Response Mechanism of cbbM Carbon Sequestration Microbial Community Characteristics in Different Wetland Types in Qinghai Lake
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Soil Sample Collection
2.3. Determination of Soil Physical and Chemical Properties
2.4. DNA Extraction and Illumina MiSeq Sequencing
2.5. Statistical Analysis
3. Results
3.1. Community Diversity of cbbM Carbon Sequestration Microorganisms in Different Wetland Types
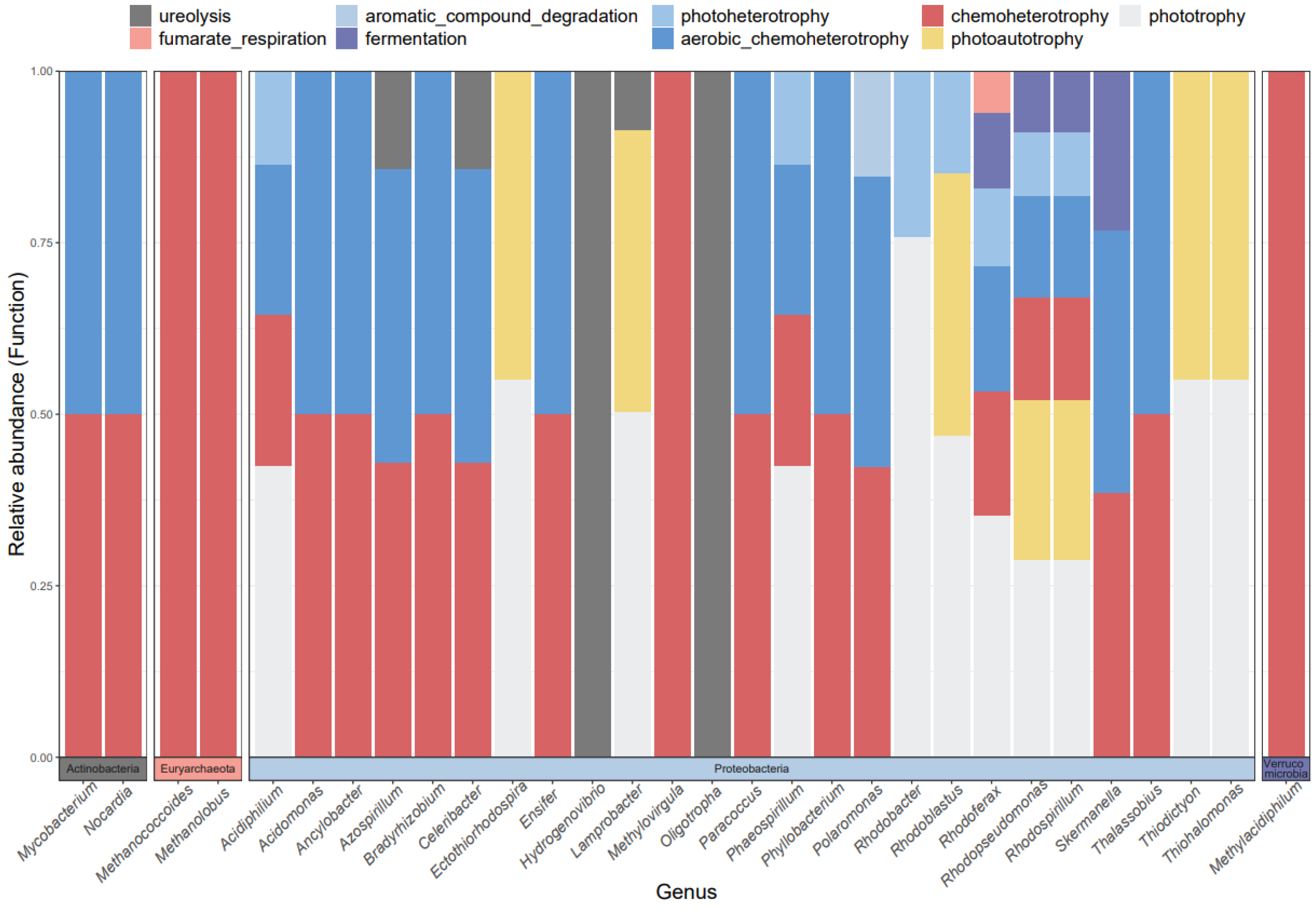
3.2. Composition of cbbM Carbon Sequestration Microbial Communities in Different Wetland Types
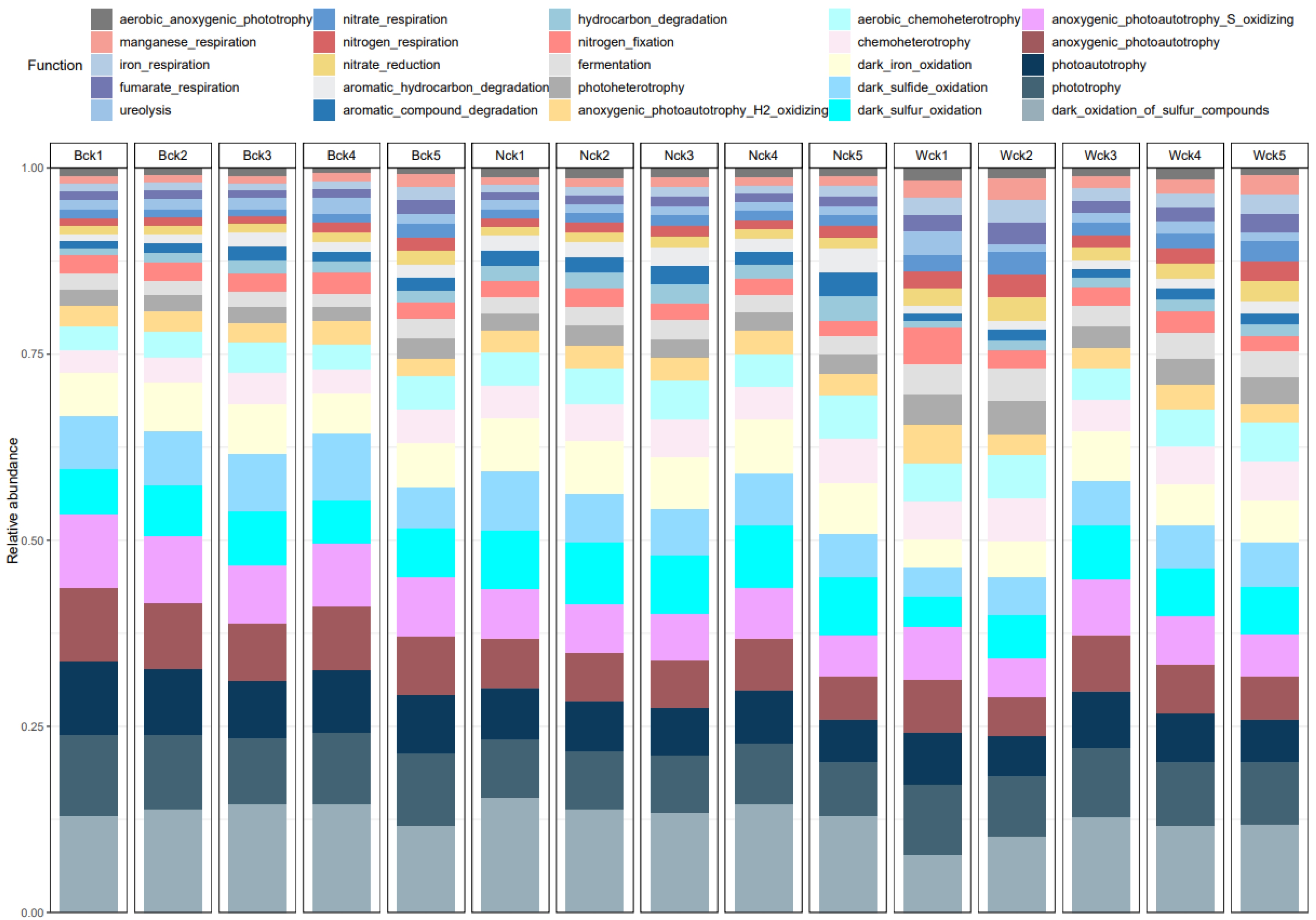
3.3. Functional Groups of cbbM Carbon Sequestration Microbial Community in Qinghai Lake Wetlands
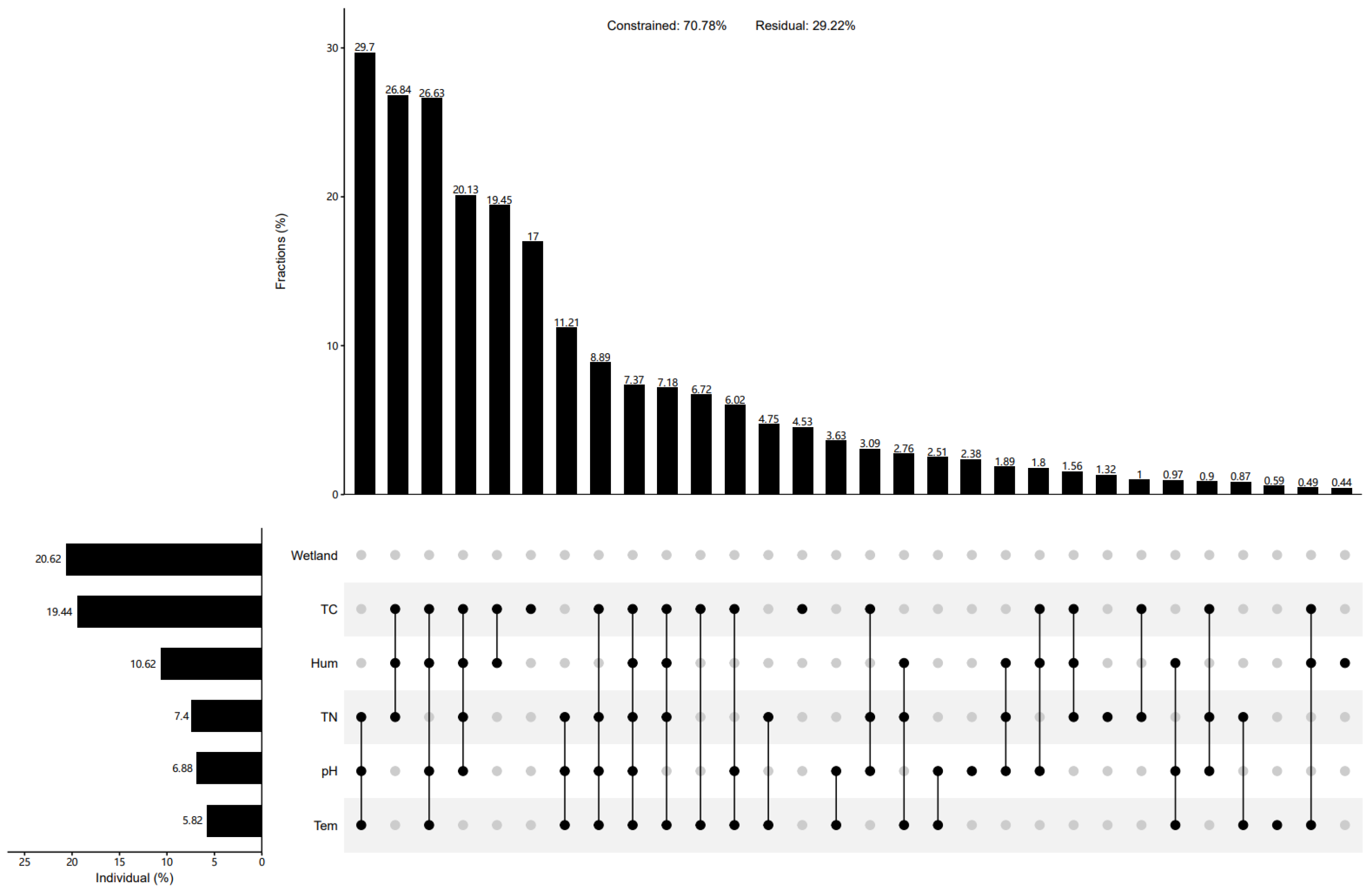
3.4. Correlations between the cbbM Carbon Sequestration Microbial Community and Soil Environmental Factors in the Qinghai Lake Wetlands
4. Discussion
4.1. Effects of Wetland Type Changes on cbbM Carbon Sequestration Microbial Community Diversity
4.2. Effects of Wetland Type Changes on cbbM Carbon Sequestration Microbial Community Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Sheehan, L.; Sherwood, E.T.; Moyer, R.P.; Radabaugh, K.R.; Simpson, S. Blue carbon: An additional driver for restoring and preserving ecological services of coastal wetlands in Tampa Bay (Florida, USA). Wetlands 2019, 39, 1317–1328. [Google Scholar] [CrossRef]
- Asanopoulos, C.; Baldock, J.; Macdonald, L.; Cavagnaro, T. Quantifying blue carbon and nitrogen stocks in surface soils of temperate coastal wetlands. Soil Res. 2021, 59, 619–629. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.; Blum, L.K. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosci. Discuss. 2011, 8, 707–722. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, R.; Zhang, H.; Ge, X.; Liu, J. Distribution of organic carbon in the sediments of Xinxue river and the Xinxue river constructed wetland, China. PLoS ONE 2015, 10, e0134713. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Megonigal, J.P.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916. [Google Scholar] [CrossRef]
