Projecting the Global Potential Geographical Distribution of Ceratitis capitata (Diptera: Tephritidae) under Current and Future Climates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. CLIMEX Model
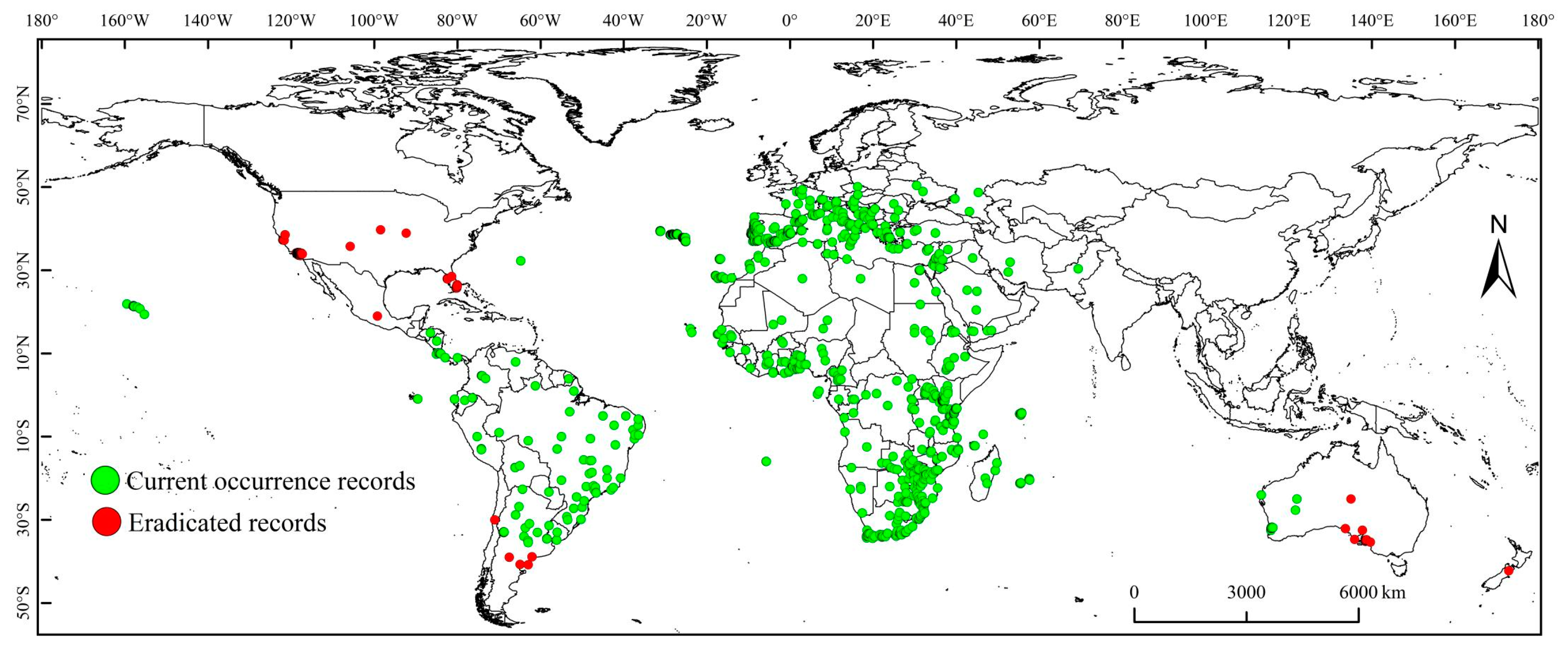
2.2. Species Occurrence Records
2.3. Climate Data
2.4. Irrigation Data
2.5. Fitting CLIMEX Parameters
2.5.1. Growth Index
2.5.2. Temperature Index
2.5.3. Moisture Index
2.5.4. Cold Stress
2.5.5. Heat Stress
2.5.6. Dry Stress
2.5.7. Wet Stress
2.5.8. Degree Days per Generation
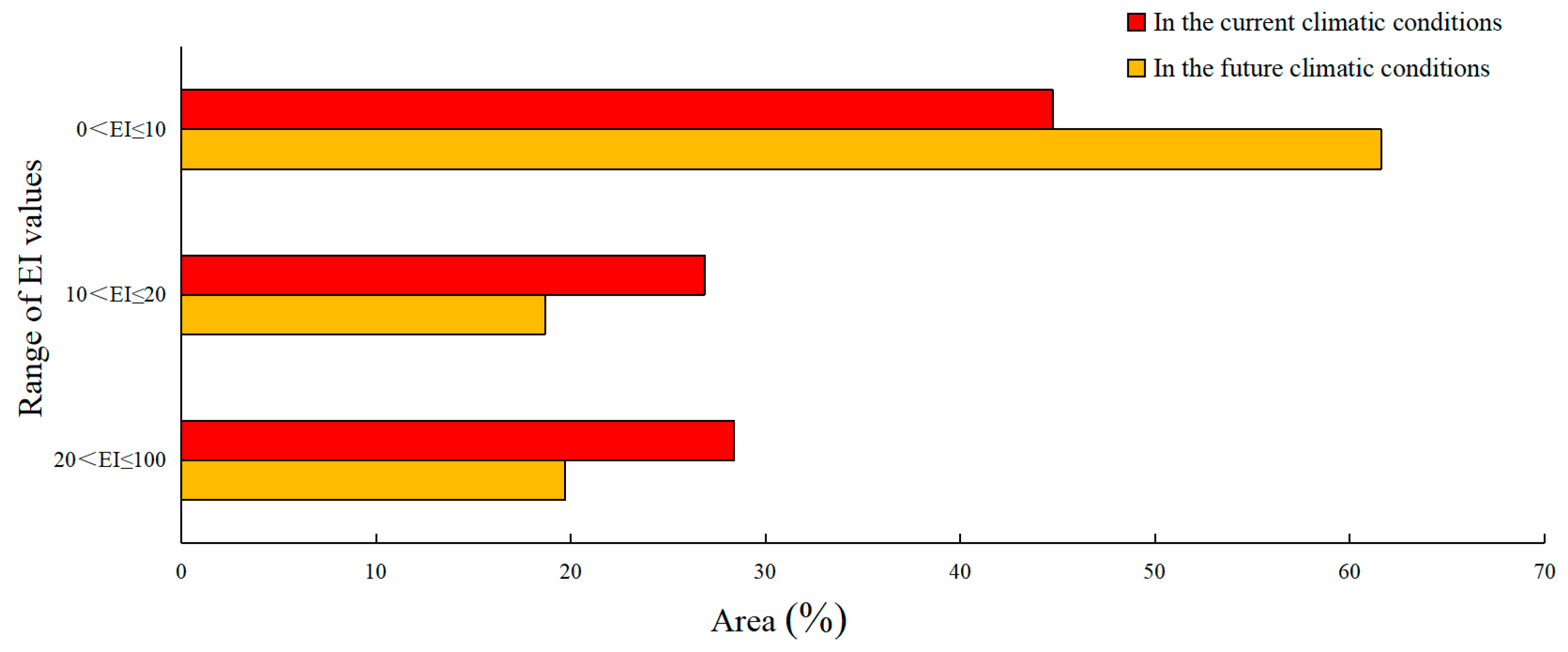
2.6. Classification of EI Values
2.7. Mapping the Potential Geographical Distribution and Spatial Variation in PGD
3. Results
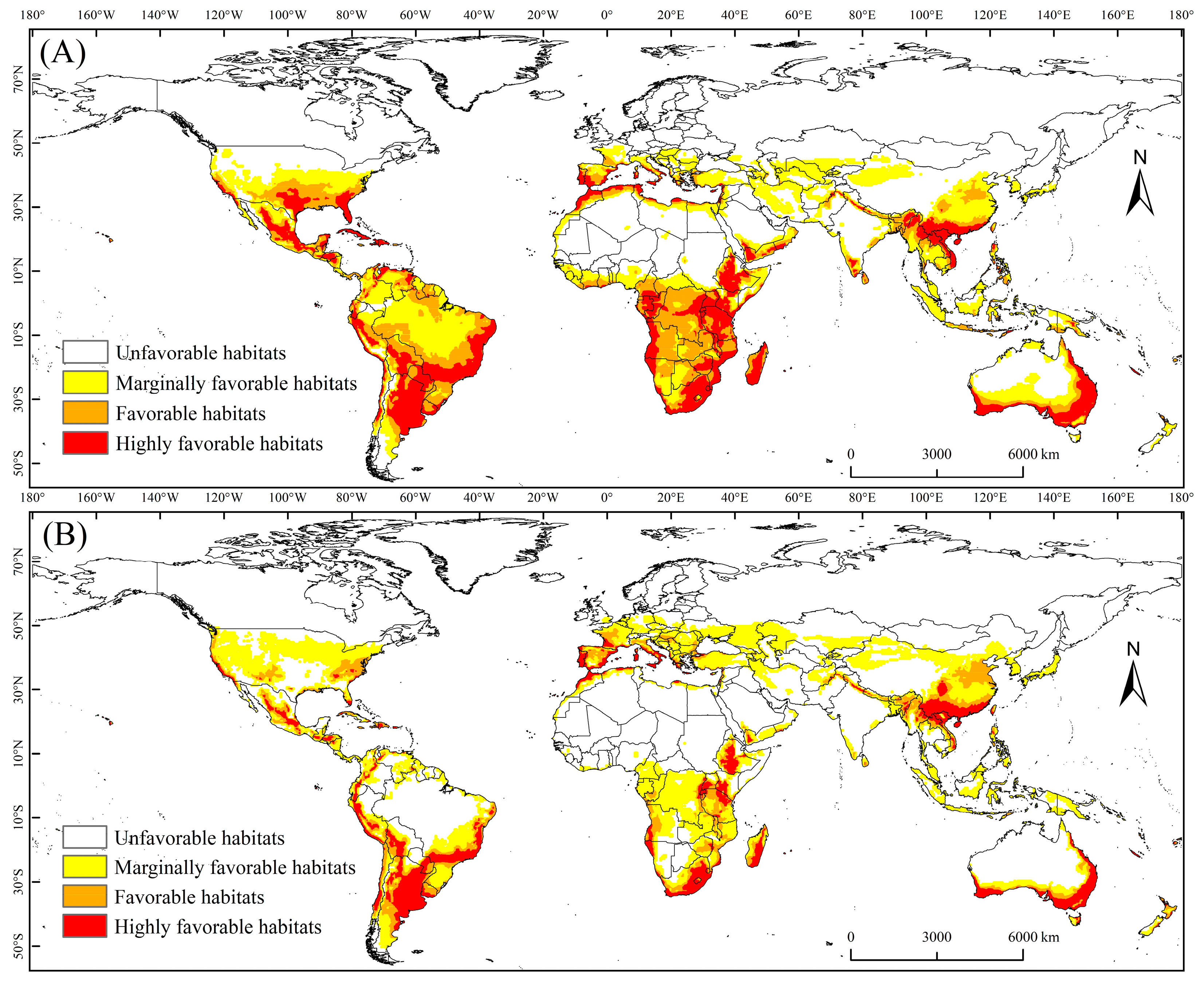
3.1. PGD of the MFF under Current Climate Conditions
3.2. PGD of the MFF under the Future Climate Scenario
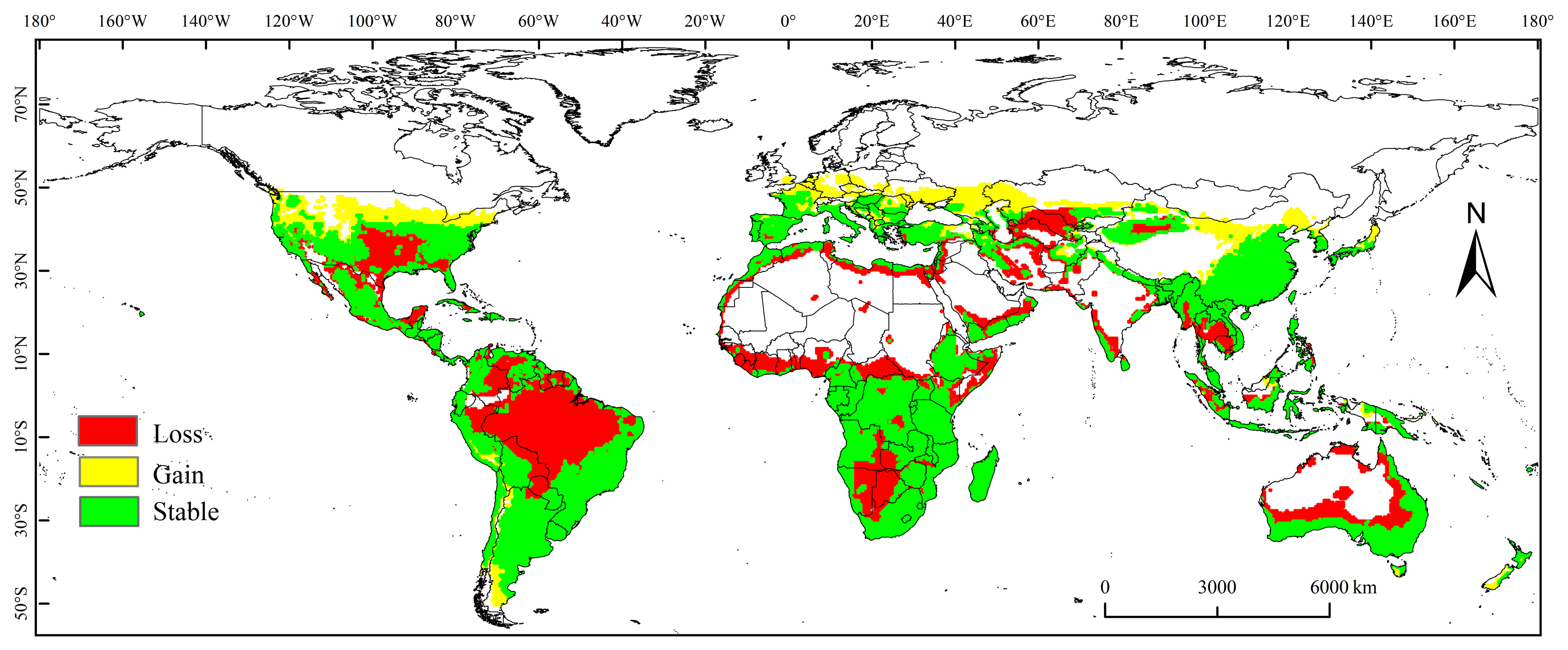
3.3. Spatial Variation in PGD under Future Climate Conditions
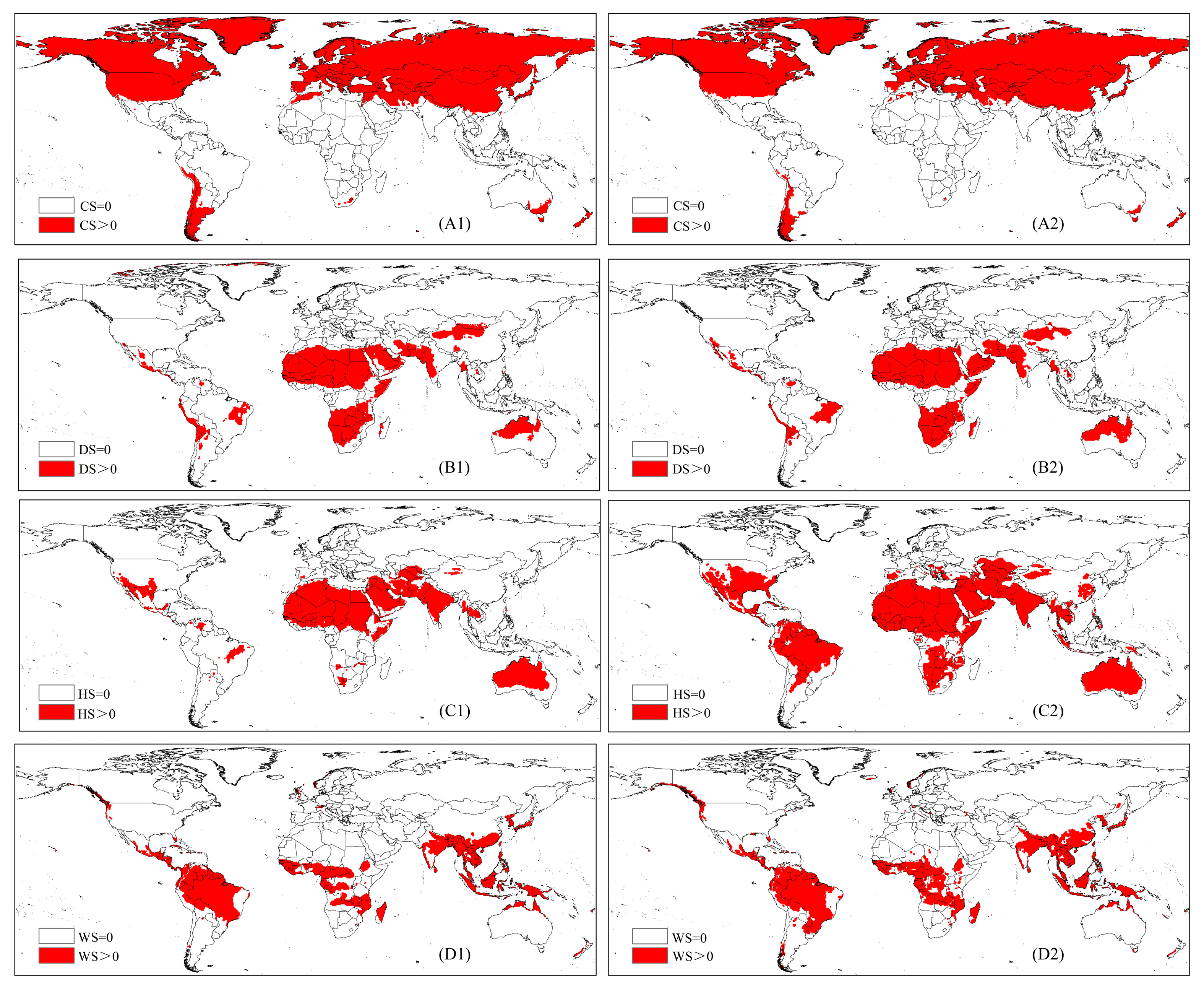
