Amorphous Calcium Carbonate from Plants Can Promote Bone Growth in Growing Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Bone Turnover Biochemical Markers (BTMs)
2.4. Micro-Computed Tomography (μCT)
2.5. Bone Length and Weight
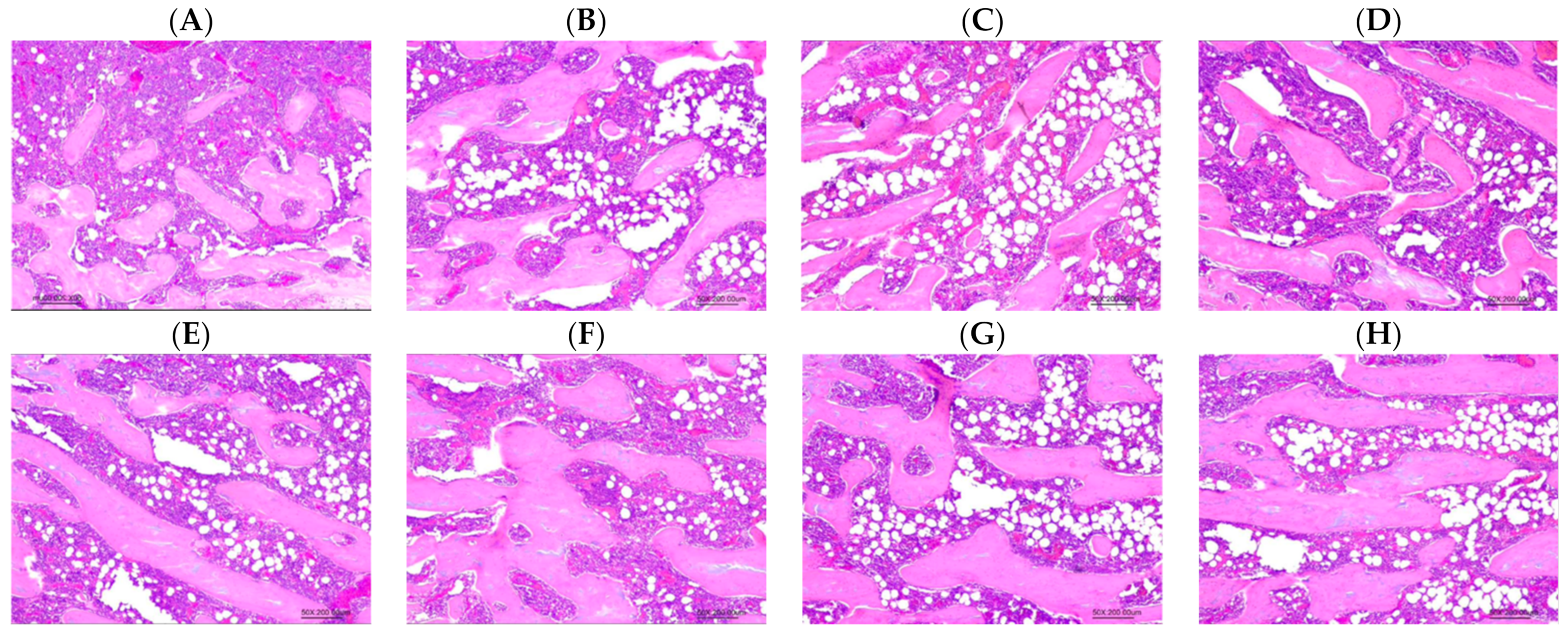
2.6. Histopathological Examination
2.7. Statistical Analysis
3. Results
3.1. Body Weight (BW)
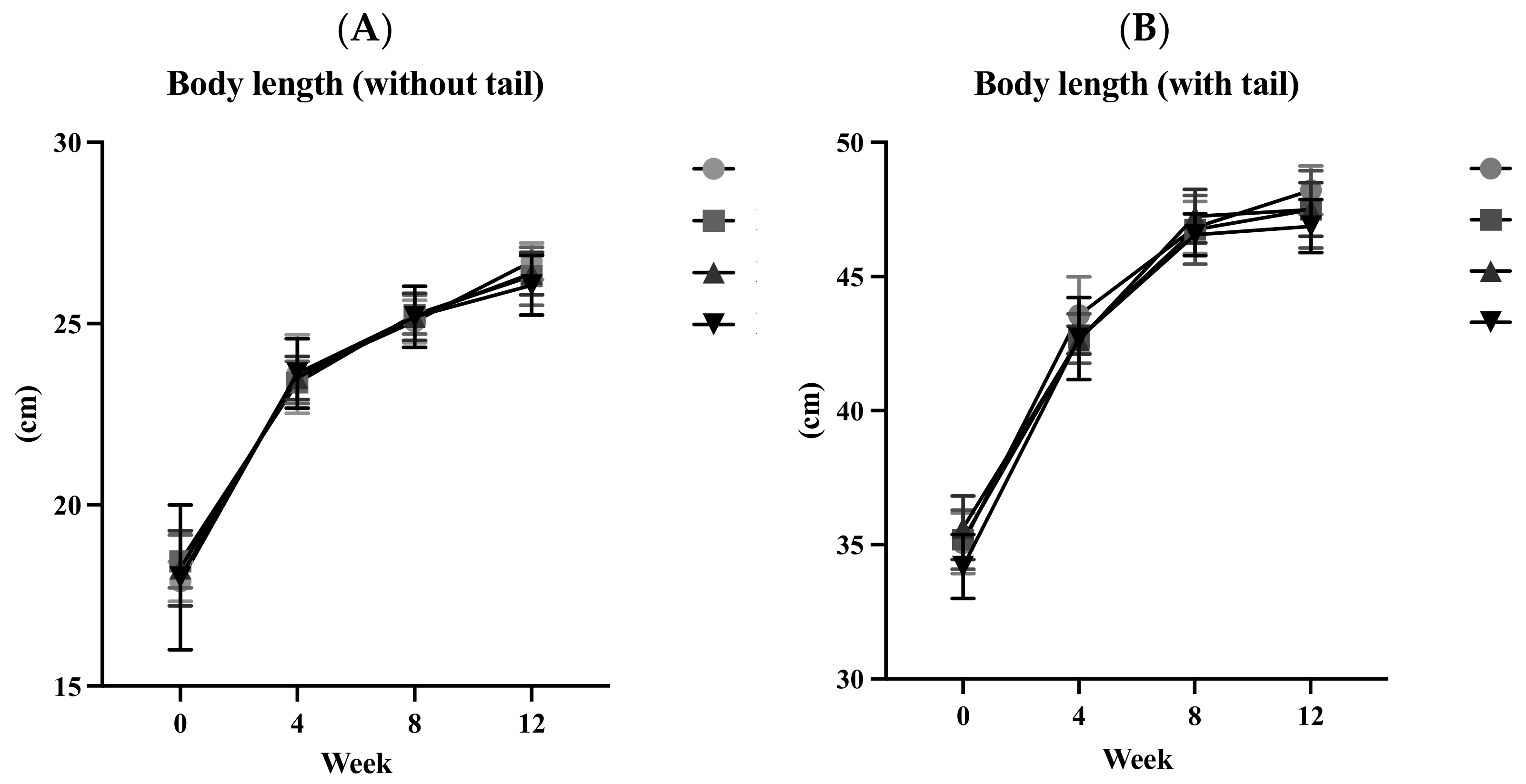
3.2. Body Length (BL)
3.3. Lee’s Index (LI)
3.4. Food Intake
3.5. Bone Turnover Biochemical Markers (BTMs)
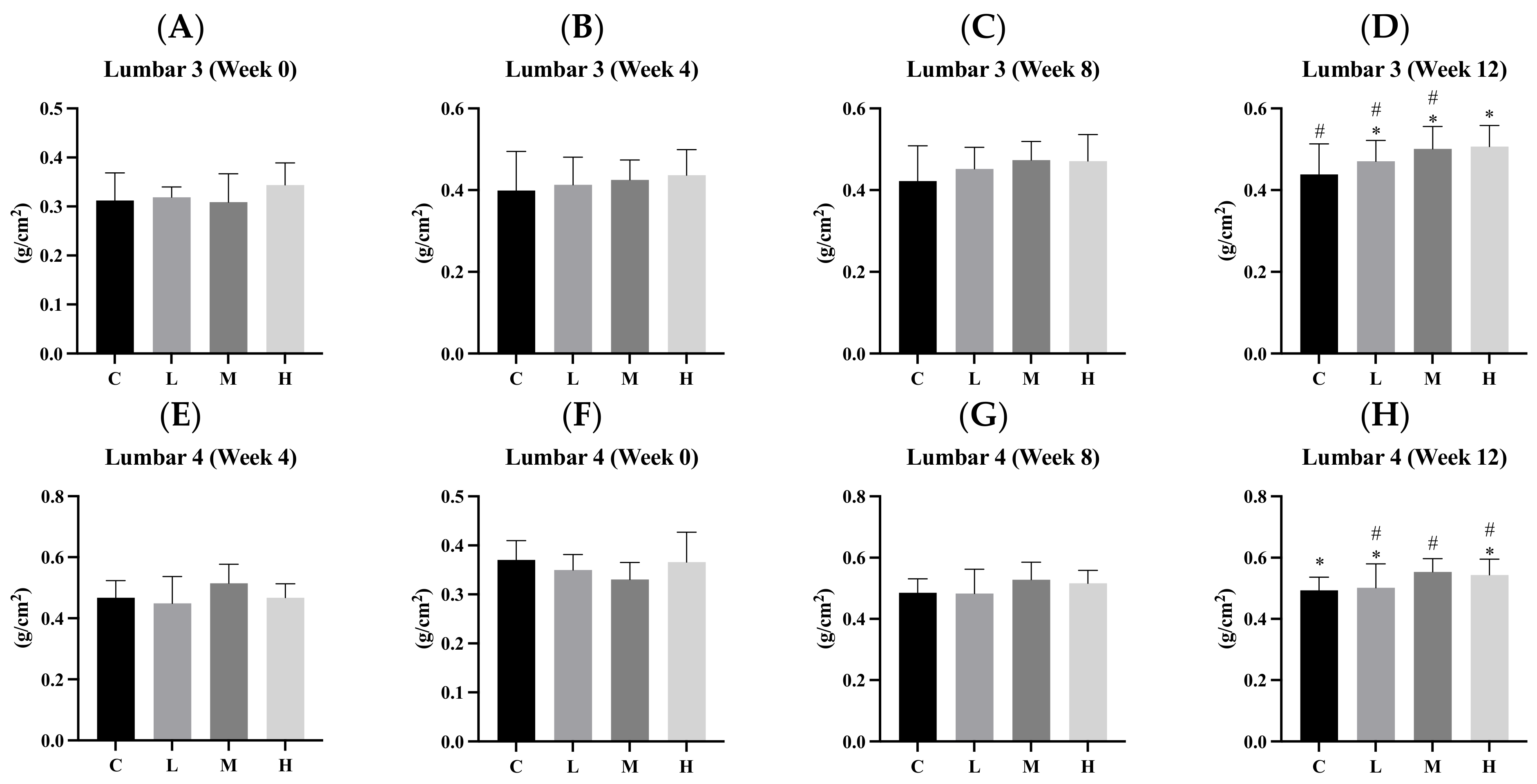
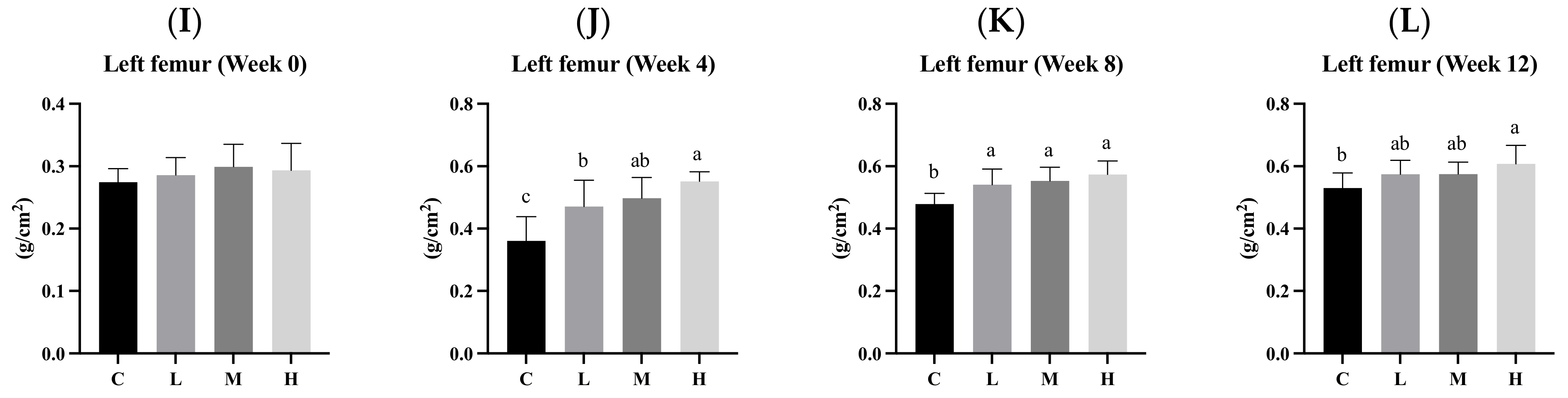
3.6. Bone Mineral Density (BMD)
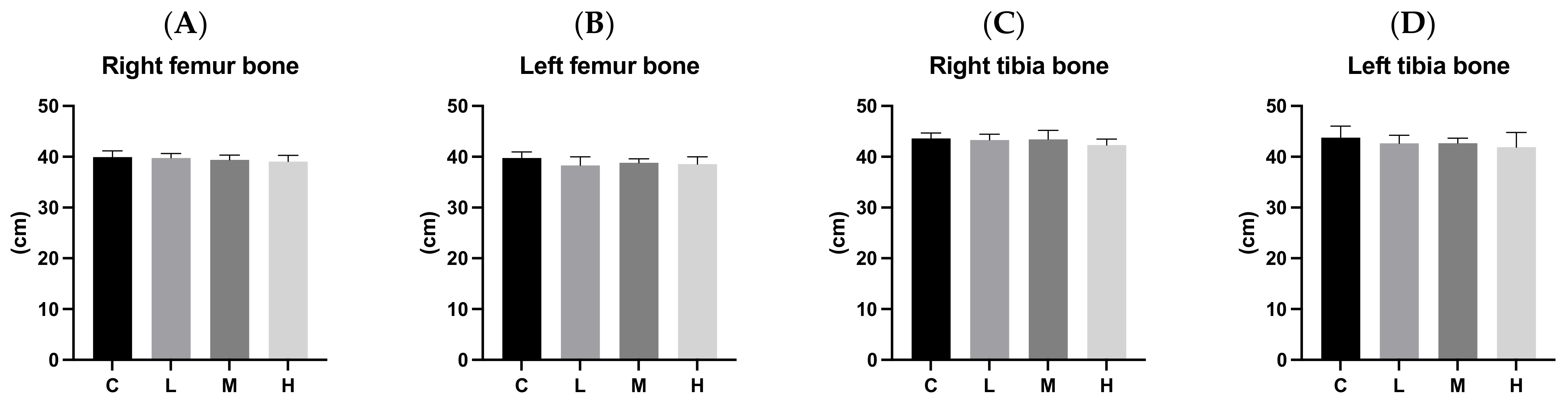
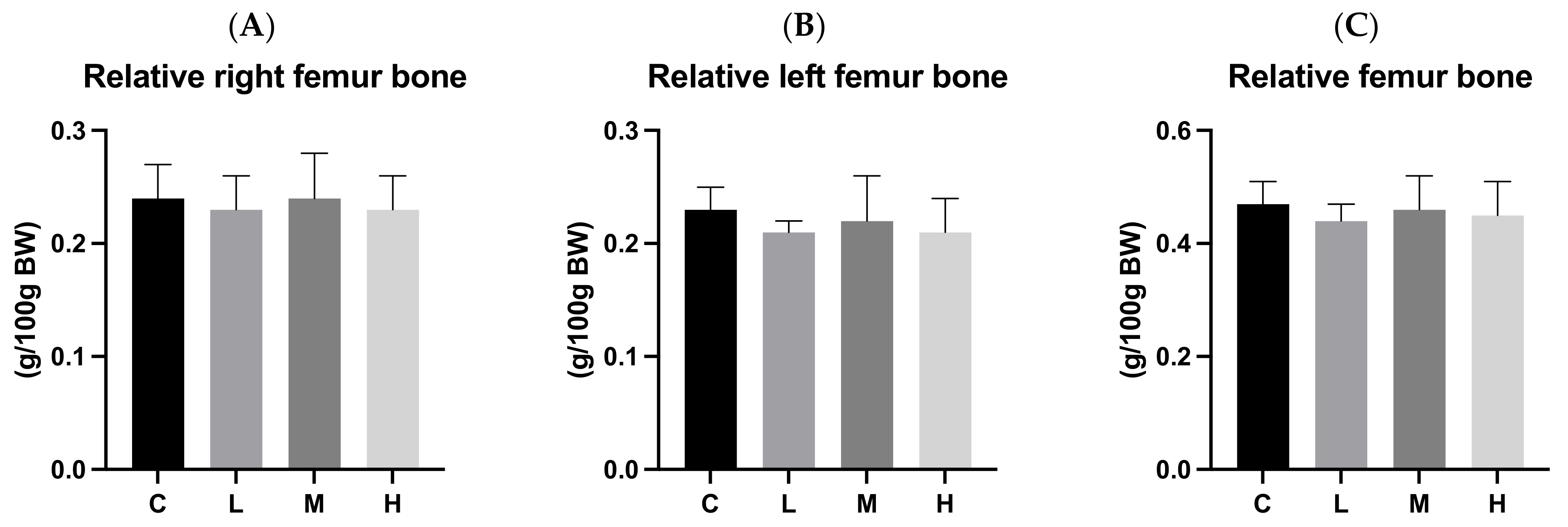
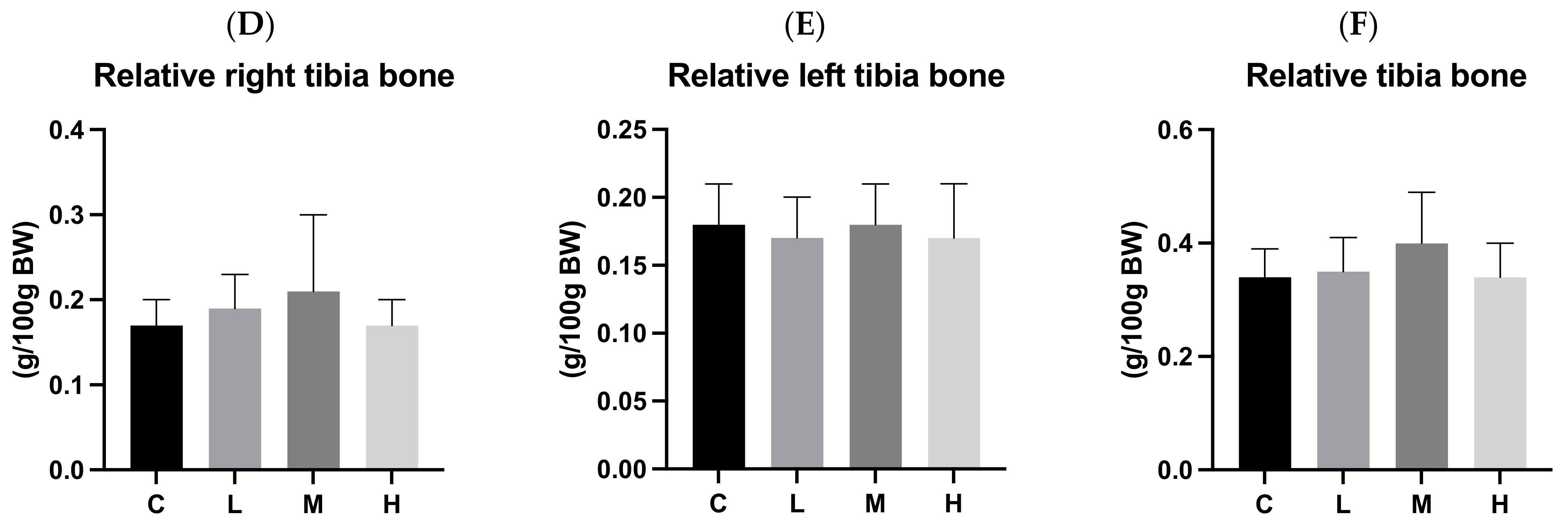
3.7. Bone Length and Weight
3.8. Cortical Bone Thickness
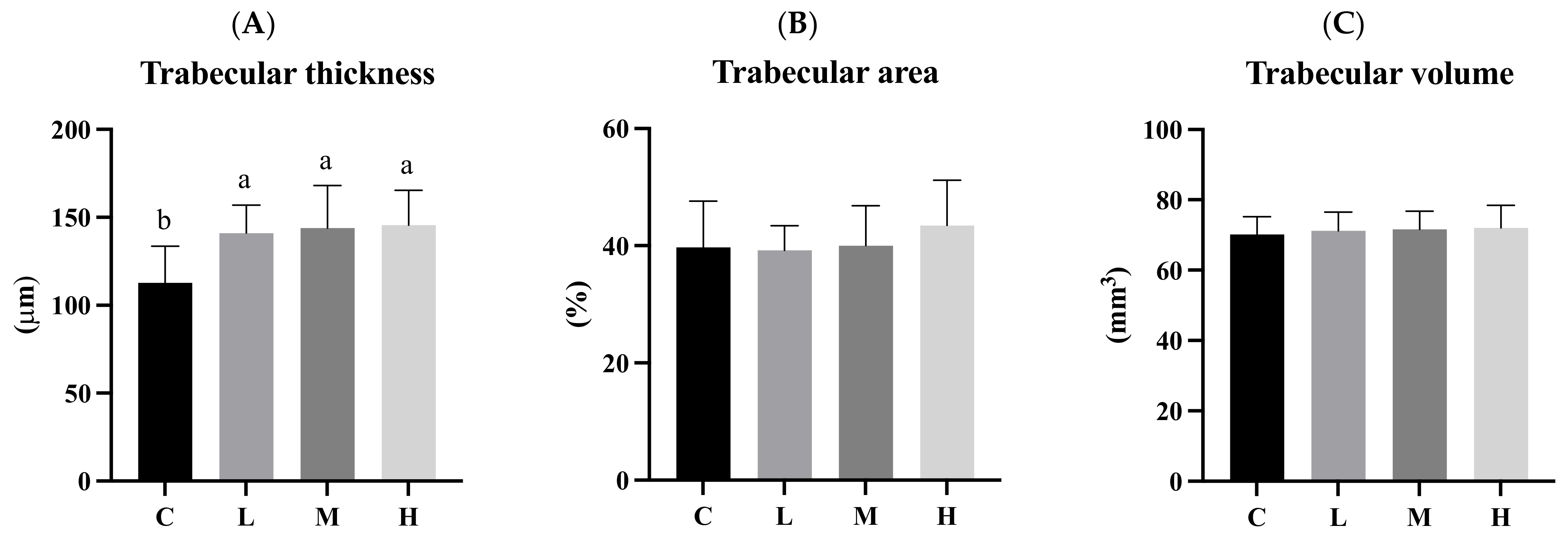
3.9. Trabecular Bone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, J.S.; Chan, D.C.; Chen, J.F.; Cheng, T.T.; Wu, C.H.; Soong, Y.K.; Tsai, K.S.; Yang, R.S. Clinical practice guidelines for the prevention and treatment of osteoporosis in Taiwan: Summary. J. Bone Miner. Metab. 2014, 32, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhai, Y.; Zhang, J.; Chen, J.Y.; Liu, D.; Zhao, W.H. Combined effects of physical activity and calcium on bone health in children and adolescents: A systematic review of randomized controlled trials. World J. Pediatr. 