Fire-Induced Changes in Soil Properties and Bacterial Communities in Rotational Shifting Cultivation Fields in Northern Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
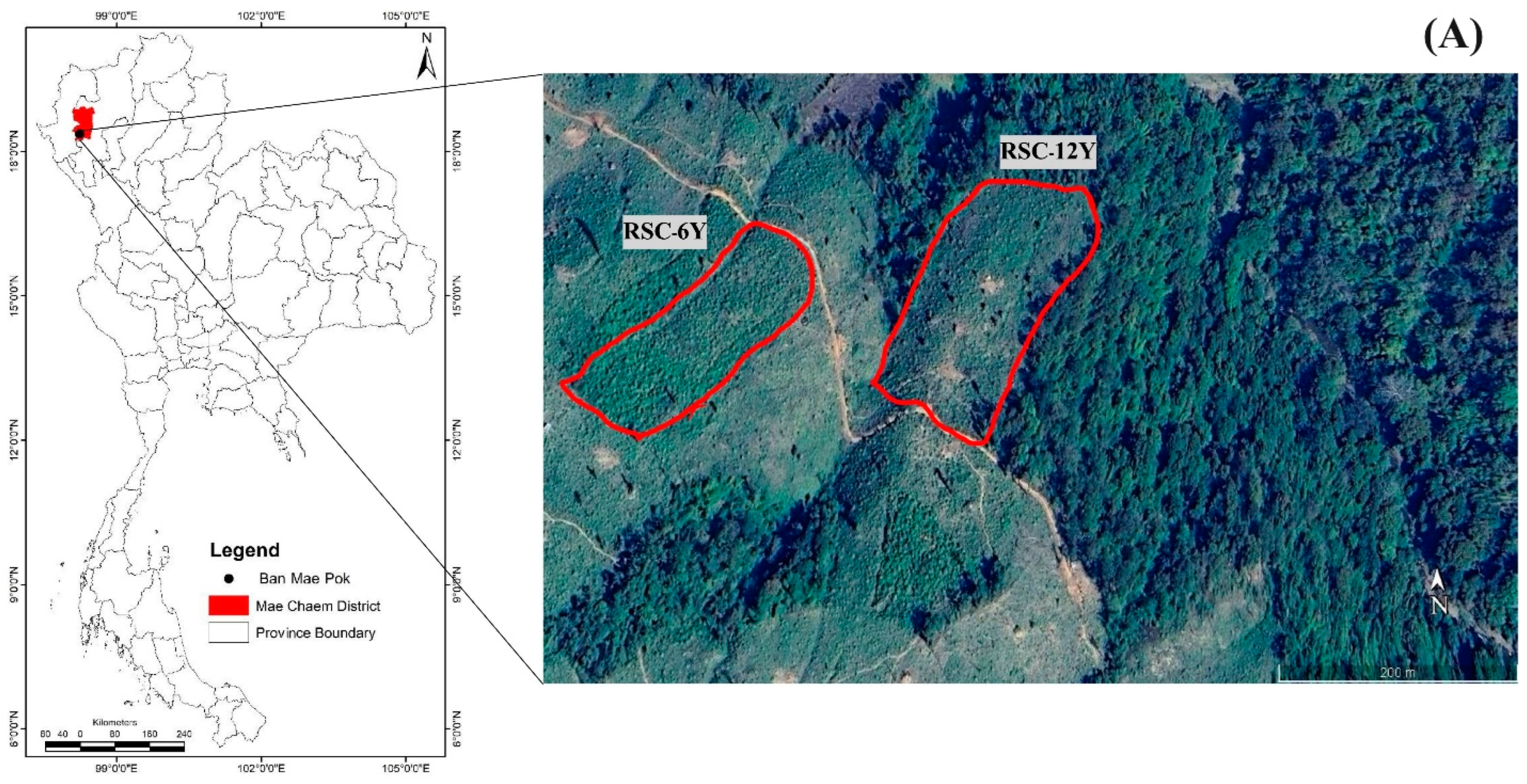
2.1. Study Areas
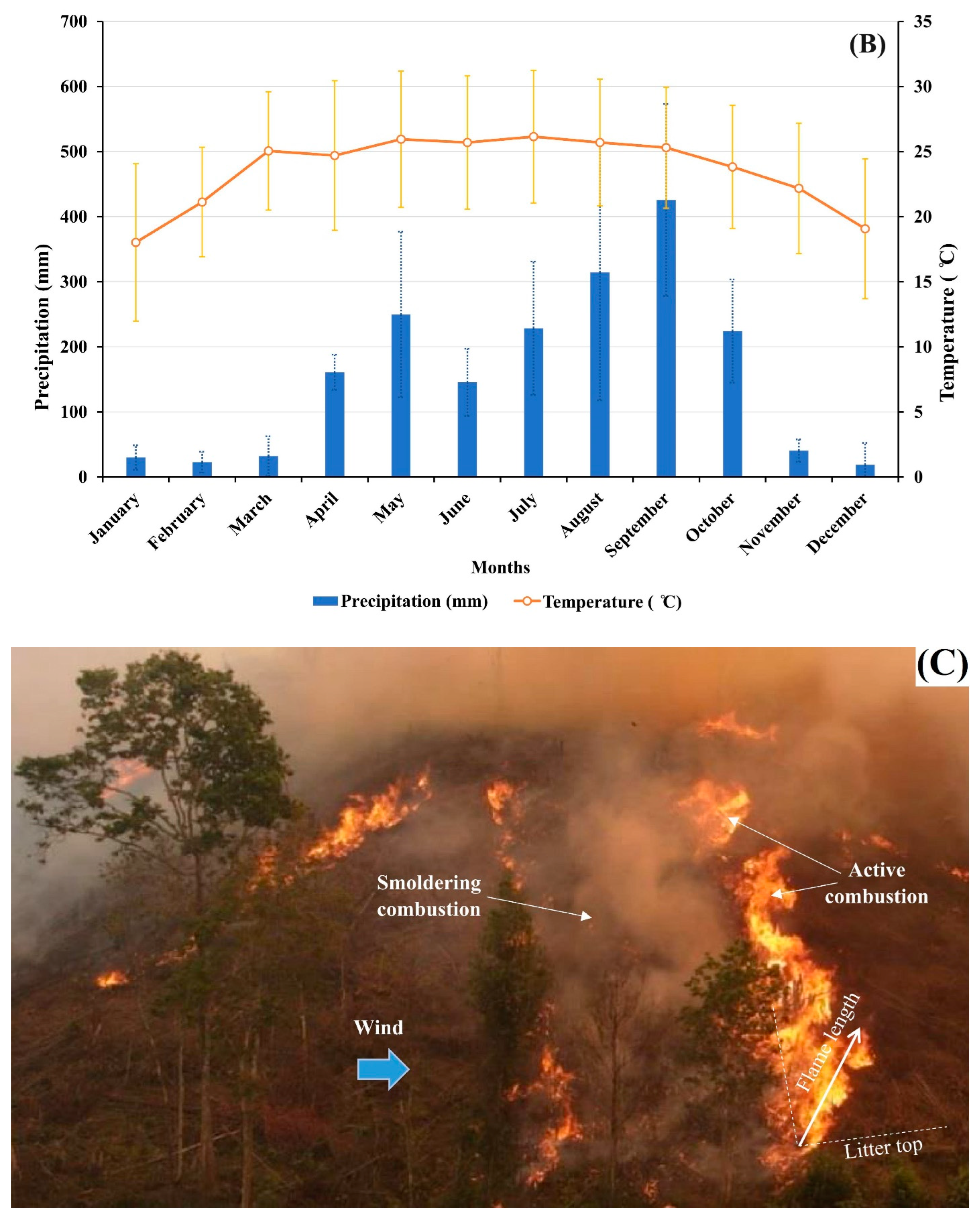
2.2. Experimental Design, Fire Measurements and Soil Sampling
2.3. Analysis of Soil Physical and Chemical Properties
2.4. DNA Extraction, Bacterial 16s Amplification, and Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Moisture, Soil Temperature, Fire Behaviors, and Soil Physicochemical Properties
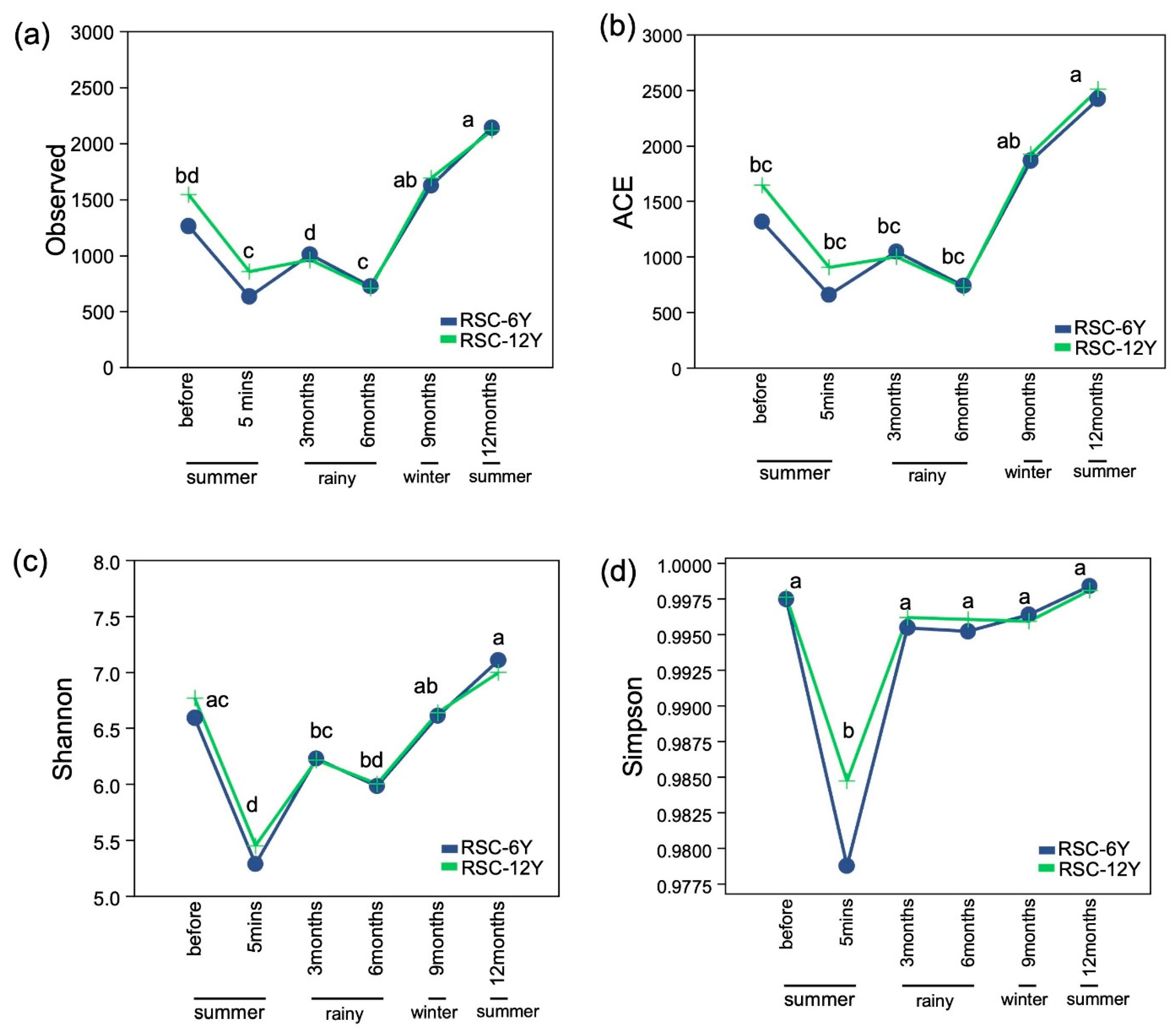
3.2. Bacterial Richness and Diversity
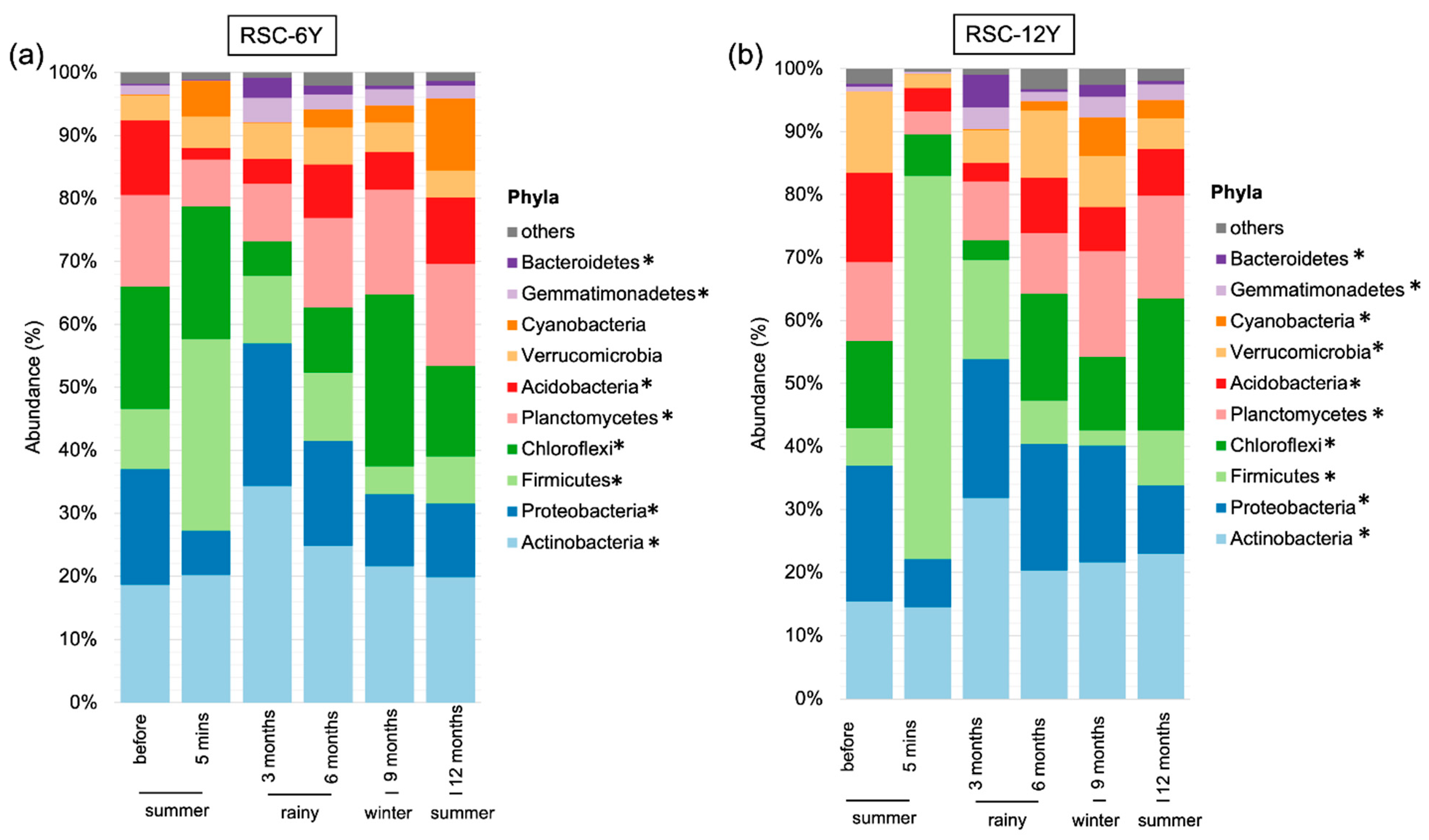
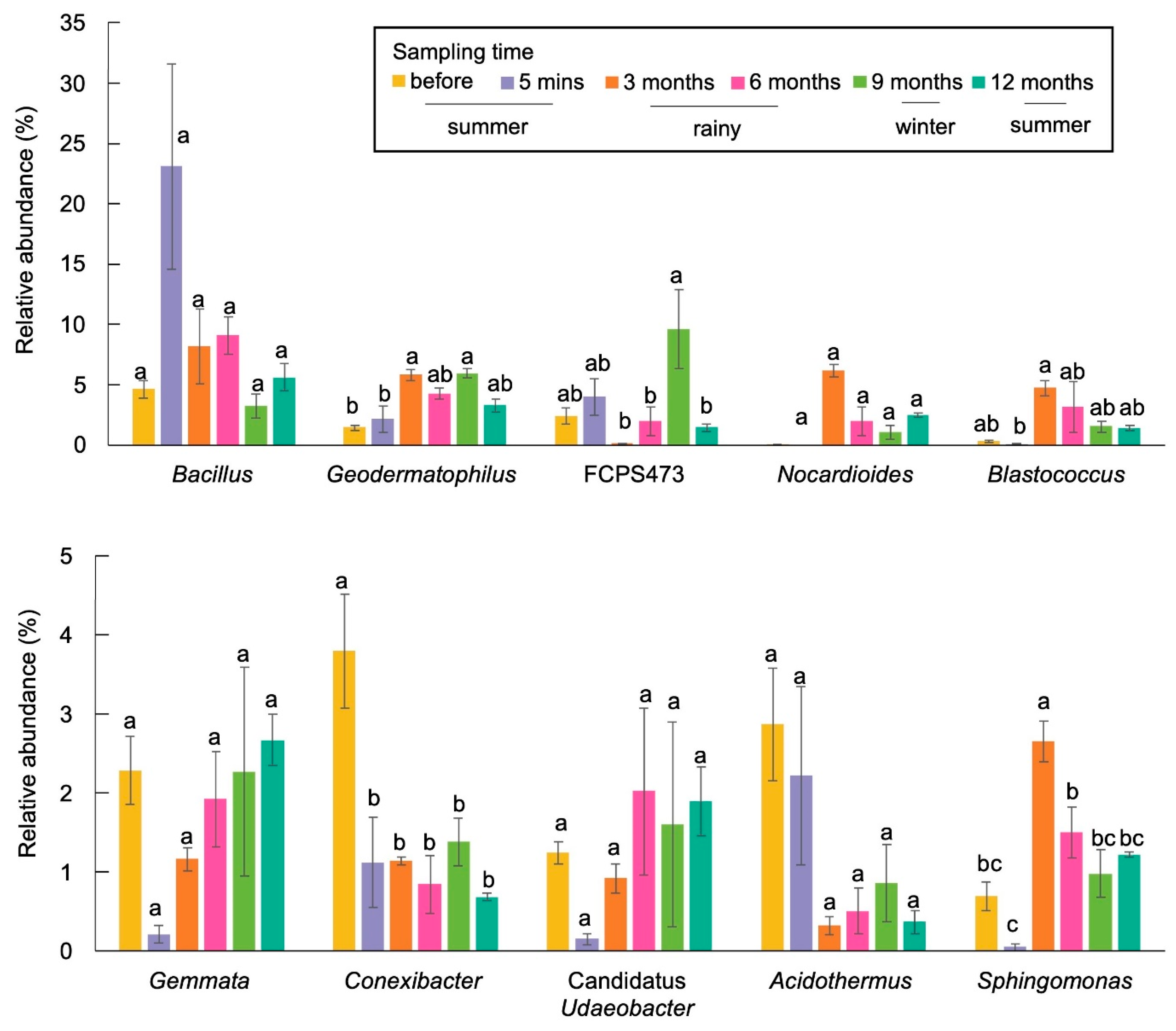
3.3. Bacterial Taxonomic Distribution
3.3.1. Taxonomic Distribution in RSC-6Y
3.3.2. Taxonomic Distribution in RSC-12Y
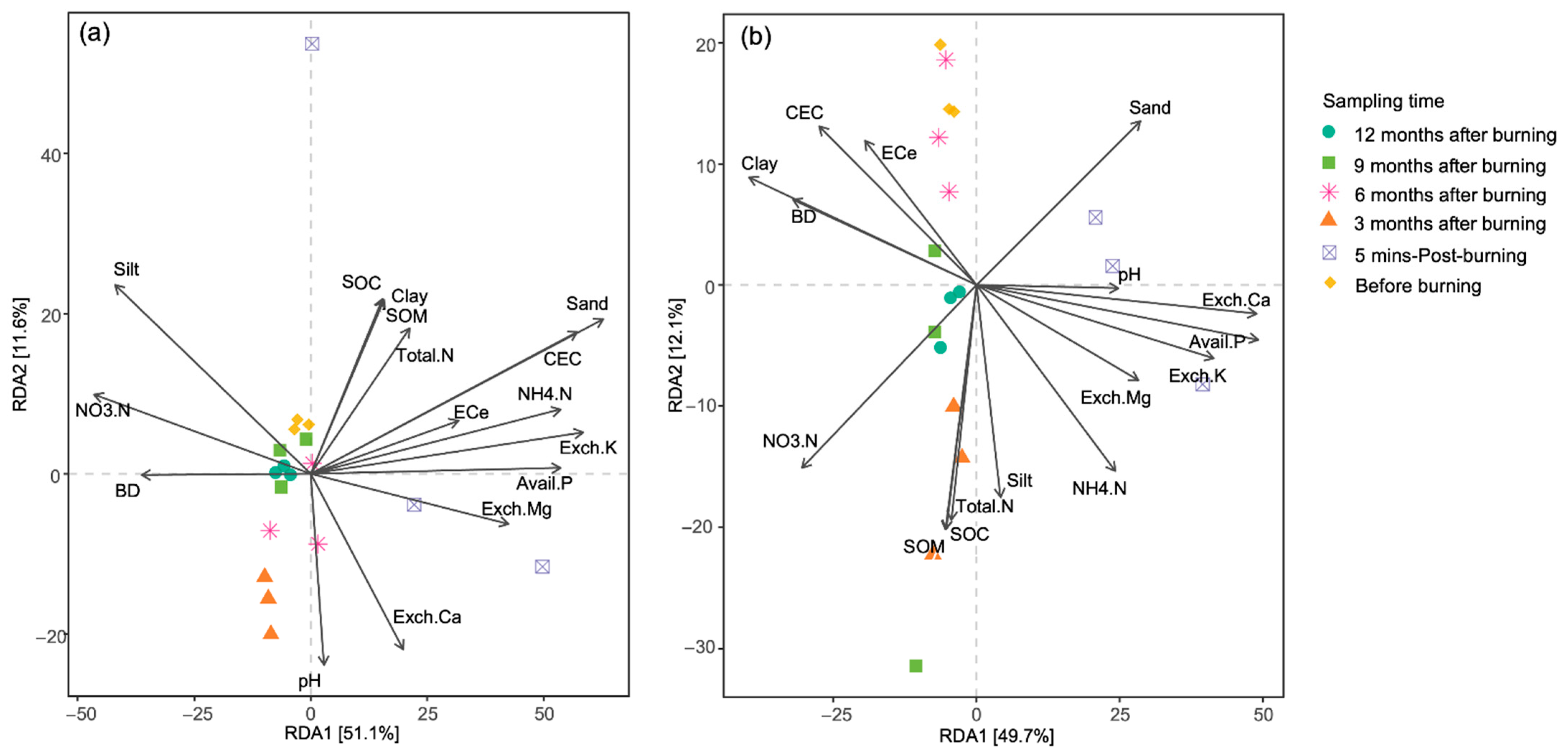
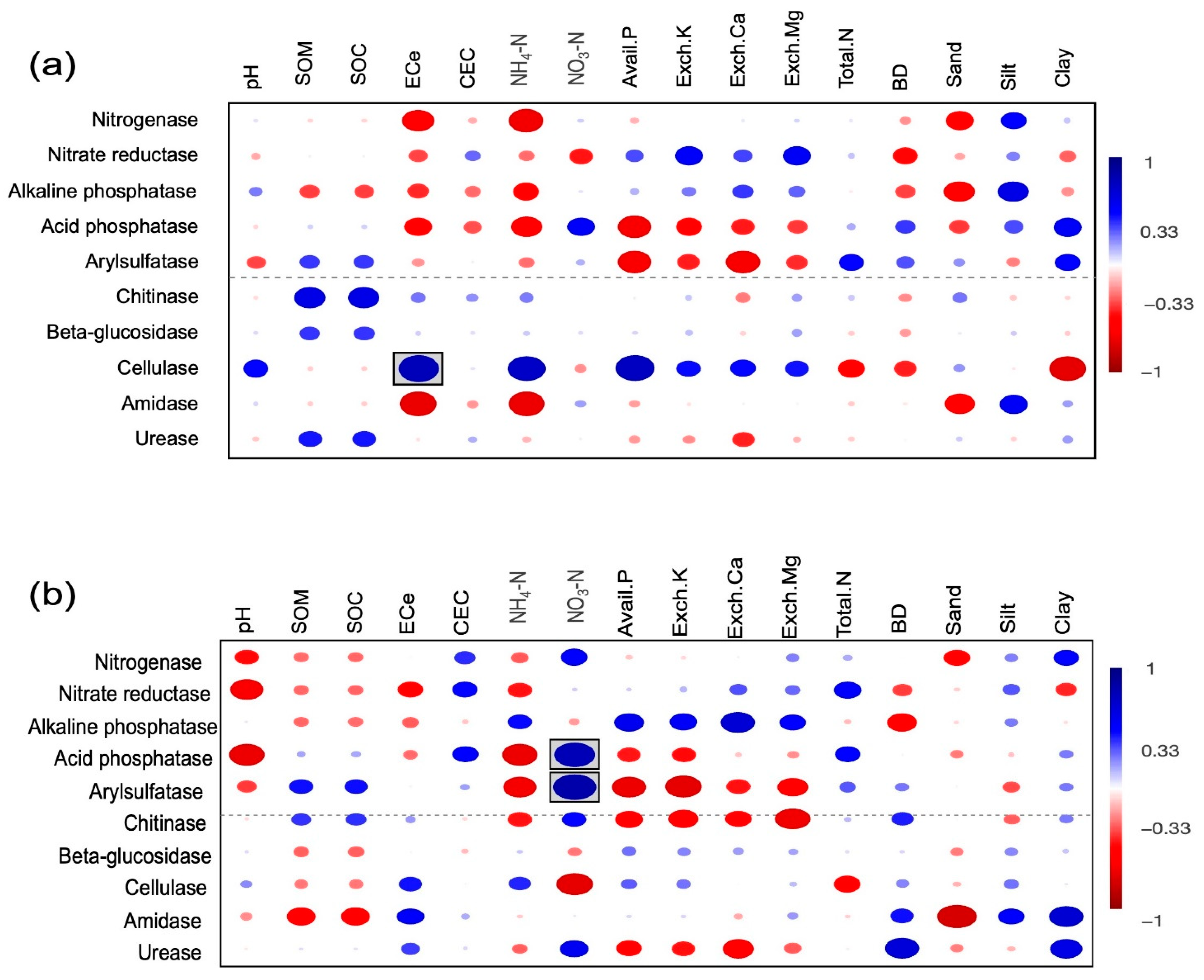
3.4. Community Composition and Correlation with Soil Properties
3.5. Predictive Function
4. Discussion
4.1. Effects of Seasonal Variations on Soil Microbial Communities