- Song, C.C. Advance in research on carbon cycling in wetlands. Sci. Geogr. Sin. 2003, 23, 622–628. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Allison, M.A.; Zhao, J.; Li, X.; Comeaux, R.S.; Feagin, R.A.; Kulawardhana, R.W. Historical reconstruction of mangrove expansion in the Gulf of Mexico: Linking climate change with carbon sequestration in coastal wetlands. Estuar. Coast. Shelf Sci. 2013, 119, 7–16. [Google Scholar] [CrossRef]
- Saintilan, N.; Rogers, K.; Kelleway, J.; Ens, E.; Sloane, D. Climate change impacts on the coastal wetlands of Australia. Wetlands 2019, 39, 1145–1154. [Google Scholar] [CrossRef]
- IPCC. Special Report: Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef]
- IPCC. Central and South America: Impacts, Adaptation, and Vulnerability Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Fu, X.; Shao, M.; Wei, X.; Horton, R. Soil organic carbon and total nitrogen as affected by vegetation types in Northern Loess plateau of China. Geoderma 2010, 155, 31–35. [Google Scholar] [CrossRef]
- Albaladejo, J.; Ortiz, R.; Garcia-Franco, N.; Navarro, A.R.; Almagro, M.; Pintado, J.G.; Martinez-Mena, M. Land use and climate change impacts on soil organic carbon stocks in semi-arid Spain. J. Soils Sediments 2013, 13, 265–277. [Google Scholar] [CrossRef]
- Cheng, M.; Xiang, Y.; Xue, Z.; An, S.; Darboux, F. Soil aggregation and intra-aggregate carbon fractions in relation to vegetation succession on the Loess plateau, China. Catena 2015, 124, 77–84. [Google Scholar] [CrossRef]
- Craft, C.; Vymazal, J.; Kröpfelová, L. Carbon sequestration and nutrient accumulation in floodplain and depressional wetlands. Ecol. Eng. 2018, 114, 137–145. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Lynn, T.M.; Ge, T.; Yuan, H.; Wei, X.; Wu, X.; Xiao, K.; Kumaresan, D.; Yu, S.S.; Wu, J.; Whiteley, A.S. Soil carbon-fixation rates and associated bacterial diversity and abundance in three natural ecosystems. Microb. Ecol. 2017, 73, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Claassens, N.J.; Sousa, D.Z.; Dos Santos, V.A.; De Vos, W.M.; Van Der Oost, J. Harnessing the power of microbial autotrophy. Nat. Rev. Microbiol. 