3.4. Driving Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, I.M. Taxonomy of the Dacina (Diptera: Tephritidae) of Africa and the Middle East, Afr. Entomol. 2006, 1–156. [Google Scholar]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive Phytophagous Pests Arising through a Recent Tropical Evolutionary Radiation: The Bactrocera dorsalis Complex of Fruit Flies. Annu. Rev. Entomol. 2005, 50, 293. [Google Scholar] [CrossRef]
- Rwomushana, I.; Ekesi, S.; Gordon, I.; Ogol, C.K.P.O. Host Plants and Host Plant Preference Studies for Bactrocera invadens (Diptera: Tephritidae) in Kenya, a New Invasive Fruit Fly Species in Africa. Ann. Entomol. Soc. Am. 2008, 101, 331–340. [Google Scholar] [CrossRef]
- Yee, W.L.; Goughnour, R.B. Host Plant Use by and New Host Records of Apple Maggot, Western Cherry Fruit Fly, and Other Rhagoletis Species (Diptera: Tephritidae) in Western Washington State. Pan-Pac. Entomol. 2008, 84, 179–193. [Google Scholar] [CrossRef]
- Schutze, M.K.; Mahmood, K.; Pavasovic, A.N.; Bo, W.; Newman, J.; Clarke, A.R.; Krosch, M.N.; Cameron, S.L. One and the Same: Integrative Taxonomic Evidence that Bactrocera invadens (Diptera: Tephritidae) is the Same Species as the Oriental Fruit Fly Bactrocera dorsalis. Syst. Entomol. 2015, 40, 472–486. [Google Scholar] [CrossRef]
- Karsten, M.; Hansen van Vuuren, B.; Addison, P.; Terblanche, J.S. Deconstructing Intercontinental Invasion Pathway Hypotheses of the Mediterranean Fruit Fly (Ceratitis Capitata) Using a Bayesian Inference Approach: Are Port Interceptions and Quarantine Protocols Successfully Preventing New Invasions? Divers. Distrib. 2015, 21, 813–825. [Google Scholar] [CrossRef]
- Quayle, H.J. Citrus Fruit Insects in Mediterranean Countries; No. 134; U.S. Department of Agriculture: Washington, DC, USA, 1914. [Google Scholar]
- Gasperi, G.; Bonizzoni, M.; Gomulski, L.M.; Murelli, V.; Torti, C.; Malacrida, A.R.; Guglielmino, C.R. Genetic Differentiation, Gene Flow and the Origin of Infestations of the Medfly, Ceratitis capitata. Genetica 2002, 116, 125–135. [Google Scholar] [CrossRef]