2020, 16, 356–365. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, H. Factors influencing peak bone mass gain. Front. Med. 2021, 15, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Meiron, O.E.; Bar-David, E.; Aflalo, E.D.; Shechter, A.; Stepensky, D.; Berman, A.; Sagi, A. Solubility and bioavailability of stabilized amorphous calcium carbonate. J. Bone Miner. Res. 2011, 26, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Nebel, H.; Neumann, M.; Mayer, C.; Epple, M. On the structure of amorphous calcium carbonate--a detailed study by solid-state NMR spectroscopy. Inorg. Chem. 2008, 47, 7874–7879. [Google Scholar] [CrossRef] [PubMed]
- Kawano, J.; Shimobayashi, N.; Kitamura, M.; Shinoda, K.; Aikawa, N. Formation process of calcium carbonate from highly supersaturated solution. J. Cryst. Growth 2002, 237, 419–423. [Google Scholar] [CrossRef]
- Shaltiel, G.; Bar-David, E.; Meiron, O.E.; Waltman, E.; Shechter, A.; Aflalo, E.D.; Stepensky, D.; Berman, A.; Martin, B.R.; Weaver, C.M. Bone loss prevention in ovariectomized rats using stable amorphous calcium carbonate. Health 2013, 5, 18–29. [Google Scholar] [CrossRef]
