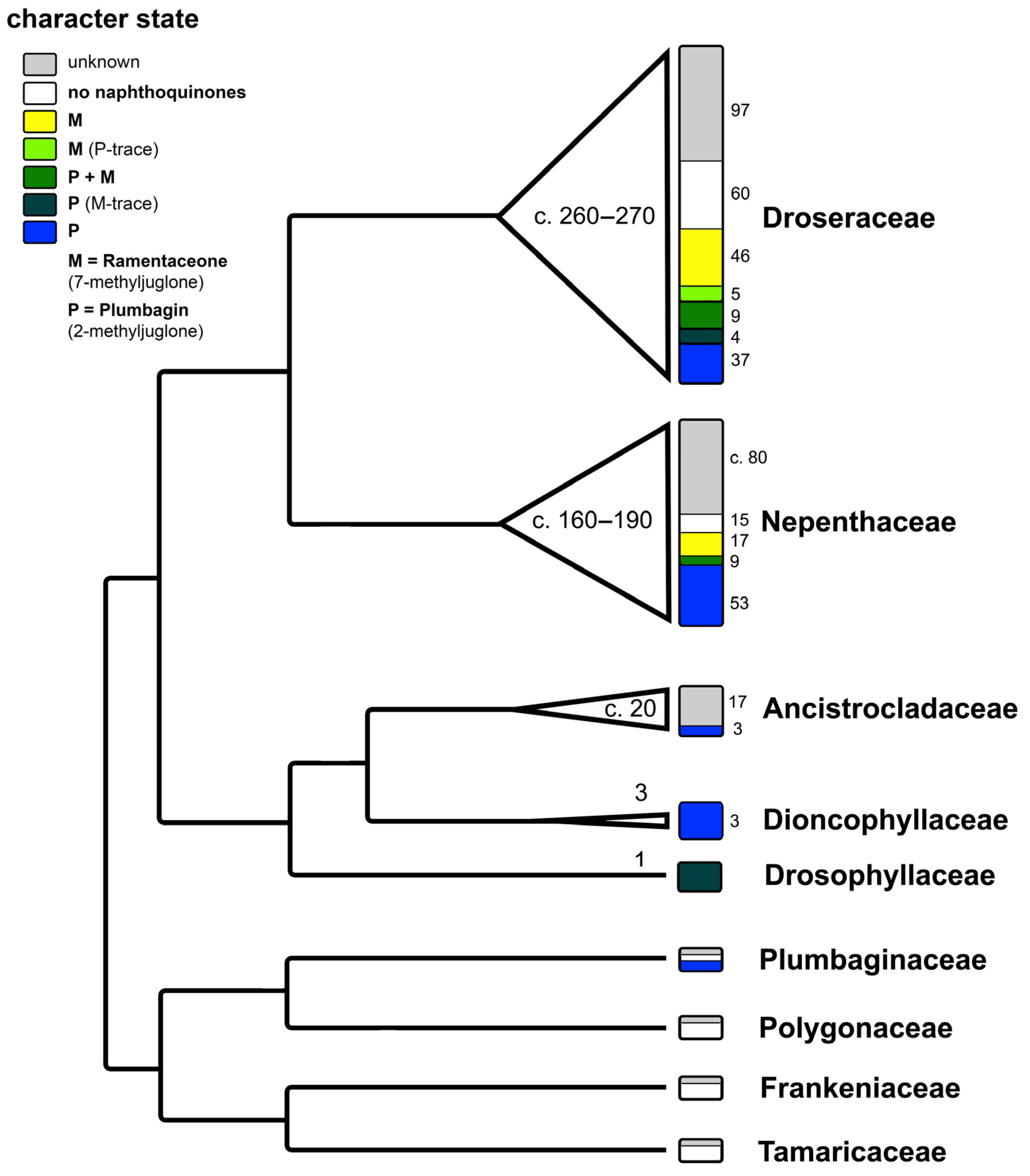
The regioisomers plumbagin (2-methyljuglone) and ramentaceone (7-methyljuglone) are characteristic naphthoquinones within the angiosperm order Nepenthales or “non-core Caryophyllales” (recorded so far in families Plumbaginaceae, Nepenthaceae, Droseraceae, Drosophyllaceae, Dioncophyllaceae and Ancistrocladaceae, Figure 2). As these 1,4-naphthoquinones demonstrably possess biological activities [1,2], preparations from sundew species (Droserae herba) used to be listed in several pharmacopoeias and are currently evaluated by European Pharmacopoeia standards [4]. Structurally and biosynthetically similar anthraquinones (e.g., emodin) are known from Polygonaceae within the same order [5]. Apart from genetic evidence, these characteristic metabolites clearly support the separation of Nepenthales from the sister order Caryophyllales (sensu stricto), which is in turn characterized by the common presence of betalains [6] that are lacking in Nepenthales [7,8]. Another noteworthy coincidence is the presence of at least six genera of carnivorous plants in four families of Nepenthales (Nepenthes of Nepenthaceae, Dionaea, Aldrovanda and Drosera of Droseraceae, Drosophyllum of Drosophyllaceae, Triphyophyllum of Dioncophyllaceae; carnivory is monophyletic in Nepenthales and became lost at least twice in this lineage, in two out of the three genera of Dioncophyllaceae and in all Ancistrocladaceae), while no carnivorous members of Caryophyllales are known [9].