- Szyniszewska, A.M.; Tatem, A.J. Global Assessment of Seasonal Potential Distribution of Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae). PLoS ONE 2014, 9, e111582. [Google Scholar] [CrossRef]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and Fruitfly Invasion and Expansion: The Medfly Paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/CERTCA (accessed on 15 September 2023).
- Papadopoulos, N.T.; Katsoyannos, B.I.; Kouloussis, N.A.; Hendrichs, J.; Carey, J.R.; Heath, R.R. Early Detection and Population Monitoring of Ceratitis capitata (Diptera: Tephritidae) in a Mixed-Fruit Orchard in Northern Greece. J. Econ. Entomol. 2001, 94, 971–978. [Google Scholar] [CrossRef]
- Cayol, J.P.; Causse, R. Mediterranean Fruit Fly Ceratitis capitata Wiedemann (Dipt., Trypetidae) back in Southern France. J. Appl. Entomol. 1993, 116, 94–100. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. The Mediterranean Fruit Fly (Diptera: Tephritidae), a Key Pest of Citrus in Egypt. J. Integr. Pest. Manag. 2021, 12, 28. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F.; Bourne, A.S. Including Species Interactions in Risk Assessments for Global Change. Glob. Chang. Biol. 2007, 13, 1843–1859. [Google Scholar] [CrossRef]
- Early, R.; Rwomushana, I.; Chipabika, G.; Day, R. Comparing, Evaluating and Combining Statistical Species Distribution Models and CLIMEX to Forecast the Distributions of Emerging Crop Pests. Pest. Manag. Sci. 2022, 78, 671–683. [Google Scholar] [CrossRef]
- Falla, C.M.; Avila, G.A.; McColl, S.T.; Minor, M.; Najar-Rodríguez, A.J. The Current and Future Potential Distribution of Gargaphia decoris: A Biological Control Agent for Solanum mauritianum (Solanaceae). Biol. Control 2021, 160, 104637. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Kean, J.M.; Phillips, C.B.; Senay, S.D.; Acosta, H.; Haye, T. The Potential Global Distribution of the Brown Marmorated Stink Bug, Halyomorpha halys, a Critical Threat to Plant Biosecurity. J. Pest. Sci. 2017, 90, 1033–1043. [Google Scholar] [CrossRef]
- CABI Compendium. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.12367 (accessed on 15 September 2023).