- Vaisman, N.; Shaltiel, G.; Daniely, M.; Meiron, O.E.; Shechter, A.; Abrams, S.A.; Niv, E.; Shapira, Y.; Sagi, A. Increased Calcium Absorption From Synthetic Stable Amorphous Calcium Carbonate: Double-Blind Randomized Crossover Clinical Trial in Postmenopausal Women. J. Bone Miner. Res. 2014, 29, 2203–2209. [Google Scholar] [CrossRef]
- Weaver, C.M.; Peacock, M. Calcium. Adv. Nutr. 2011, 2, 290–292. [Google Scholar] [CrossRef]
- Baird, G.S. Ionized calcium. Clin. Chim. Acta 2011, 412, 696–701. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Winzenberg, T.; Shaw, K.; Fryer, J.; Jones, G. Calcium supplements in healthy children do not affect weight gain, height, or body composition. Obesity 2007, 15, 1789–1798. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Hoppler, M.; Membrez Scalfo, F.; Vuichoud, J.; Vigo, M.; Offord, E.A.; Ammann, P. Peak bone strength is influenced by calcium intake in growing rats. Bone 2014, 68, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624. [Google Scholar] [PubMed]
- Hernández-Becerra, E.; Jímenez-Mendoza, D.; Mutis-Gonzalez, N.; Pineda-Gomez, P.; Rojas-Molina, I.; Rodríguez-García, M.E. Calcium Deficiency in Diet Decreases the Magnesium Content in Bone and Affects Femur Physicochemical Properties in Growing Rats. Biol. Trace Elem. Res. 2020, 197, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Bollen, A.-M.; Bai, X.