2016, 14, 692–706. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Zhang, Y.; Feng, W.; Lai, Z.; Fa, K.; Qin, S. Metagenomic and 13C tracing evidence for autotrophic atmospheric carbon absorption in a semiarid desert. Soil Biol. Biochem. 2018, 125, 156–166. [Google Scholar] [CrossRef]
- Rubin-Blum, M.; Dubilier, N.; Kleiner, M. Genetic evidence for two carbon fixation pathways (the calvin-benson-bassham cycle and the reverse tricarboxylic acid cycle) in symbiotic and free-living bacteria. mSphere 2019, 4, e00394-18. [Google Scholar] [CrossRef] [PubMed]
- Hügler, M.; Sievert, S.M. Beyond the calvin cycle: Autotrophic carbon fixation in the ocean. Ann. Rev. Mar. Sci. 2011, 3, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.B.; Thomas, B.C.; Alvarez, W.; Banfield, J.F. Metagenomic analysis of a high carbon dioxide subsurface microbial community populated by chemolithoautotrophs and bacteria and archaea from candidate phyla. Environ. Microbiol. 2016, 18, 1686–1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hou, L.; Zheng, Y.; Zhang, Z.; Tang, X.; Mao, T.; Du, J.; Bi, Q.; Dong, H.; Yin, G.; et al. Dark carbon fixation in intertidal sediments: Controlling factors and driving microorganisms. Water Res. 2022, 216, 118381. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Xi, D.; He, J.Z.; Hu, H.W.; Fang, Y.T.; Zhang, L.M. Activity, abundance and community structure of anammox bacteria along depth profiles in three different paddy soils. Soil Biol. Biochem. 2015, 91, 212–221. [Google Scholar] [CrossRef]
- Pan, B.T.; Li, J.J. Qinghai-Tibetan Plateau: A Driver and Amplifier of the Global Climatic Change—III. The effects of the uplift of Qinghai-Tibetan Plateau on Climatic Changes. J. Lanzhou Univ. (Nat. Sci.) 1996, 32, 108–115. [Google Scholar]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Wang, Y.; Fang, X.; Gao, Y.; Zhu, D.; Yang, G.; Tian, J.; et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob. Chang. Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, K.K.; Williams, J.J.; Craine, J.M.; Jeffers, E.S. Changes in global nitrogen cycling during the holocene epoch. Nature 2013, 495, 352–355. [Google Scholar] [CrossRef]