- BOLD Systems. Available online: https://v3.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=25698 (accessed on 15 September 2023).
- CliMond. Available online: https://www.climond.org/Core/Authenticated/Climex.aspx (accessed on 15 September 2023).
- Liu, K.; Wang, Q.; Wang, M.; Koks, E.E. Global Transportation Infrastructure Exposure to the Change of Precipitation in a Warmer World. Nat. Commun. 2023, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.T.; Rodriguez, R.; Segura, D.F.; Cladera, J.L.; Sutherst, R.W. Potential Geographical Distribution of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae), with Emphasis on Argentina and Australia. Environ. Entomol. 2002, 31, 1009–1022. [Google Scholar] [CrossRef]
- Guo, S.; Ge, X.; Zou, Y.; Zhou, Y.; Wang, T.; Zong, S. Projecting the Global Potential Distribution of Cydia pomonella (Lepidoptera: Tortricidae) under Historical and RCP4.5 Climate Scenarios. J. Insect Sci. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Szyniszewska, A.M.; Leppla, N.C.; Manoukis, N.C.; Collier, T.C.; Hastings, J.M.; Kriticos, D.J.; Bigsby, K.M. CLIMEX and MED-FOES Models for Predicting the Variability in Growth Potential and Persistence of Mediterranean Fruit Fly (Diptera: Tephritidae) Populations. Ann. Entomol. Soc. Am. 2020, 113, 114–124. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global High-Resolution Historical and Future Scenario Climate Surfaces for Bioclimatic Modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Duyck, P.F.; Quilici, S. Survival and Development of Different Life Stages of Three Ceratitis spp. (Diptera: Tephritidae) Reared at Five Constant Temperatures. Bull. Entomol. Res. 2002, 92, 461–469. [Google Scholar] [CrossRef]
- Back, E.A.; Pemberton, C.E. Susceptibility of Citrous Fruits to the Attack of the Mediterranean Fruit Fly. J. Agric. Res. 1915, 3, 311–330. [Google Scholar]
- Thomas, M.C.; Heppner, J.B.; Woodruff, R.E.; Weems, H.V., Jr.; Steck, G.J.; Fasulo, T.R. Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann) (Insecta: Diptera: Tephritidae): EENY-214/IN371, rev. 9/2001. EDIS 2004, 2004. [Google Scholar] [CrossRef]
- Elnagar, S.; Smith, D.; Peterson, A.T. Mapping the Risk of West Nile Virus in North America. Int. J. Health Geogr. 2010, 9, 71. [Google Scholar]
- Zou, Y.; Ge, X.; Guo, S.; Zhou, Y.; Wang, T.; Zong, S. Impacts of Climate Change and Host Plant Availability on the Global Distribution of Brontispa longissima (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2020, 76, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Liu, Y.L.; Qin, H.; Meng, Q.X. Prediction on Spatial Migration of Suitable Distribution of Elaeagnus mollis under Climate Change Conditions in Shanxi Province, China. J. Appl. Ecol. 2019, 30, 496–502. [Google Scholar]
- Liu, H.; Zhang, C.; Hou, B.H.; Ou-Yang, G.C.; Ma, J. Interspecific Competition Between Ceratitis capitata and Two Bactrocera spp. (Diptera: Tephritidae) Evaluated via Adult Behavioral Interference under Laboratory Conditions. J. Econ. Entomol. 2017, 110, 1145–1155. [Google Scholar] [CrossRef]
- Ruiz-Arce, R.; Todd, T.N.; Deleon, R.; Barr, N.B.; Virgilio, M.; De Meyer, M.; McPheron, B.A. Worldwide Phylogeography of Ceratitis capitata (Diptera: Tephritidae) Using Mitochondrial DNA. J. Econ. Entomol. 2020, 113, 1455–1470. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Q.; Zhao, W.; Zhang, H. Predicting the Potential Distributions of the Invasive Cycad Scale Aulacaspis yasumatsui (Hemiptera: Diaspididae) under Different Climate Change Scenarios and the Implications for Management. PeerJ 2018, 6, e4832. