Quantification of Thermal Acclimation in Immune Functions in Ectothermic Animals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Literature Review
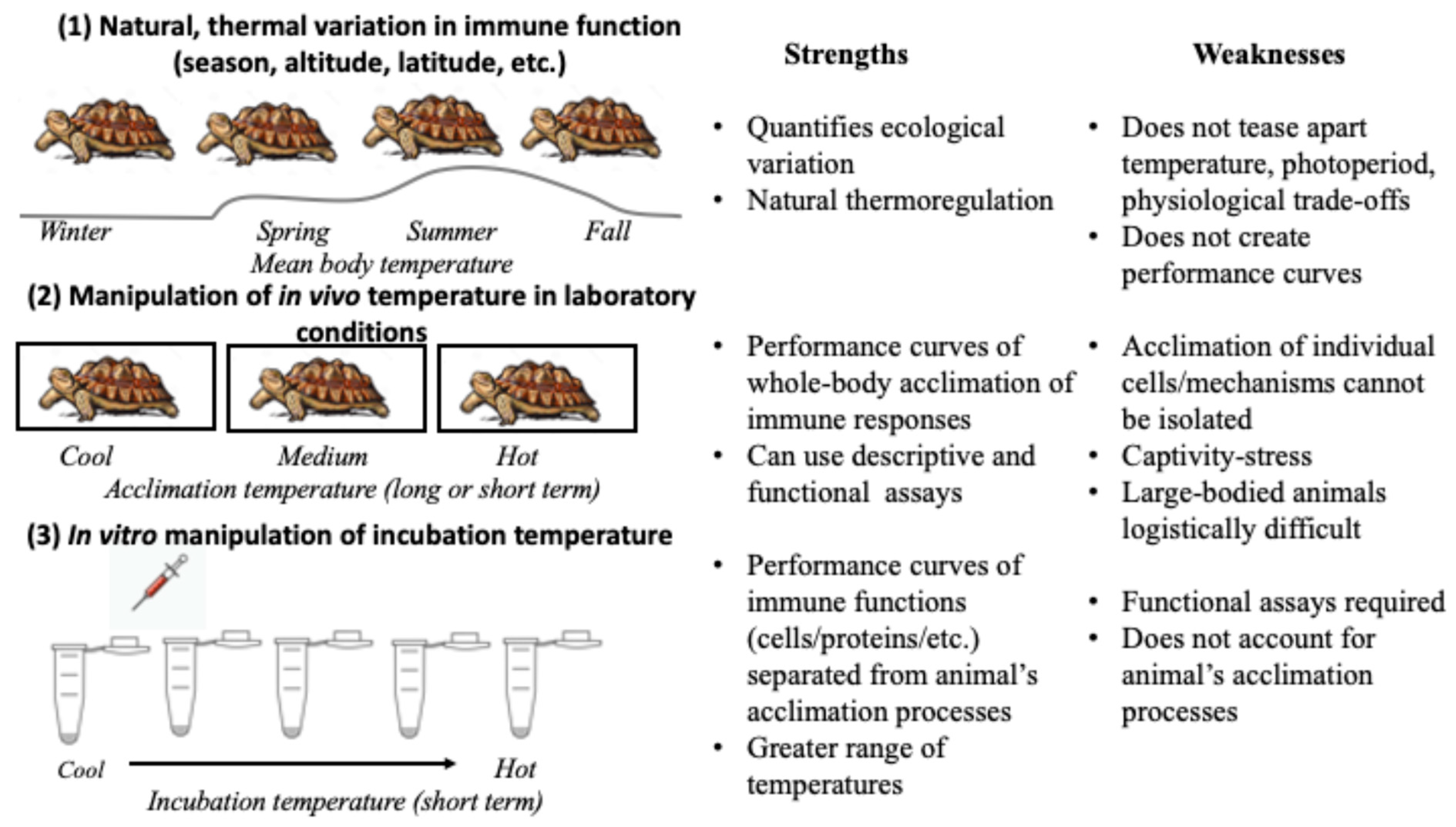
3. Considerations for Research Design
| Ecoimmunological Assay | Immune Parameter Quantified | Functional Assay | Differentiation of Quantity and Function of Molecules/Cells | Direct Interaction with Microbes | General or Taxa-Specific | References (Examples) |
|---|---|---|---|---|---|---|
| Antibody titers (ELISA) | Combined amount and avidity of antibodies (can include induced and NAbs) | No | No | No | Vertebrate | [47] |
| Quantification of other molecules (cytokines/antioxidants/antimicrobial peptides, etc.) | Levels of proteins with immune-support | No | No | No | General | [17,49,50] |
| Differential immune cell count | Cells in interstitial fluid/blood | No | No | No | General | [51,52] |
| Red blood cell agglutionation/lysis | Antimicrobial proteins: complement, NAbs, lysozyme, acute phase proteins, others | Yes | No | No | Predominantly used in vertebrates | [53] |
| PHA-induced inflammation | Components of inflammation: leukocytes, antibodies, cytokines, chemokines | Yes | No | No | Vertebrate | [54] |
| Fluorescence-based phagocytic assay | Acidification by phagocytes | Yes | No | Yes and No (substrate dependent) | General | [55] |
| Melanization/phenoloxidase activity | Melanization of foreign objects | Yes | No | Yes | Arthropods | [2,17] |
| Survival due to microbial challenge | Whole-organism ability to stay alive | Yes | No | Yes | General | [2] |
| Microbial killing assays (plasma/hemolymph-based) | Antimicrobial proteins: complement, NAbs, lysozyme, acute phase proteins, others | Yes | No | Yes | General | [53] |
| Antibody secretion by cells (ELISpot) | Antibody secretion by B lymphocytes | Yes | Yes | No | Vertebrate | [56] |
| Microbial killing assays (cell-based) | Phagocytosis/entrapment by cells | Yes | Yes | Yes | General | [53] |
| Phagocytic assay (cell-specific or all immune-related cells) | Engulfment of substrate by specific or all leukocytes | Yes | Yes | Yes and No (substrate dependent) | General | [57,58] |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlman, K.A.; Tuberville, T.T.; Metts, B.S.; Greene, J.W.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The global decline of reptiles, déjà vu amphibians. BioScience 2000, 50, 653–666. [Google Scholar] [CrossRef]
- Ferguson, L.V.; Kortet, R.; Sinclaire, B.J. Eco-immunology in the cold: The role of immunity in shaping the overwintering survival of ectotherms. J. Exp. Biol. 2018, 221, 163873. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.S.; Novarro, A.J.; Kohl, K.K. Environmental temperature alters digestive performance and gut microbiota of a terrestrial amphibian. J. Exp. Biol. 2018, 221, 187559. [Google Scholar] [CrossRef] [PubMed]
- Trevelline, B.K.; Fontaine, S.S.; Hartup, B.K.; Kohl, K.D. Conservation biology needs a microbial renaissance: A call for consideration of host-associated microbiota in wildlife management practices. Proc. R. Soc. B 2019, 286, 20182448. [Google Scholar] [CrossRef]
- Ferguson, L.V.; Heinrich, D.E.; Sinclair, B.J. Paradoxical acclimation responses in the thermal performance of insect immunity. Oecologia 2016, 181, 77–85. [Google Scholar] [CrossRef]