-Q. Effects of long-term calcium intake on body weight, body fat and bone in growing rats. Osteoporos. Int. 2005, 16, 1864–1870. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; Gutiérrez-Cortez, E.; Del Real, A.; Rojas-Molina, A.; Rodríguez-García, M.; Rubio, E.; Quintero-García, M.; Rojas-Molina, I. Bone Mineral Density, Mechanical, Microstructural Properties and Mineral Content of the Femur in Growing Rats Fed with Cactus Opuntia ficus indica (L.) Mill. (Cactaceae) Cladodes as Calcium Source in Diet. Nutrients 2017, 9, 108. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Liao, E.Y.; Dai, R.C.; Wu, X.P.; Genant, H.K. Application of micro-CT assessment of 3-D bone microstructure in preclinical and clinical studies. J. Bone Miner. Metab. 2005, 23 (Suppl. S1), 122–131. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Diemar, S.S.; Møllehave, L.T.; Quardon, N.; Lylloff, L.; Thuesen, B.H.; Linneberg, A.; Jørgensen, N.R. Effects of age and sex on osteocalcin and bone-specific alkaline phosphatase-reference intervals and confounders for two bone formation markers. Arch. Osteoporos. 2020, 15, 26. [Google Scholar] [CrossRef]
- Tripathi, T.; Gupta, P.; Rai, P.; Sharma, J.; Gupta, V.K.; Singh, N.; Verma, M. Longitudinal evaluation of the association between Insulin-like growth factor-1, Bone specific alkaline phosphatase and changes in mandibular length. Sci. Rep. 2019, 9, 11582. [Google Scholar] [CrossRef]