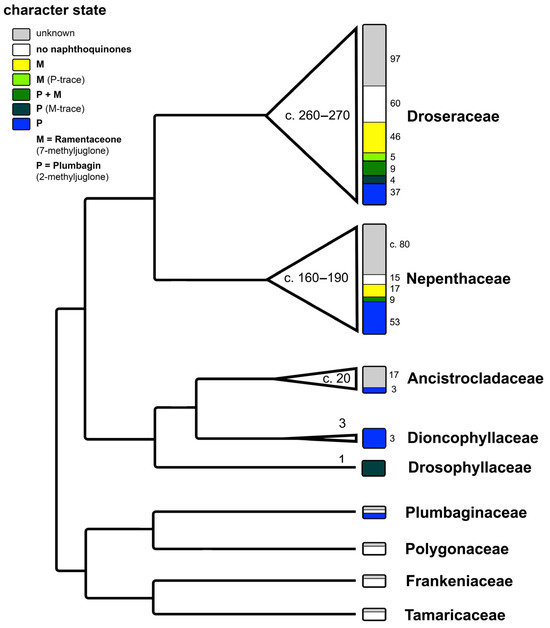
Figure 2.
Phylogenetic pattern of naphthoquinone distribution in Nepenthales. Cladogram modified from [9]. Numbers within the triangles and width of the triangles correspond to species number of each lineage. Numbers in small font correspond to the number of species showing the respective character state. Illustration by Andreas Fleischmann.
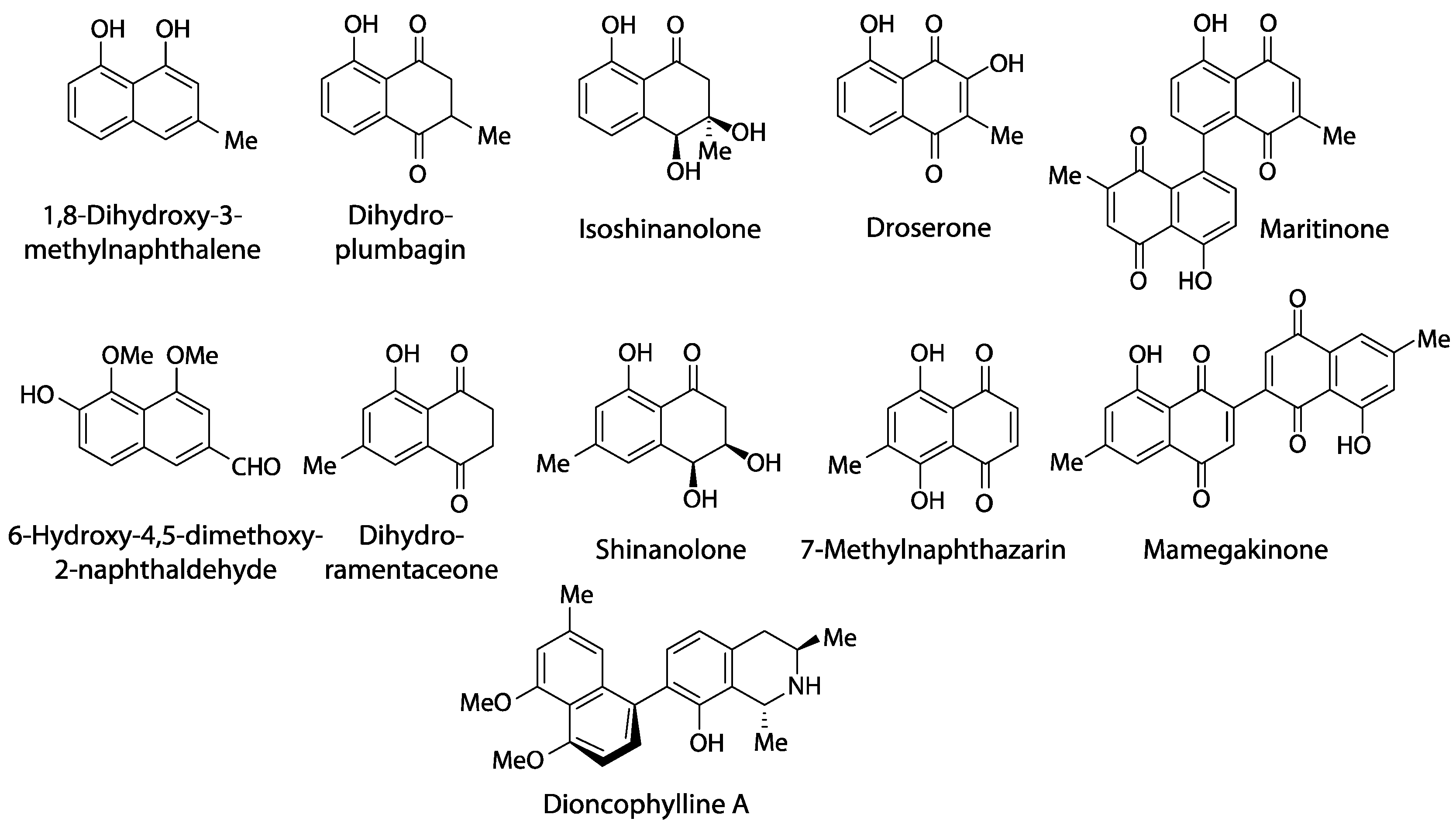
Outside Nepenthales, noteworthy records of plumbagin and/or ramentaceone are limited to the phylogenetically distant families Ebenaceae (Ericales) and Iridaceae (Asparagales) [3]. Due to their indicative substitution patterns, it is assumed that the characteristic naphthoquinones in Nepenthales, Ebenaceae and Iridaceae are formed via the acetate-polymalonate route with hexaketide and 1,8-dihydroxy-3-methylnaphthalene intermediates [10]. A number of biosynthetic precursors, structural variants and di- or polymers have been described (e.g., droserone, isoshinanolone, shinanolone, 7-methylnaphthazarin, dihydroplumbagin, maritinone, mamegakinone [1,11], Figure 3). Because: 1. their structural affinities to the respective naphthoquinone are obvious; 2. they usually co-occur with the same regioisomer; and 3. their distribution has been portrayed satisfactorily before [1,11], the additional presence of one or several of these derivatives is of less chemotaxonomic interest here. Thus, they will not be addressed in detail in this review that will instead focus on plumbagin and ramentaceone in the Droseraceae. It should, however, be noted that naphthaldehydes and their polymers appear to be particularly common in Ebenaceae only, where they may contribute to the coloration of the heartwood (ebony, [12,13]).