- IPPC (Intergovernmental Panel on Climate Change). Climate change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral impacts. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Baros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014; p. 1132. [Google Scholar]
- Vasseur, D.A.; DeLong, J.P.; Gilbert, B.; Greig, H.S.; Harley, C.D.G.; McCann, K.S.; Savage, V.; Tunney, T.D.; O’Connor, M.I. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 2014, 281, 20132612. [Google Scholar] [CrossRef]
- Martin, L.B.; Weil, Z.M.; Nelson, R.J. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Phil. Trans. R. Soc. B 2008, 363, 321–339. [Google Scholar] [CrossRef]
- Altman, K.A.; Paull, S.H.; Johnson, P.T.J.; Golembieski, M.N.; Stephens, L.P.; LaFonte, B.E.; Raffel, T.R. Host and parasite thermal acclimation responses depend on the stage of infection. J. Anim. Ecol. 2016, 85, 1014–1024. [Google Scholar] [CrossRef]
- Stahlschmidt, Z.R.; French, S.S.; Ahn, A.; Webb, A.; Butler, M.W. A simulated heat wave has diverse effects on immune function and oxidative physiology on the corn snake (Pantherophis guttatus). Physiol. Biochem. Zool. 2017, 90, 434–444. [Google Scholar] [CrossRef]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Cohen, J.M.; Venesky, M.D.; Sauer, E.L.; Civitello, D.J.; McMahon, T.A.; Roznik, E.A.; Rohr, J.R. The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol. Lett. 2017, 20, 184–193. [Google Scholar] [CrossRef]
- Molnár, P.K.; Sckrabulis, J.P.; Altman, K.A.; Raffel, T.R. Thermal performance curves and the metabolic theory of ecology—A practical guide to models and experiments for parasitologists. J. Parasitol. 2017, 103, 423–439. [Google Scholar] [CrossRef]
- Pxytycz, B.; Józkowicz, A. Differential effects of temperature on macrophages of ecothermic vertebrates. J. Leukoc. Biol. 1994, 56, 729–731. [Google Scholar] [CrossRef]
- Baker, S.; Merchant, M.E. Antibacterial properties of plasma from the prairie rattlesnake (Crotalus viridis). Dev. Comp. Immunol. 2018, 84, 273–278. [Google Scholar] [CrossRef]
- Sanhueza, N.; Fuentes, R.; Aguilar, A.; Carnicero, B.; Vega, K.; Muñoz, D.; Contreras, D.; Moreno, N.; Tronscoso, E.; Mercado, L.; et al. Behavioral fever promotes an inflammatory reflex circuit in ectotherms. Int. J. Mol. Sci. 2021, 22, 8860. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.V.; Adamo, S.A. From perplexing to predictive: Are we ready to forecast insect disease susceptibility in a warming world? J. Exp. Biol. 2023, 226, jeb244911. [Google Scholar] [CrossRef] [PubMed]
- Weil, Z.M.; Nelson, R.J. Neuroendocrine mechanisms of seasonal changes in immune function. In Ecoimmunology; Demas, G.E., Nelson, R.J., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 297–325. [Google Scholar]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of responses of ecotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Morvan, C.L.; Troutaud, D.; Deschaux, P. Differential effects of temperature on specific and nonspecific immune defences in fish. J. Exp. Biol. 1998, 201, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Parham, W.W. The Immune System, 5th ed.; Norton and Company: New York, NY, USA, 2021. [Google Scholar]
- Baker, S.J.; Kessler, E.J.; Merchant, M.E. Antimicrobial activities of plasma from the common (Chelydra serpentina) and alligator snapping turtle (Macrochelys temminckii). J. Exp. Zool. 2019, 331, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Raffel, T.R.; Rohr, J.R.; Kiesecker, J.M.; Hudson, P.J. Negative effects of changing temperature on amphibian immunity under field conditions. Func. Ecol. 2006, 20, 819–828. [Google Scholar] [CrossRef]