4.2. Relationship between Soil Physical and Chemical Properties, and Soil Bacterial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherrier, J. Shifting Cultivation: Misconception of the Asian Governments. J. Int. Dev. Coop. 2018, 24, 71–82. [Google Scholar]
- Rasul, G.; Thapa, G.B. Shifting cultivation in the mountains of South and Southeast Asia: Regional patterns and factors influencing the change. Land Degrad. Dev. 2003, 14, 495–508. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Hatano, R. Effects of fire on soil organic carbon, soil total nitrogen, and soil properties under rotational shifting cultivation in northern Thailand. J. Environ. Manag. 2022, 302, 113978. [Google Scholar] [CrossRef]
- Stygler, E.; Harivelo, M.R.; Max, J.; Pfefer, M.J.; Fernandes, E.C.M.; Bates, D.M. Infuence of slash and burn farming practices on fallow succession and land degradation in the rainforest region of Madagascar. Agric. Ecosyst. Environ. 2007, 19, 257–269. [Google Scholar] [CrossRef]
- Mertz, O.; Padoch, C.; Fox, J.; Cramb, R.A.; Leisz, S.J.; Lam, N.I.; Vien, T.D. Swidden change in Southeast Asia: Understanding causes and consequences. Hum. Ecol. 2009, 37, 259–264. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and process in neotropical secondary rain forests: The first 100 years of succession. Trends Ecol. 1996, 1, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Hamman, S.T.; Burke, I.C.; Stromberger, M.E. Relationships between microbial community structure and soil environmental conditions in a recently burned system. Soil Biol. Biochem. 2007, 39, 1703–1711. [Google Scholar] [CrossRef]
- Emery, S.M.; Uwimbabazi, J.; Flory, S.L. Fire intensity effects on seed germination of native and invasive Eastern deciduous forest understory plants. For. Ecol. Manag. 2011, 261, 1401–1408. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Tri Wibowo, T.; van Noordwijk, M.; Penot, E. Farmers’ perspectives on slash-and-burn as a land clearing method for small-scale rubber producers in Sepunggur, Jambi Province, Sumatra, Indonesia. For. Ecol. Manag. 1999, 120, 157–169. [Google Scholar] [CrossRef]