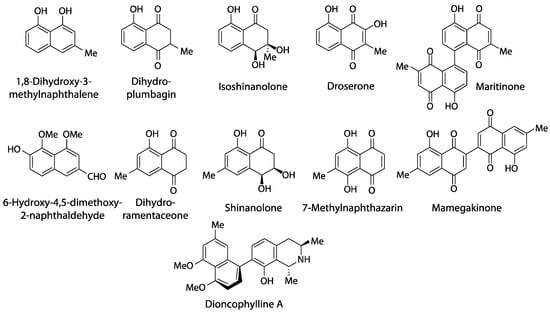
Figure 3.
Naphthalene derivatives related to plumbagin and ramentaceone.
Droseraceae are among the most diverse families of carnivorous plants, containing three genera, of which two are monotypic (Aldrovanda, a rare but widely distributed-azonal-aquatic in the Old World, and Dionaea, endemic to few localities in the southeastern United States). The third genus, Drosera, contains about 260 described species of almost cosmopolitan distribution but with only a few representatives (essentially from just a single section) in the northern hemisphere. A certain degree of motility in the leaf lamina and/or its substructures is usually associated with the adhesive (Drosera) or snap trap (Aldrovanda, Dionaea) mechanisms for prey capture. This is unique within carnivorous Nepenthales, of which the other members use passive (pitcher or adhesive) trap types [9].
Throughout the past decade, significant progress has been made in the screening for naphthoquinones in Droseraceae [14,15,16,17,18,19,20,21,22], and although several papers aimed to address these specific metabolites at various degrees of accuracy [1,23,24,25], none of the recent reviews represented their meanwhile known isomer distribution satisfactorily. It is the aim of the present account to fill this gap, so that chemotaxonomic considerations can be based on adequate data.
2. Review of Naphthoquinone Data from Droseraceae
The distribution of individual naphthoquinone isomers that have been detected in identified species of Droseraceae is summarized in Table 1 that also contains information on the classification of the investigated taxa, the provenance of the investigated samples and comments that essentially relate to the (few) differences between subsequent, independent studies.

Table 1.
Naphthoquinone distribution and classification of Droseraceae taxa.
3. Discussion
3.1. Screening Approaches to Naphthoquinones in Droseraceae
Since the first comparative study that covered a representative selection of (19) species of Droseraceae [26], Thin Layer Chromatography (TLC) has been established as a simple yet reliable, sensitive and highly specific detection and separation method for plumbagin and ramentaceone. This has been confirmed convincingly by a subsequent, more inclusive (63 species) survey that already outlined major distribution trends for either main quinone in several of the larger lineages within Drosera [27]. A recent review on secondary metabolites from Nepenthales [25] summarized “investigations based on modern identification techniques” to the explicit and deliberate exclusion of results from TLC, arguably the most efficient tool ever applied in comparative studies on these same metabolites. While more detailed analyses using additional methods, such as High-Performance Liquid Chromatography (HPLC) or Ultra-High-Performance Liquid Chromatography (UPLC) with Mass Spectrometry (MS) and Nuclear Magnetic Resonance spectroscopy (NMR), have indeed contributed to the detection and structural elucidation of several derivatives and potential biosynthetic intermediates of plumbagin and ramentaceone [11], TLC is and remains the principal source of secondary metabolite distribution data throughout Nepenthales and in Droseraceae.
3.2. Role of Naphthoquinones in Carnivory
While naphthoquinones may aid in preserving animal prey and protecting plant traps during digestion [44], and precursors acquired by carnivory are channeled into their biosynthetic pathway [45], the speculation that this may be an “important adaptation” across all carnivorous Nepenthales [46] is flawed by the fact that there are a few species of Nepenthes [47] and a comparatively large number of species of Drosera (Table 1, [21,22,27]) in which neither plumbagin nor ramentaceone have been detected. Although it was mentioned that the acetogenic naphthoquinones of carnivorous Nepenthales are lacking in all other lineages of carnivorous plants [46] where, interestingly, other characteristic acetogenic polyketides occur in some families such as the alkaloid coniine in Sarracenia [48], it was not appropriately considered that plumbagin is present in the closely related but non-carnivorous Plumbaginaceae (consecutive sister to the carnivorous clade of Nepenthales [9]) and Ancistrocladaceae (carnivory lost secondarily [9]). Both plumbagin and ramentaceone are known outside Nepenthales from the likewise non-carnivorous but phylogenetically distant Ebenaceae and Iridaceae [1], where they can obviously not constitute any adaptation to carnivory.
Carnivory has obviously been lost in most Dioncophyllaceae and throughout Ancistrocladaceae [9]. However, even these families retained the ability to form acetogenic naphthoquinones and/or tetralones [1,49], and they have additionally acquired the ability to form naphthylisoquinoline alkaloids (e.g., dioncophylline A, Figure 3), evidently from common biosynthetic precursors [50]. The erroneous statements that there “are no reports on the biosynthesis of this type of alkaloid” and “the origin of these alkaloids is phenylalanine” [51] are obviously untenable and incomprehensible in the light of the quite extensive relevant literature [50] that has been outright ignored.