- Goessling, J.M.; Koler, S.A.; Overman, B.D.; Hiltbold, E.M.; Guyer, C.; Mendonça, M. Lag of immunity across seasonal acclimation states in gopher tortoises (Gopherus polyphemus). J. Exp. Zool. 2017, 327, 235–242. [Google Scholar] [CrossRef]
- Slama, S.L.; Williams, G.S.; Painter, M.N.; Sheedy, M.D.; Sandmeier, F.C. Temperature and season influence phagocytosis by B1 lymphocytes in the Mojave desert tortoise. Integr. Comp. Biol. 2022, 62, 1683–1692. [Google Scholar] [CrossRef]
- Kiel, D.; Luebke, R.W.; Pruett, S.B. Quantifying the relationship between multiple immunological parameters and host resistance: Probing the limits of reductionism. J. Immunol. 2001, 167, 4543–4552. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Ngor, L.; Nevarez, R.B.; Rothman, J.A.; Raffel, T.R.; McFrederick, Q.S. Temperature dependence of parasitic infection and gut bacterial communities in bumble bees. Environ. Microbiol. 2019, 21, 470604723. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Ivey, K.; Cornwall, M.B.; Herr, K.; Rede, J.; Taylor, E.N.; Gunderson, A.R. Lizard gut microbiome changes with temperature and is associated with heat tolerance. Appl. Environ. Microbiol. 2020, 86, e01181. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.D.; Bletz, M.C.; Le Sage, M.; LaBumbard, B.; Rollins-Smith, L.A.; Woodhams, D.C.; Miller, D.L.; Gray, M.J. Winter is coming—Temperature affects immune defenses and susceptibility to Batrachochytridium salamandrivorans. PLOS Pathog. 2021, 17, e1009234. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.C.; Brandt, H.; Baumgartner, S.; Kielgast, J.; Kupfer, E.; Tobler, U.; Davis, L.R.; Schmidt, B.R.; Bel, C.; Hodel, S.; et al. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 2014, 9, e96375. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Hablützel, P.I.; Brown, M.; Watson, H.V.; Parker-Norman, S.; Tober, A.V.; Thomason, A.G.; Friber, I.M.; Cable, J.; Jackson, J.A. Half the story: Thermal effects on within-host infectious disease progression in a warming climate. Glob. Chang. Biol. 2017, 24, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Shiu, J.; Keshavmurthy, S.; Chiang, P.; Chen, H.; Lou, S.; Tseng, C.; Hsieh, H.J.; Chen, C.A.; Tang, S. Dynamics of coral-associated bacterial communities acclimated to temperature stress based on recent thermal history. Sci. Rep. 2017, 7, 14933. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.; Leiva, L.; Wörheide, G. Short-term exposure to high-temperature water causes a shift in the microbiome of the common aquarium sponge Lendenfeldia chondrodes. Invert. Microbiol. 2021, 81, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, W.; Zhao, T.; Chen, H.; Zhao, C.; Xu, L.; Chang, Q.; Jiang, J. Environmental temperatures affect the gastrointestinal microbes of the Chinese giant salamander. Front. Microbiol. 2021, 12, 543767. [Google Scholar] [CrossRef]
- Palackdharry, S.; Sadd, B.M.; Vogel, L.A.; Bowden, R.M. The effect of environmental temperatrue on reptilian peripheral blood B cell functions. Horm. Behav. 2017, 88, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sandmeier, F.C.; Leonard, K.L.; Tracy, C.R.; Drake, K.K.; Esque, T.E.; Nussear, K.; Germano, J.M. Tools to understand seasonality in health: Quantification of microbe loads and analyses of compositional ecoimmunological data reveal complex patterns in tortoise populations. Can. J. Zool. 2019, 97, 841–848. [Google Scholar] [CrossRef]