- Barreiro, A.; Díaz-Raviña, M. Fire impacts on soil microorganisms: Mass, activity, and diversity. Curr. Opin. Environ. Sci. Health 2021, 2, 100264. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Caon, L.; Vallejo, V.R.; Ritsema, C.J.; Geissen, V. Effects of wildfire on soil nutrients in Mediterranean ecosystems. Earth-Sci. Rev. 2014, 139, 47–58. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S.; Gawroński, S. Impact of fire severity on soil properties and the development of tree and shrub species in a Scots pine moist forest site in southern Poland. For. Ecol. Manag. 2015, 342, 56–63. [Google Scholar] [CrossRef]
- Pierson, D.N.; Robichaud, P.R.; Rhoades, C.C.; Brown, R.E. Soil carbon nitrogen eroded after severe wildfire erosion mitigation treatments. Int. J. Wildland Fire 2019, 28, 814. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Sun, L.; Qi, G.; Chen, S.; Zhao, X. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smith Nancy, R.; Kishchuk Barbara, E.; Mohn William, W. Effects of Wildfire and Harvest Disturbances on Forest Soil Bacterial Communities. Appl. Environ. Microbiol. 2008, 74, 216–224. [Google Scholar] [CrossRef]
- Miah, S.; Dey, S.; Sirajul Haque, S.M. Shifting cultivation effects on soil fungi and bacterial population in Chittagong Hill Tracts, Bangladesh. J. For. Res. 2010, 21, 311–318. [Google Scholar] [CrossRef]
- Arunrat, N.; Sansupa, C.; Sereenonchai, S.; Hatano, R. Stability of soil bacteria in undisturbed soil and continuous maize cultivation in Northern Thailand. Front. Microbiol. 2023, 14, 1285445. [Google Scholar] [CrossRef] [PubMed]
- Arunrat, N.; Sansupa, C.; Sereenonchai, S.; Hatano, R. Short-term response of soil bacterial and fungal communities to fire in rotational shifting cultivation, Northern Thailand. Appl. Soil Ecol. 2024, 196, 105303. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbrück, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining seasonal marine microbial community dynamics. ISME J. 2012, 6, 298–308. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Yuttitham, M.; Hatano, R. Variations of soil properties and soil surface loss after fire in rotational shifting cultivation in Northern Thailand. Front. Environ. Sci. 2023, 11, 1213181. [Google Scholar] [CrossRef]