- O’Beirne, M.D.; Werne, J.P.; Hecky, R.E.; Johnson, T.C.; Katsev, S.; Reavie, E.D. Anthropogenic climate change has altered primary productivity in lake superior. Nat. Commun. 2017, 8, 15713. [Google Scholar] [CrossRef]
- Zhang, N.; Bao, H.; Zuo, D.Z.; Cui, B.L.; Chen, K.L. Community characteristics of methanogenic bacteria in different types of alpine wetlands around Qinghai Lake. J. Appl. Environ. Biol. 2022, 28, 283–289. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, K.; Wang, S.; Qi, D.; Zhou, Z.; Xie, C.; Liu, X. Dynamic Response of the cbbL Carbon Sequestration Microbial Community to Wetland Type in Qinghai Lake. Biology 2023, 12, 1503. [Google Scholar] [CrossRef]
- Liu, J.F.; Mbadinga, S.M.; Sun, X.B.; Yang, G.C.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Microbial communities responsible for fixation of CO2 revealed by using mcrA, cbbM, cbbL, fthfs, fefe-hydrogenase genes as molecular biomarkers in petroleum reservoirs of different temperatures. Int. Biodeterior. Biodegrad. 2016, 114, 164–175. [Google Scholar] [CrossRef]
- Liang, S.; Deng, J.; Jiang, Y.; Wu, S.; Zhou, Y.; Zhu, W. Functional Distribution of Bacterial Community under Different Land Use Patterns Based on FaProTax Function Prediction. Pol. J. Environ. Stud. 2020, 29, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fang, J.; Liu, J.; Yang, X.; Lyu, T.; Wang, L.; Zhou, S.; Dou, H.; Zhang, H. Differences in sediment carbon-fixation rate and associated bacterial communities in four wetland types in Hulun lake basin. Catena 2022, 213, 106167. [Google Scholar] [CrossRef]
- Xiang, X.; Gibbons, S.M.; Li, H.; Shen, H.; Fang, J.; Chu, H. Shrub encroachment is associated with changes in soil bacterial community composition in a temperate grassland ecosystem. Plant Soil 2018, 425, 539–551. [Google Scholar] [CrossRef]