- Tobiume, H.; Kanzaki, S.; Hida, S.; Ono, T.; Moriwake, T.; Yamauchi, S.; Tanaka, H.; Seino, Y. Serum bone alkaline phosphatase isoenzyme levels in normal children and children with growth hormone (GH) deficiency: A potential marker for bone formation and response to GH therapy. J. Clin. Endocrinol. Metab. 1997, 82, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, G.; Eickelberg, E.; Elveback, L.R. Alkaline phosphatase activity in the plasma of children and adolescents. Clin. Chem. 1977, 23, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Seibel, M.J. Biochemical markers of bone turnover: Part I: Biochemistry and variability. Clin. Biochem. Rev. 2005, 26, 97–122. [Google Scholar] [PubMed]
- Rosen, H.N.; Moses, A.C.; Garber, J.; Iloputaife, I.D.; Ross, D.S.; Lee, S.L.; Greenspan, S.L. Serum CTX: A new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif. Tissue Int. 2000, 66, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Seibel, M.J. The amino- and carboxyterminal cross-linked telopeptides of collagen type I, NTX-I and CTX-I: A comparative review. Clin. Chim. Acta 2008, 393, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Kapinas, K.; Delany, A.M. MicroRNA biogenesis and regulation of bone remodeling. Arthritis