3.3. Taxonomic Implications
3.3.1. Quinone Relationships of Droseraceae and Nepenthales
Three biosynthetic routes towards naphthoquinones are specific to a few angiosperm orders phylogenetically distant from Nepenthales, viz the 4-hydroxybenzoate/geranyl-pyrophosphate pathway leading to shikonin and alkannin in Boraginaceae (Boraginales), the homogentisate/mevalonate route to yield chimaphilin that is unique to Ericaceae subfamily Pyroloideae (Ericales), and the farnesyl-pyrophosphate pathway to mansonones and related cadinane sesquiterpenoids in Malvaceae (Malvales), Aristolochiaceae (Piperales), Ulmaceae (Rosales), Zingiberaceae (Zingiberales) [1,3,52,53].
Additionally, the geranylgeranyl-pyrophosphate pathway is used to form serrulatane diterpenoids (“extended cadinanes”) that may be converted to naphthoquinones (e.g., biflorin) in a similar fashion in Scrophulariaceae and Plantaginaceae (both Lamiales) [54]. These are noteworthy examples of convergence in chemical structure [3] without a common phylogenetic or biochemical background.
Several further naphthoquinones of unknown or unconfirmed biosynthetic origin [3] or of limited taxonomic distribution are scattered across the plant kingdom [1]; therefore, little can be said about their taxonomic significance at present.
Interestingly, a mutual exclusivity of emodin type octaketides and plumbagin type hexaketides is found both in Nepenthales (see below) and in the monocot order Asparagales, where the octaketide quinones are confined to Asphodelaceae (incl. Aloaceae, Dianellaceae, Eccremidaceae, Geitonoplesiaceae, Hemerocallidaceae, Johnsoniaceae, Phormiaceae, Xanthorrhoeaceae) [5], while hexaketide quinones are confined to Iridaceae [1,3]. This type of chemical similarity differs from the examples above because within the orders a common biosynthetic route (in this case, the acetate-polymalonate pathway) leads to quinone structures of different size but still retains characteristics that suggest phylogenetic relationship; thus, it does not appear to be convergent. Between the distant orders, the dualism between octaketides and hexaketides may indicate similar selective pressures that have resulted in similar developments; thus, the dualism as such is supposedly convergent. The genetics of these polyketides is, however, only poorly understood, and beyond a few expectable enzymes catalyzing individual biosynthetic steps [10], no comparative sequence data are known to reflect the obvious metabolic similarities nor the anticipated genetic differences.
No dualism between different polyketide classes is known with certainty in Ericales, where Ebenaceae stand isolated with their hexaketide-derived naphthoquinones [1,3]. Occasional reports of emodin from Actinidiaceae, Balsaminaceae and Primulaceae subfam. Myrsinoideae [5] are questionable and require confirmation of both the chemical structures and the biosynthetic routes. Ericales is nevertheless the order with the highest known and confirmed naphthoquinone diversity, as it also includes Ericaceae subfam. Pyroloideae containing the unique chimaphilin (see above), and Balsaminaceae containing lawsone (also known from Lythraceae, Myrtales). The latter is produced from o-succinylbenzoate [1,3], a common intermediate among plants because it also leads to phylloquinone, to juglone in Juglandaceae (Fagales) [1,3], and to the characteristic naphtho- and anthraquinones in numerous families of Lamiales (e.g., dunnione and lapachol), Proteaceae (Proteales, e.g., lomatiol), and of Rubiaceae (Gentianales, predominantly alizarin type anthraquinones) that are o-succinylbenzoate derivatives with a hemiterpene substituent at the position that is occupied by a diterpene chain in phylloquinone [2].
In Nepenthales, the early branching families Tamaricaceae and Frankeniaceae are not known to yield any characteristic quinones [1,5].
Polygonaceae is the only family in Nepenthales characterized by various octaketides like the anthraquinone emodin [5] instead of hexaketide-derived naphthoquinones.
In Plumbaginaceae, possibly the sister family of Polygonaceae, plumbagin has been recorded in all investigated species of subfam. Plumbaginoideae [1,55,56]. Ramentaceone has not been found yet in Plumbaginaceae, and none of the isomers are known so far from the subfam. Staticoideae [1].
In Ancistrocladaceae, Dioncophyllaceae and Drosophyllaceae, plumbagin and several of its derivatives and/or potential biosynthetic precursors have been detected [1,49,57]. This strongly indicates that plumbagin formation is a symplesiomorphic character state among those members of Nepenthales that contain acetogenic naphthoquinones.
In Drosophyllum (Drosophyllaceae), dihydroramentaceone has been identified as an additional constituent besides plumbagin [11]. A previous report of ramentaceone itself [27] was not confirmed afterwards but it may possibly be formed spontaneously from the dihydroquinone.
The tendency that both monotypic genera (Aldrovanda, Dionaea) of Droseraceae and the earliest branching lineage in Drosera (D. regia) contain plumbagin (Table 1, Figure 4) confirms the general trend that this isomer is also more widespread outside Droseraceae. Within Nepenthales, the only other family in which a reasonable number of species have been identified to contain the other isomer (ramentaceone) is Nepenthaceae [47], the immediate sister of Droseraceae [9].