- Butler, M.; Stahlschmidt, Z.R.; Ardia, D.R.; Davies, S.; Davis, J.; Guillette, L.J., Jr.; Johnson, N.; McCormick, D.; McGraw, K.J.; DeNardo, D.J. Thermal sensitivity of immune function: Evidence against a generalist-specialist trade-off among endothermic and ectothermic vertebrates. Am. Nat. 2013, 181, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.E. Marine parasites and disease in the era of global climate change. Ann. Rev. Mar. Sci. 2020, 13, 397–420. [Google Scholar] [CrossRef] [PubMed]
- Goessling, J.M.; Guyer, C.; Mendonça, M. Seasonal acclimation of constitutive immunity in gopher tortoises Gopherus polyphemus. Physiol. Biochem. Zool 2016, 89, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.P.; Fielman, K.T.; Mendonça, M.T. Thermal performance and acclimatization of a component of snake (Agkistrodon piscivorus) innate immunity. J. Exp. Zool. 2017, 327, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A.; Lovett, M.M. Some like it hot: The effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J. Exp. Biol. 2011, 214, 1997–2004. [Google Scholar] [CrossRef]
- Rohr, J.R.; Civitello, D.J.; Cohen, J.M.; Roznik, E.A.; Sinervo, B.; Dell, A.I. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 2018, 21, 1425–1439. [Google Scholar] [CrossRef]
- Lima, A.S.; Ferreira, L.F.; Silva, D.P.; Gomes, F.R.; Titon, S.C.M. Thermal sensitivity of bullfrog’s immune response kept at different temperatures. J. Exper. Zool. 2020, 333, 767–778. [Google Scholar] [CrossRef]
- Goessling, J.M.; Mendonça, M.T. Physiological responses of gopher tortoises (Gopherus polyphemus) to trapping. Conserv. Physiol. 2021, 9, coab003. [Google Scholar] [CrossRef]
- Boltana, S.; Aguilar, A.; Sanhueza, N.; Donoso, A.; Mercado, L.; Imarai, M.; McKenzie, S. Behavioral fever drives epigenetic modulation of the immune response in fish. Front. Immunol. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Tirard, C.T.; Grossfeld, R.M.; Levine, J.F.; Kennedy-Stoskopf, S. Effect of hyperthermia in vitro on stress protein synthesis and accumulation in oyster haemocytes. Fish Shellfish Immunol. 1995, 5, 9–25. [Google Scholar] [CrossRef]
- Sandmeier, F.C.; Leonard, K.L.; Weitzman, C.L.; Tracy, C.R. Potential facilitation between a commensal and a pathogenic microbe in a wildlife disease. Ecohealth 2022, 19, 427–438. [Google Scholar] [CrossRef]
- Demas, G.E.; Nelson, R.G. (Eds.) Ecoimmunology; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Sandmeier, F.C.; Tracy, C.R.; DuPré, S.; Hunter, K. A trade-off between natural and acquired antibody production in a reptile: Implication for long-term resistance to disease. Biol. Open 2012, 1, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Costantini, D.; Eens, M. Impacts of rising temperatures and water acidification on oxidative status and immune system of aquatic ectothermic vertebrates: A metanalysis. Sci. Total Environ. 2023, 868, 161580. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Func. Ecol. 2008, 22, 275–286. [Google Scholar] [CrossRef]