- Liao, Q.; Liu, H.; Lu, C.; Liu, J.; Waigi, M.G.; Ling, W. Root exudates enhance the PAH degradation and degrading gene abundance in soils. Sci. Total Environ. 2021, 764, 144436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, G. Responses of plant, soil bacterial and fungal communities to grazing vary with pasture seasons and grassland types, northern Tibet. Land Degrad. Dev. 2021, 32, 1821–1832. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.; Gao, H.; An, S. Response and driving factors of soil microbial diversity related to global nitrogen addition. Land Degrad. Dev. 2020, 31, 190–204. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Niu, B.; Chen, Q.; Wang, J.; Zhao, J.; Luo, T.; Zhang, G. Effect of increasing precipitation and warming on microbial community in Tibetan alpine steppe. Environ. Res. 2020, 189, 109917. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Y.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Effects of environmental factors on the distribution of microbial communities across soils and lake sediments in the Hoh Xil Nature Reserve of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2022, 838, 156148. [Google Scholar] [CrossRef]
- Wang, Z. Diversity of Carbon Sequestration Microbial Communities in Meadow Soil of Qinghai-Tibet Plateau and Its Influencing Factors. Master’s Thesis, China University of Geosciences, Wuhan, China, 2019. [Google Scholar]
- Wang, B.C. Study on Carbon Sequestration Microbial Community Structure, Carbon Sequestration Function and Environmental Influencing Factors in Lake Sediments of Northern Tibetan Plateau. Master’s Thesis, China University of Geosciences, Wuhan, China, 2019. [Google Scholar]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.; Fierer, N. Temporal variability in soil microbial communities across land-use types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef]
- Yuan, H.; Ge, T.; Chen, C.; O’Donnell, A.G.; Wu, J. Significant role for microbial autotrophy in the sequestration of soil carbon. Appl. Environ. Microbiol. 2012, 78, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Xiao, Y.; Cheng, A.; Shen, T.; Zhu, M.; Yu, L.J. Abundance and diversity of carbon-fixing bacterial communities in karst wetland soil ecosystems. Catena 2021, 204, 105418. [Google Scholar] [CrossRef]