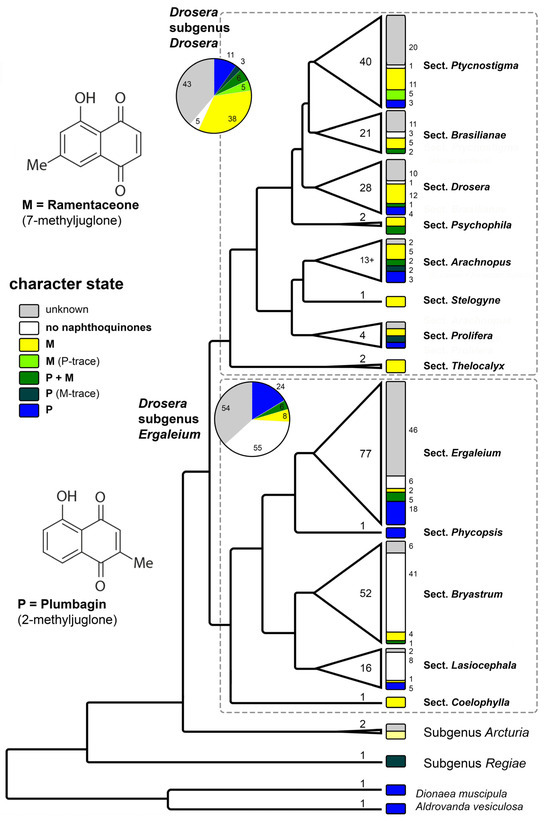
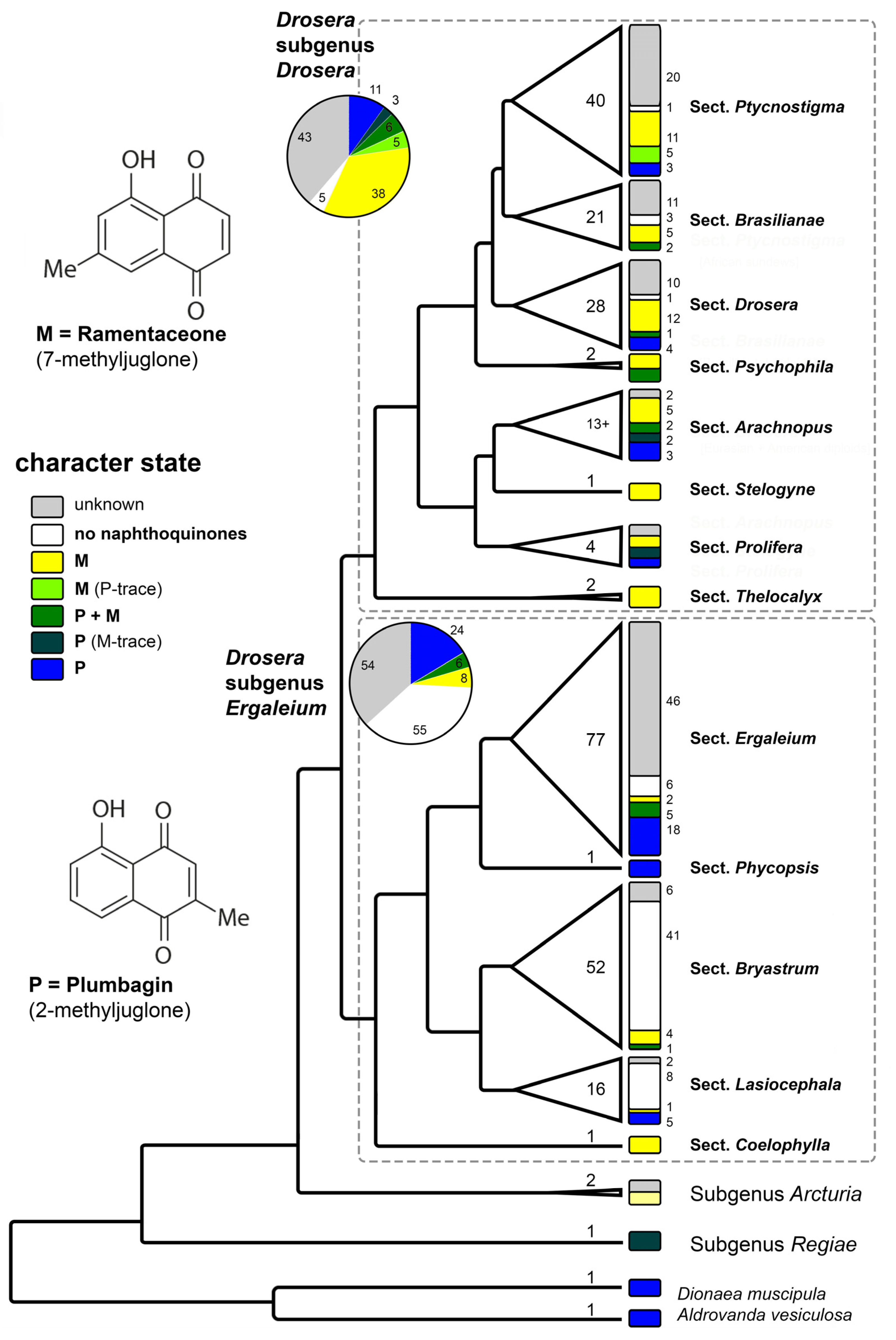
Figure 4.
Phylogenetic pattern of naphthoquinone distribution in Droseraceae. Cladogram modified from [43]. Numbers within the triangles and width of the triangles correspond to species number of each lineage. Numbers in small font correspond to the number of species showing the respective character state. For this diagram, only measurements from [11,14,15,16,17,18,19,20,21,22] were considered. Illustration by Andreas Fleischmann.
3.3.2. Quinone Relationships within Drosera
The different sections in the predominantly Australian Drosera subgenus Ergaleium show noteworthy correlations between quinone patterns and life-forms.
D. sect. Coelophylla (a single species of therophytes confined to southwestern and southern Australia) contains ramentaceone and is thus phytochemically characterized as a distinct, supposedly early branching clade within the subgenus (supported phylogenetically [43]). Additionally, the section also features a unique, one-shot catapult-flypaper trap [58].
The three morphologically (but not phylogenetically) separated groups Ergaleium, Stoloniferae, and Erythrorhiza of D. sect. Ergaleium are composed of stem tuber geophytes, and most of which are confined to southwestern Australia. The delimitation of the three subgroups is based on morphological characteristics, of which some may be convergent; thus, the assignment of a few species in Table 1 is tentative. In this group plumbagin is usually the dominant naphthoquinone. The detection of ramentaceone in two taxa of D. sect. Stoloniferae indicates that screening this group further will yield taxonomic information.