- Oliver, J.D.; Loy, J.D.; Parikh, G.; Bartholomay, L. Comparative analysis of hemocyte phagocytosis between six species of arthropods as measured by flow cytometry. J. Invertebr. Pathol. 2011, 108, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Matson, K.D.; Tieleman, B.I.; Klasing, K.C. Capture stress and bactericidal competence of blood and plasma in five species of tropical birds. Physiol. Biochem. Zool. 2006, 79, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.; Han, P.; Lewittes, J.; Kuhlman, J.R.; Klasing, K.C.; Wikelski, M. Phytohemagglutinin-induced swelling in birds: Histological support for a classic immunological technique. Func. Ecol. 2006, 20, 290–299. [Google Scholar] [CrossRef]
- Goessling, J.M.; Ward, C.; Mendonça, M.T. Rapid thermal immune acclimation in common musk turtles (Sternotherus odoratus). J. Exp. Zool. 2019, 331, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, L.M.; Clairardin, S.G.; Paitz, R.T.; Hicke, J.W.; LaMagdeleine, K.A.; Vogel, L.A.; Bowden, R.M. Humoral responses are maintained with age in a long-lived ectotherm, the red-eared slider. J. Exp. Biol. 2013, 216, 185–191. [Google Scholar] [CrossRef]
- Graham, A.L.; Shuker, D.M.; Pollitt, L.C.; Auld, S.K.J.R.; Wilson, A.J.; Little, T.J. Fitness consequences of immune responses: Strengthening the empirical framework for ecoimmunology. Func. Ecol. 2011, 25, 5–17. [Google Scholar] [CrossRef]
- Slama, S.L.; Painter, M.N.; Sheedy, M.D.; Sandmeier, F.C. Quantifying phagocytic lymphocytes in ectothermic vertebrates: A simplified technique for assessing immune function. Methods Ecol. Evol. 2021, 12, 548–552. [Google Scholar] [CrossRef]
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef]
- Teti, G.; Biondo, C.; Beninati, C. The phagocyte, Metchnivoff, and the foundation of immunology. Microbiol. Spectrum 2016, 4, MHCD-009-2015. [Google Scholar] [CrossRef]
- Millet, S.; Bennett, J.; Lee, K.A.; Hau, M.; Klasing, K.C. Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 2007, 31, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.W.; Anderson, M.; Cavanaugh, D. Animal Physiology, 5th ed.; Oxford University Press: New York, NY, USA, 2021. [Google Scholar]
- Sandmeier, F.C.; Tracy, R.C. The metabolic pace-of-life model: Incorporating ectothermic organisms into the theory of vertebrate ecoimmunology. Integr. Comp. Biol. 2014, 54, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Peck, L.S.; Morley, S.A.; Richard, J.; Clark, M.S. Acclimation and thermal tolerance in Antarctic marine ectotherms. J. Exp. Biol. 2014, 217, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Andreassen, A.H.; Åsheim, E.; Finnøen, M.H.; Dresler, G.; Brembu, T.; Loh, A.; Miest, J.J.; Jutfelt, F. Reduced physiological plasticity in a fish adapted to stable temperatures. Proc. Natl. Acad. Sci. USA 2022, 22, e2201919119. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandmeier, F.C. Quantification of Thermal Acclimation in Immune Functions in Ectothermic Animals. Biology 2024, 13, 179. https://doi.org/10.3390/biology13030179
Sandmeier FC. Quantification of Thermal Acclimation in Immune Functions in Ectothermic Animals. Biology. 2024; 13(3):179. https://doi.org/10.3390/biology13030179
Chicago/Turabian StyleSandmeier, Franziska C. 2024. "Quantification of Thermal Acclimation in Immune Functions in Ectothermic Animals" Biology 13, no. 3: 179. https://doi.org/10.3390/biology13030179
APA StyleSandmeier, F. C. (2024). Quantification of Thermal Acclimation in Immune Functions in Ectothermic Animals. Biology, 13(3), 179. https://doi.org/10.3390/biology13030179