- Gao, J.; Said, M.; Yue, L.; Yongtao, H.; Dorji, T.; Zhang, X. Changes in CO2-fixing microbial community characteristics with elevation and season in alpine meadow soils on the Northern Tibetan plateau. Acta Ecol. Sin. 2018, 38, 3816–3824. [Google Scholar] [CrossRef]
- Yousuf, B.; Kumar, R.; Mishra, A.; Jha, B. Unravelling the carbon and sulphur metabolism in coastal soil ecosystems using comparative cultivation-independent genome-level characterisation of microbial communities. PLoS ONE 2014, 9, e107025. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, H.; Leng, X.; Cheng, X.; An, S. Soil organic carbon and nitrogen dynamics following Spartina alterniflora invasion in a coastal wetland of Eastern China. Catena 2017, 156, 281–289. [Google Scholar] [CrossRef]
- Schippers, A. Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. In Microbial Processing of Metal Sulfides; Donati, E., Sand, W., Eds.; Springer: Heidelberg, NY, USA, 2007. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Chen, K.; Wang, X.; Ji, W.; Yang, Z.; Wang, X.; Li, J. Response Mechanism of cbbM Carbon Sequestration Microbial Community Characteristics in Different Wetland Types in Qinghai Lake. Biology 2024, 13, 333. https://doi.org/10.3390/biology13050333
Zhang N, Chen K, Wang X, Ji W, Yang Z, Wang X, Li J. Response Mechanism of cbbM Carbon Sequestration Microbial Community Characteristics in Different Wetland Types in Qinghai Lake. Biology. 2024; 13(5):333. https://doi.org/10.3390/biology13050333
Chicago/Turabian StyleZhang, Ni, Kelong Chen, Xinye Wang, Wei Ji, Ziwei Yang, Xia Wang, and Junmin Li. 2024. "Response Mechanism of cbbM Carbon Sequestration Microbial Community Characteristics in Different Wetland Types in Qinghai Lake" Biology 13, no. 5: 333. https://doi.org/10.3390/biology13050333
APA StyleZhang, N., Chen, K., Wang, X., Ji, W., Yang, Z., Wang, X., & Li, J. (2024). Response Mechanism of cbbM Carbon Sequestration Microbial Community Characteristics in Different Wetland Types in Qinghai Lake. Biology, 13(5), 333. https://doi.org/10.3390/biology13050333