With plumbagin as the main quinone D. sect., Phycopsis (hemicryptophytes from E Australia to New Zealand) is chemically close to the tuberous sections.
Sections Lasiocephala and Bryastrum are largely hemicryptophytes; a few species of the former are also bulbous geophytes. While D. sect. Bryastrum is almost confined to SW Australia (but two species, one, D. pygmaea extending to southern Australia and NZ, and the other, D. meristocaulis, has a disjunct distribution confined to a single mountain peak in the Amazonian part of the Guayana Highlands); D. sect. Lasiocephala is distributed from northern Australia to New Guinea. In the D. sect., transformed Bryastrum leaves (gemmae) are seasonally formed for vegetative reproduction, while gemmae are missing in D. sect. Lasiocephala (and in D. meristocaulis). Although the two groups are morphologically, ecologically and chorologically distinct, both sections are phylogenetic sister lineages and are notable for the absence of naphthoquinones in most species (including the disjunct D. meristocaulis), clearly a secondary loss. The presence of a few species that contain quinones in both sections indicates that the loss has either occurred twice independently or that quinone production is easily restored.
The other large subgenus, D. subgen. Drosera is far more widespread (subcosmopolitan) and is composed predominantly of hemicryptophytes adapted to various climatic conditions.
D. sect. Thelocalyx has a tropical distribution in Australasia and South America. Its two species are therophytes that are so similar to each other that it can become difficult to identify them if the provenance is unknown. This is also reflected in their common formation of ramentaceone.
D. sect. Prolifera is another tropical group but this one is restricted to northern Queensland. Ramentaceone is found in this section as well, but D. prolifera contains plumbagin instead.
D. sect. Arachnopus consists of therophytes with one species from Africa to Eastern Asia, three species in SE Asia and Australia and about a dozen endemic to Australia. Besides the adaptation to an annual life cycle, this section has developed an amazing diversity of quinone patterns. It is noteworthy that morphologically similar and geographically overlapping sister species (D. aquatica/D. nana and D. hartmeyerorum/D. barrettiorum) usually also exhibit similar quinone patterns (ramentaceone dominant in the mentioned species). The widespread D. indica contains both quinone isomers, which is a rare feature in Drosera, usually resulting from hybridization [14].
D. sect. Stelogyne, a monotypic section comprising a single relict species from SW Australia containing ramentaceone (like in the more speciose sections mentioned below), occupies a morphologically and phylogenetically isolated position in D. subgen. Drosera.
D. sect. Psychophila has a “Gondwanan” distribution with one species in New Zealand and the other from southern South America (cf. the decidedly more tropical D. sect. Thelocalyx that likewise has a gap in Africa). Their quinone patterns involve both isomers at various proportions and do not provide clear taxonomic clues.
D. sect. Drosera is distributed throughout the northern hemisphere and in South America, with four species extending into tropical Asia and Malesia and one of them further to Australia and New Zealand. The section is characterized by a dominance of ramentaceone in most species. Most members of this lineage are diploid. Where hybridization involves the few species of this sections that contain plumbagin, the hybrids usually contain both isomers [14].
Essentially the same situation (ramentaceone dominant) is also present in D. sect. Ptycnostigma (Africa) and in D. sect. Brasilianae (Brazil)—both sections largely comprise tetraploids or polyploids. D. sect. Brasilianae shows an additional trend by several species lacking naphthoquinones whatsoever, which may be another (and very probably independent) secondary loss involving several related species in the genus.
4. Conclusions
As detailed in the discussion, the acetogenic naphthoquinones plumbagin and ramentaceone are valuable phytochemical markers that aid in the delimitation, identification and classification of taxa in Droseraceae (and beyond). Their presence/absence is stable enough within and between populations and taxa to provide reliable taxonomic information and phylogenetic signal.
In comparison to other groups of comparable distribution and size, plants of the order Nepenthales belonging to other families than Droseraceae have been subject to rather thorough investigation with respect to their secondary metabolites [1,47,49,55,56,57]. However, whereas the naphthylisoquinoline alkaloids of Ancistrocladaceae are fairly well known [59], only three of 21 species of Ancistrocladus have been investigated for naphthoquinones so far [1].