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yee, W.L.; Neven, L.G. Mapping Global Potential Risk of Establishment of Rhagoletis pomonella (Diptera: Tephritidae) Using MaxEnt and CLIMEX Niche Models. J. Econ. Entomol. 2016, 109, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castañeda, G.; MacVean, C.; Cardona, C.; Hof, A.R. What Limits the Distribution of Liriomyza Huidobrensis and Its Congener Liriomyza Sativae in Their Native Niche: When Temperature and Competition Affect Species’ Distribution Range in Guatemala. J. Insect Sci. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, J.; Hu, X.; Liu, H.; Zhang, R. Potential Geographical Distributions of the Fruit Flies Ceratitis capitata, Ceratitis cosyra, and Ceratitis rosa in China. J. Econ. Entomol. 2009, 102, 1781–1790. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Potter, K.J.B.; Alexander, N.S.; Gibb, A.R.; Suckling, D.M. Using a Pheromone Lure Survey to Establish the Native and Potential Distribution of an Invasive Lepidopteran, Uraba lugens. J. Appl. Ecol. 2007, 44, 853–863. [Google Scholar] [CrossRef]
- van Lierop, P.; Lindquist, E.; Sathyapala, S.; Franceschini, G. Global Forest Area Disturbance from Fire, Insect Pests, Diseases and Severe Weather Events. For. Ecol. Manag. 2015, 352, 78–88. [Google Scholar] [CrossRef]
- Canelles, Q.; Aquilue, N.; James, P.M.A.; Lawler, J.; Brotons, L. Global Review on Interactions between Insect Pests and Other Forest Disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- da Silva Tavares, P.; Acosta, R.; Nobre, P.; Resende, N.C.; Chou, S.C.; de Arruda Lyra, A. Water Balance Components and Climate Extremes over Brazil under 1.5 °C and 2.0 °C of Global Warming Scenarios. Reg. Environ. Chang. 2023, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, H.; Wen, J. Climate Change Impacts on the Potential Distribution of Apocheima cinerarius (Erschoff) (Lepidoptera: Geometridae). Insects 2022, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Shabani, F.; Kumar, L.; Taylor, S. Climate Change Impacts on the Future Distribution of Date Palms: A Modeling Exercise Using CLIMEX. PLoS ONE 2012, 7, e48021. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, S.; Zhu, C.; Wang, T.; Xu, Z.; Zong, S. Projecting the Current and Future Potential Global Distribution of Hyphantria cunea (Lepidoptera: Arctiidae) Using CLIMEX. Pest. Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Szyniszewska, A.M.; Leppla, N.C.; Huang, Z.; Tatem, A.J. Analysis of Seasonal Risk for Importation of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera: Tephritidae), via Air Passenger Traffic Arriving in Florida and California. J. Econ. Entomol. 2016, 109, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wei, D.; Dou, W.; Hu, F.; Liu, W.F.; Wang, J.J. Toxicities and Synergistic Effects of Several Insecticides Against the Oriental Fruit Fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 970–978. [Google Scholar] [CrossRef]
- Vargas, R.I.; Stark, J.D.; Kido, M.H.; Ketter, H.M.; Whitehand, L.C. Methyl Eugenol and Cue-Lure Traps for Suppression of Male Oriental Fruit Flies and Melon Flies (Diptera: Tephritidae) in Hawaii: Effects of Lure Mixtures and Weathering. J. Econ. Entomol. 2000, 93, 81–87. [Google Scholar] [CrossRef]
- Douglas, A.E. Symbiotic Microorganisms: Untapped Resources for Insect Pest Control. Trends Biotechnol. 2007, 25, 338–342. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, A.; Chen, R.; Zeng, L.; Xu, Y. Predation of the Oriental Fruit Fly, Bactrocera dorsalis Puparia by the Red Imported Fire Ant, Solenopsis invicta: Role of Host Olfactory Cues and Soil Depth. Biocontrol Sci. Technol. 2012, 22, 551–557. [Google Scholar] [CrossRef]