Res. Ther. 2011, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Lee, W.T.; Leung, S.S.; Leung, D.M.; Cheng, J.C. A follow-up study on the effects of calcium-supplement withdrawal and puberty on bone acquisition of children. Am. J. Clin. Nutr. 1996, 64, 71–77. [Google Scholar] [CrossRef]
- Matkovic, V.; Goel, P.K.; Badenhop-Stevens, N.E.; Landoll, J.D.; Li, B.; Ilich, J.Z.; Skugor, M.; Nagode, L.A.; Mobley, S.L.; Ha, E.J.; et al. Calcium supplementation and bone mineral density in females from childhood to young adulthood: A randomized controlled trial. Am. J. Clin. Nutr. 2005, 81, 175–188. [Google Scholar] [CrossRef]
- Safadi, F.F.; Barbe, M.F.; Abdelmagid, S.M.; Rico, M.C.; Aswad, R.A.; Litvin, J.; Popoff, S.N. Bone structure, development and bone biology. In Bone Pathology; Humana Press: Totowa, NJ, USA, 2009; pp. 1–50. [Google Scholar]
- Schmidutz, F.; Yan, S.G.; Schopf, C.; Ihle, C.; Ahrend, M.D.; Sprecher, C.M. Cortical bone thickness predicts the quantitative bone mineral density of the proximal humerus. Arch. Osteoporos. 2021, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, E.; Matsukawa, M.; Mano, I.; Matsui, D.; Yoneda, Y.; Masumura, M.; Koyama, T.; Watanabe, I.; Maekawa, M.; Tomida, S. Growth of cortical bone thickness and trabecular bone density in Japanese children. Bone 2020, 141, 115669. [Google Scholar] [CrossRef] [PubMed]