A few species groups in Drosera are still underinvestigated, e.g., D. subgen. Arcturia, D. sect. Ergaleium—“Stoloniferae”, D. sect. Prolifera, and, to some extent, D. sect. Arachnopus. Access to suitable plant material for investigation is the main bottleneck for chemotaxonomic progress that can be expected in these cases.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hook, I.; Mills, C.; Sheridan, H. Bioactive naphthoquinones from higher plants. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 119–160. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.W.; Bahamon Naranjo, M.A.; Widhalm, J.R. Convergent evolution of plant specialized 1,4-naphthoquinones: Metabolism, trafficking, and resistance to their allelopathic effects. J. Exp. Bot. 2021, 72, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Gerschler, S.; Neumann, N.; Schultze, N.; Guenther, S.; Schulze, C. Quality parameters for the medicinal plant Drosera rotundifolia L.: A new approach with established techniques. Arch. Pharm. 2023, 357, e2300436. [Google Scholar] [CrossRef] [PubMed]
- Izhaki, I. Emodin—A secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217. [Google Scholar] [CrossRef]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Guo, R.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Henarejos-Escudero, P.; Guadarrama-Flores, B.; García-Carmona, F.; Gandía-Herrero, F. Digestive glands extraction and precise pigment analysis support the exclusion of the carnivorous plant Dionaea muscipula Ellis from the Caryophyllales order. Plant Sci. 2018, 274, 342–348. [Google Scholar] [CrossRef]
- Dávila-Lara, A.; Reichelt, M.; Wang, D.; Vogel, H.; Mithöfer, A. Proof of anthocyanins in the carnivorous plant genus Nepenthes. FEBS Open Bio 2021, 11, 2576–2585. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schlauer, J.; Smith, S.A.; Givnish, T.J. Evolution of carnivory in angiosperms. In Carnivorous Plants: Physiology, Ecology, and Evolution; Adamec, L., Ellison, A., Eds.; Oxford University Press: London, UK, 2017; pp. 22–42. [Google Scholar] [CrossRef]
- Vasav, A.P.; Meshram, B.G.; Pable, A.A.; Vitthal, T.; Barvkar, V.T. Artificial microRNA mediated silencing of cyclase and aldo–keto reductase genes reveal their involvement in the plumbagin biosynthetic pathway. J. Plant Res. 2023, 136, 47–62. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Seppänen-Laakso, T.; Rischer, H. Contrasting dihydronaphthoquinone patterns in closely related Drosera (sundew) species enable taxonomic distinction and identification. Plants 2021, 10, 1601. [Google Scholar] [CrossRef]
- Brown, A.G.; Thomson, R.H. Ebenaceae Extractives. Part II Naphthaldehydes from Diospyros ebenum Koen. J. Chem. Soc. 1965, 4292–4295. [Google Scholar] [CrossRef]
- Matsushita, Y.; Jang, I.; Imai, T.; Fukushima, K.; Lee, S. Naphthalene derivatives from Diospyros kaki. J. Wood Sci. 2010, 56, 418–421. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A. Chemical evidence for hybridity in Drosera (Droseraceae). Biochem. Syst. Ecol. 2016, 66, 33–36. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Unexpected discovery of Ramentaceone (7-Methyljuglone) in several Australian sundews. Carniv. Pl. Newslett. 2017, 46, 20–22. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Hennern, H.; Hennern, A. Sundew chemistry and emergence updates. Carniv. Plant Newsl. 2018, 47, 10–17. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Hennern, H.; Hennern, A. New sundew quinone and emergence data. Carniv. Plant Newsl. 2019, 48, 6–12. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A.; Carow, T. Quinones from “Gondwanan” Drosera taxa. Carniv. Plant Newsl. 2019, 48, 13–17. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Chemistry and surface micromorphology of the Queensland sundews. Carniv. Plant Newsl. 2019, 48, 111–116. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Quinone Patterns and Identification of Japanese Spider Leg Sundews. Carniv. Plant Newsl. 2019, 48, 161–163. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A. Naphthoquinones in Pygmy Sundews (Drosera sect. Bryastrum). Carniv. Plant Newsl. 2021, 50, 111–117. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A. Refined Taxon Sampling Discloses New Quinone Patterns and Relationships among Sundews (Drosera, Droseraceae). Carniv. Plant Newsl. 2022, 51, 70–73. [Google Scholar] [CrossRef]
- Devi, S.P.; Kumaria, S.; Rao, S.R.; Tandon, P. Carnivorous Plants as a Source of Potent Bioactive Compound: Naphthoquinones. Trop. Plant Biol. 2016, 9, 267–279. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Kumari, L.; Nakhate, K.; Verma, V.S.; Sakure, K. Phytoconstituent plumbagin: Chemical, biotechnological and pharmaceutical aspects. Stud. Nat. Prod. Chem. 2019, 63, 415–460. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Carnivorous Plants from Nepenthaceae and Droseraceae as a Source of Secondary Metabolites. Molecules 2023, 28, 2155. [Google Scholar] [CrossRef] [PubMed]
- Zenk, M.H.; Fürbringer, M.; Steglich, W. Occurrence and distribution of ramentaceone and plumbagin in the Droseraceae. Phytochemistry 1969, 8, 2199–2200. [Google Scholar] [CrossRef]
- Culham, A.; Gornall, R.J. The taxonomic significance of naphthoquinones in the Droseraceae. Biochem. Syst. Ecol. 1994, 22, 507–515. [Google Scholar] [CrossRef]
- Schlauer, J. Literature Reviews. Carniv. Plant Newsl. 2012, 41, 121. Available online: https://cpn.carnivorousplants.org/articles/CPNv41n3p121.pdf (accessed on 30 January 2024).
- Länger, R.; Pein, I.; Kopp, B. Glandular hairs in the genus Drosera (Droseraceae). Plant Syst. Evol. 1995, 194, 163–172. [Google Scholar] [CrossRef]
- Durand, R.; Zenk, M.H. Homogentisate ring-cleavage pathway in the biosynthesis of acetate-derived naphthoquinones of the Droseraceae. Phytochemistry 1974, 13, 1483–1492. [Google Scholar] [CrossRef]
- Nahrstedt, A. Absence of cyanogenesis from Droseraceae. Phytochemistry 1980, 19, 2757–2758. [Google Scholar] [CrossRef]
- Kovacik, J.; Repcak, M. Naphthoquinones Content of Some Sundews (Drosera L.). Carniv. Plant Newsl. 2006, 35, 49–51. [Google Scholar] [CrossRef]
- Egan, P.A.; van der Kooy, F. Phytochemistry of the Carnivorous Sundew Genus Drosera (Droseraceae)—Future Perspectives and Ethnopharmacological Relevance. Chem. Biodivers. 2013, 10, 1774–1790. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, J. Naphthoquinone Glucosides of Drosera gigantea from in vitro Cultures. Planta Med. 2000, 66, 667–669. [Google Scholar] [CrossRef]
- Le Clercq, J.; Angenot, L. À propos du Drosera peltata et de la standardisation de la teinture de Drosera. J. Pharm. Belg. 1984, 39, 269–274. Available online: https://hdl.handle.net/2268/41270 (accessed on 30 January 2024).