- Bokonon-Ganta, A.H.; McQuate, G.T.; Messing, R.H.; Jang, E.B. Release and Establishment of the Parasitoid Diachasmimorpha kraussii against the Tephritid Fruit Fly Bactrocera latifrons in Hawaii. J. Insect Sci. 2013, 13, 302–311. [Google Scholar] [CrossRef]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W. Medfly Areawide Sterile Insect Technique Programmes for Prevention, Suppression or Eradication: The Importance of Mating Behavior Studies. Fla. Entomol. 2002, 85, 1–13. [Google Scholar] [CrossRef]

| Parameter | Szyniszewska et al. (2020) [26] Parameter Values | Vera et al. (2002) [24] Parameter Values | Final Parameter Values |
|---|---|---|---|
| Lower threshold of soil moisture (SM0) | 0.10 | 0.10 | 0.10 |
| Lower limit of optimum soil moisture (SM1) | 0.30 | 0.30 | 0.30 |
| Upper limit of optimum soil moisture (SM2) | 1.00 | 1.00 | 1.00 |
| Upper threshold of soil moisture (SM3) | 1.50 | 1.50 | 1.50 |
| Lower threshold temperature (DV0) | 10.0 °C | 12.0 °C | 10.0 °C |
| Lower optimum temperature (DV1) | 21.0 °C | 22.0 °C | 21.0 °C |
| Upper optimum temperature (DV2) | 26.5 °C | 30.0 °C | 26.0 °C |
| Upper threshold temperature (DV3) | 35.0 °C | 35.0 °C | 35.0 °C |
| Cold stress temperature threshold (TTCS) | 10.0 °C | 12.0 °C | 10.0 °C |
| Cold stress accumulation rate (THCS) | 0/week | 0/week | |
| Heat stress temperature threshold (TTHS) | 39.0 °C | 39.0 °C | 35.0 °C |
| Heat stress accumulation rate (THHS) | 0.01/week | 0.01/week | 0.01/week |
| Dry stress soil moisture threshold (SMDS) | 0.02 | 0.02 | 0.02 |
| Dry stress accumulation rate (HDS) | −0.05/week | - | −0.05/week |
| Wet stress soil moisture threshold (SMWS) | 1.6 | 1.6 | 1.5 |
| Wet stress accumulation rate (HWS) | 0.0015/week | 0.0015/week | 0.0015/week |
| Degree days taken to complete one generation (PDD) | 662 °C·d | 622 °C·d | 616 °C·d |
| Irrigation scenario | 1.5 mm day−1 in summer |
| Continent | Current Climate | Future Climate | ||||
|---|---|---|---|---|---|---|
| Highly Favorable Area (105 km2) | Favorable Area (105 km2) | Marginally Favorable Area (105 km2) | Highly Favorable Area (105 km2) | Favorable Area (105 km2) | Marginally Favorable Area (105 km2) | |
| Africa | 63.5 | 58.3 | 44.1 | 24.1 | 23.1 | 66.7 |
| Asia | 20 | 35.3 | 123.8 | 15.2 | 25.3 | 123.6 |
| North America | 25 | 23.7 | 38.2 | 7.2 | 17.2 | 60.4 |
| South America | 46.7 | 47.3 | 56.6 | 31.3 | 22.3 | 41.7 |
| Europe | 11.4 | 6 | 14.5 | 13.6 | 8.4 | 33.5 |
| Oceania | 22 | 8.1 | 20.3 | 15.9 | 5.3 | 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, J.; Zhang, Y.; Zhao, H.; Guo, J.; Wan, F.; Xian, X.; Yang, N.; Liu, W. Projecting the Global Potential Geographical Distribution of Ceratitis capitata (Diptera: Tephritidae) under Current and Future Climates. Biology 2024, 13, 177. https://doi.org/10.3390/biology13030177
Rao J, Zhang Y, Zhao H, Guo J, Wan F, Xian X, Yang N, Liu W. Projecting the Global Potential Geographical Distribution of Ceratitis capitata (Diptera: Tephritidae) under Current and Future Climates. Biology. 2024; 13(3):177. https://doi.org/10.3390/biology13030177
Chicago/Turabian StyleRao, Jiawei, Yu Zhang, Haoxiang Zhao, Jianyang Guo, Fanghao Wan, Xiaoqing Xian, Nianwan Yang, and Wanxue Liu. 2024. "Projecting the Global Potential Geographical Distribution of Ceratitis capitata (Diptera: Tephritidae) under Current and Future Climates" Biology 13, no. 3: 177. https://doi.org/10.3390/biology13030177
APA StyleRao, J., Zhang, Y., Zhao, H., Guo, J., Wan, F., Xian, X., Yang, N., & Liu, W. (2024). Projecting the Global Potential Geographical Distribution of Ceratitis capitata (Diptera: Tephritidae) under Current and Future Climates. Biology, 13(3), 177. https://doi.org/10.3390/biology13030177