| C (n = 9) | L (n = 8) | M (n = 8) | H (n = 8) | |
|---|---|---|---|---|
| Initial BW (g) | 224.83 ± 20.50 | 220.19 ± 15.78 | 222.88 ± 15.54 | 226.63 ± 16.46 |
| Total BW gain (g) | 367.83 ± 45.44 | 356.69 ± 52.62 | 334.50 ± 50.66 | 338.81 ± 50.39 |
| Total BL gain (with tail) (g) | 13.17 ± 0.61 | 12.31 ± 1.13 | 11.88 ± 0.74 | 12.69 ± 1.22 |
| Total BL gain (without tail) (g) | 8.83 ± 0.50 | 7.88 ± 0.83 | 8.13 ± 1.03 | 8.06 ± 1.64 |
| Food intake (g/day/rat) | 25.67 ± 2.91 | 25.99 ± 0.58 | 24.76 ± 1.32 | 24.60 ± 2.33 |
| Food efficiency (%) | 14.33 ± 0.80 | 13.72 ± 1.98 | 13.47 ± 1.53 | 13.78 ± 1.54 |
| C (n = 9) | L (n = 8) | M (n = 8) | H (n = 8) | |
|---|---|---|---|---|
| Week 0 (g/cm) | 0.340 ± 0.01 | 0.328 ± 0.02 | 0.334 ± 0.03 | 0.341 ± 0.03 |
| Week 1 (g/cm) | 0.333 ± 0.01 | 0.324 ± 0.02 | 0.325 ± 0.01 | 0.319 ± 0.08 |
| Week 2 (g/cm) | 0.322 ± 0.02 | 0.328 ± 0.03 | 0.319 ± 0.01. | 0.318 ± 0.01. |
| Week 3 (g/cm) | 0.323 ± 0.01 | 0.318 ± 0.01 | 0.315 ± 0.01 | 0.318 ± 0.02 |
| Week 4 (g/cm) | 0.318 ± 0.01 | 0.316 ± 0.01 | 0.314 ± 0.01 | 0.313 ± 0.01 |
| Week 5 (g/cm) | 0.316 ± 0.01 | 0.313 ± 0.01 | 0.311 ± 0.00 | 0.318 ± 0.01 |
| Week 6 (g/cm) | 0.326 ± 0.01 | 0.321 ± 0.01 | 0.323 ± 0.01 | 0.329 ± 0.01 |