- Krenn, L.; Länger, R.; Kopp, B. Qualitätsprüfung von Sonnentaukraut. 2. Botanische Identitätsprüfung sowie qualitative und quantitative Naphthochinonbestimmung an Handelsmustern. Dtsch. Apoth. Ztg. 1995, 135, 867–870. [Google Scholar]
- Putalun, W.; Udomsin, O.; Yusakul, G.; Juengwatanatrakul, T.; Sakamoto, S.; Tanaka, H. Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol. Lett. 2010, 32, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Trevisan Ferreira, D.; Andrei, C.C.; Ostrensky Saridakis, H.; Faria, T.J.; Vinhato, E.; Carvalho, K.E.; Feijó Souza, D.J.; Machado, S.L.; Panayotis Saridakis, D.; Braz-Filho, R. Antimicrobial Activity and Chemical Investigation of Brazilian Drosera. Mem. Inst. Oswaldo Cruz Rio Jan. 2004, 99, 753–755. [Google Scholar] [CrossRef]
- Sauerwein, M.; Schmidt, S.; Reichling, J.; Wink, M. Naphthoquinone production in in vitro cultures of Drosera communis. St. Hil. BIOforum Extra Prague 1994, 94, 26–27. [Google Scholar]
- Bonnet, M.; Coumans, M.; Hofinger, M.; Ramaut, J.L.; Gaspar, T. High-performance gas chromatography of 1,4-naphthoquinomes from Droseraceae. Chromatographia 1984, 18, 621–622. [Google Scholar] [CrossRef]
- Crouch, I.J.; Finnie, J.F.; van Staden, J. Studies on the isolation of plumbagin from in vitro and in vivo grown Drosera species. Plant Cell Tissue Organ Cult. 1990, 21, 78–82. [Google Scholar] [CrossRef]
- Egan, P.A.; van der Kooy, F. Coproduction and Ecological Significance of Naphthoquinones in Carnivorous Sundews (Drosera). Chem. Biodivers. 2012, 9, 1033–1044. [Google Scholar] [CrossRef]
- Fleischmann, A.; Cross, A.T.; Gibson, R.; Gonella, P.M.; Dixon, K.W. Systematics and Evolution of Droseraceae. In Carnivorous Plants: Physiology, Ecology, and Evolution; Adamec, L., Ellison, A., Eds.; Oxford University Press: London, UK, 2017; pp. 45–57. [Google Scholar] [CrossRef]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Rischer, H.; Hamm, A.; Bringmann, G. Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry 2002, 59, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, C.R.; Ryves, D.B.; Millett, J. The Function of Secondary Metabolites in Plant Carnivory. Ann. Bot. 2020, 125, 399–411. [Google Scholar] [CrossRef]
- Schlauer, J.; Wistuba, A.; Hartmeyer, S.R.H.; Hartmeyer, I. The taxonomic relevance of naphthoquinones in tropical pitcher plants (Nepenthes L., Nepenthaceae). Carniv. Plant Newsl. 2022, 51, 185–193. [Google Scholar] [CrossRef]
- Hotti, H.; Gopalacharyulu, P.; Seppänen-Laakso, T.; Rischer, H. Metabolite profiling of the carnivorous pitcher plants Darlingtonia and Sarracenia. PLoS ONE 2017, 12, e0171078. [Google Scholar] [CrossRef]
- Hanson, S.W.; Crawford, M.; Thanasingh, D.P.J. (+)-Isoshinanolone and 2-methylbenzofuran-4-carbaldehyde from the Fish-Stunning Plant Habropetalum dawei. Phytochemistry 1981, 20, 1162–1164. [Google Scholar] [CrossRef]
- Bringmann, G.; Wohlfarth, M.; Rischer, H.; Grüne, M.; Schlauer, J. A New Biosynthetic Pathway to Alkaloids in Plants: Acetogenic Isoquinolines. Angew. Chem. Int. Ed. 2000, 39, 1464–1466. Available online: http://10.1002/(SICI)1521-3773(20000417)39:8<1464::AID-ANIE1464>3.0.CO;2-%23 (accessed on 30 January 2024). [CrossRef]
- Funayama, S.; Cordell, G.A. Alkaloids—A Treasury of Poisons and Medicines; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 263–273. [Google Scholar] [CrossRef]
- Ogihara, K.; Zhao, J.; Higa, M.; Yogi, S. Studies on the Constituents of Aristolochia liukiuensis 2. Bull. Coll. Science. Univ. Ryukyus 1992, 54, 17–28. Available online: https://u-ryukyu.repo.nii.ac.jp/record/2005300/files/No54p017.pdf (accessed on 30 January 2024).
- Connolly, J.D.; Hill, R.A. Dictionary of Terpenoids; Chapman & Hall: London, UK, 1991. [Google Scholar]
- Forster, P.G.; Ghisalberti, E.L.; Jefferies, P.R.; Poletti, V.M.; Whiteside, N.J. Serrulatane Diterpenes from Eremophila spp. Phytochemistry 1986, 25, 1377–1383. [Google Scholar] [CrossRef]
- van der Vijver, L.M. Distribution of Plumbagin in the Plumbaginaceae. Phytochemistry 1972, 11, 3247–3248. [Google Scholar] [CrossRef]
- Shcherbanovskii, L.R. Plumbagin from Plumbagella micrantha. Chem. Nat. Compd. 1974, 10, 518. [Google Scholar] [CrossRef]
- Budzianowski, J.; Budzianowska, A.; Kromer, K. Naphthalene Glucoside and Other Phenolics from the Shoot and Callus Cultures of Drosophyllum lusitanicum. Phytochemistry 2002, 61, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.; Hartmeyer, S.R.H.; Seidel, R.; Masselter, T.; Hartmeyer, I.; Speck, T. Catapulting Tentacles in a Sticky Carnivorous Plant. PLoS ONE 2012, 7, e45735. [Google Scholar] [CrossRef]
- Feineis, D.; Bringmann, G. Ancistrocladus Naphthylisoquinoline Alkaloids. Progress. Chem. Org. Nat. Prod. 2023, 119, 1–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).