| Week 7 (g/cm) | 0.326 ± 0.01 | 0.328 ± 0.01 | 0.323 ± 0.01 | 0.319 ± 0.01 |
| Week 8 (g/cm) | 0.324 ± 0.01 | 0.319 ± 0.01 | 0.316 ± 0.01 | 0.319 ± 0.01 |
| Week 9 (g/cm) | 0.316 ± 0.01 | 0.316 ± 0.01 | 0.319 ± 0.01 | 0.321 ± 0.01 |
| Week 10 (g/cm) | 0.313 ± 0.01 | 0.318 ± 0.02 | 0.315 ± 0.01 | 0.320 ± 0.01 |
| Week 11 (g/cm) | 0.316 ± 0.01 ab | 0.318 ± 0.01 b | 0.306 ± 0.01 ab | 0.309 ± 0.01 a |
| Week 12 (g/cm) | 0.313 ± 0.01 | 0.318 ± 0.00 | 0.313 ± 0.01 | 0.318 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-K.; Lee, Y.-S.; Kong, Z.-L.; Chien, Y.-W. Amorphous Calcium Carbonate from Plants Can Promote Bone Growth in Growing Rats. Biology 2024, 13, 201. https://doi.org/10.3390/biology13030201
Chen C-K, Lee Y-S, Kong Z-L, Chien Y-W. Amorphous Calcium Carbonate from Plants Can Promote Bone Growth in Growing Rats. Biology. 2024; 13(3):201. https://doi.org/10.3390/biology13030201
Chicago/Turabian StyleChen, Chun-Kai, Yu-Shan Lee, Zwe-Ling Kong, and Yi-Wen Chien. 2024. "Amorphous Calcium Carbonate from Plants Can Promote Bone Growth in Growing Rats" Biology 13, no. 3: 201. https://doi.org/10.3390/biology13030201
APA StyleChen, C.-K., Lee, Y.-S., Kong, Z.-L., & Chien, Y.-W. (2024). Amorphous Calcium Carbonate from Plants Can Promote Bone Growth in Growing Rats. Biology, 13(3), 201. https://doi.org/10.3390/biology13030201