- LDD (Land Development Department). Preliminary Study on Highland Development Project in Northern Thailand; Soil Survey Division, Land Development Department: Bangkok, Thailand, 1992.
- Butler, B.; Quarles, S.; Standohar-Alfano, C.; Morrison, M.; Jimenez, D.; Sopko, P.; Wold, C.; Bradshaw, L.; Atwood, L.; Landon, J.; et al. Exploring fire response to high wind speeds: Fire rate of spread, energy release and flame residence time from fires burned in pine needle beds under winds up to 27 m s−1. Int. J. Wildland Fire 2019, 29, 81–92. [Google Scholar] [CrossRef]
- Gould, J.S.; Sullivan, A.L. Two methods for calculating wildland fire rate of forward spread. Int. J. Wildland Fire 2020, 29, 272–281. [Google Scholar] [CrossRef]
- Mehring, M.; Glaser, B.; de Camargo, P.B.; Zech, W. Impact of forest organic farming change on soil microbial C turnover using 13C of phospholipid fatty acids. Agron. Sustain. Dev. 2011, 31, 719–731. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhang, L.; Yin, R.; Wu, G.-L. Bacterial contributions of bio-crusts and litter crusts to nutrient cycling in the mu us Sandy land. Catena 2021, 199, 105090. [Google Scholar] [CrossRef]
- Barbour, K.M.; Weihe, C.; Allison, S.D.; Martiny, J.B.H. Bacterial community response to environmental change varies with depth in the surface soil. Soil Biol. Biochem. 2022, 172, 108761. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual; Burt, R., Soil Survey Staff, Eds.; Soil Survey Investigations Report No. 51, Version 2.0; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Jones, J.B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; ASA; SSSA: Madison, WI, USA, 1982; Chapter 9; pp. 159–165. [Google Scholar]
- Bray, R.A.; Kurtz, L.T. Determination of total organic and available form of phosphorus in soil. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Walkley, A.; Black, J.A. An examination of the dichormate method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–32. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Thomaz, E.L. Fire changes the larger aggregate size classes in slash-and-burn agricultural systems. Soil Till. Res. 2017, 165, 210–217. [Google Scholar] [CrossRef]
- Habekost, M.; Eisenhauer, N.; Scheu, S.; Steinbeiss, S.; Weigelt, A.; Gleixner, G. Seasonal changes in the soil microbial community in a grassland plant diversity gradient four years after establishment. Soil Biol. Biochem. 2008, 40, 2588–2595. [Google Scholar] [CrossRef]
- Landesman, W.J.; Freedman, Z.B.; Nelson, D.M. Seasonal, sub-seasonal and diurnal variation of soil bacterial community composition in a temperate deciduous forest. FEMS Microbiol. Ecol. 2019, 95, fiz002. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Sansupa, C.; Kongsurakan, P.; Hatano, R. Effect of rice straw and stubble burning on soil physicochemical properties and bacterial communities in Central Thailand. Biology 2023, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol. Biochem. 2013, 60, 95–104. [Google Scholar] [CrossRef]
- Yao, M.; Rui, J.; Niu, H.; Heděnec, P.; Li, J.; He, Z.; Wang, J.; Cao, W.; Li, X. The differentiation of soil bacterial communities along a precipitation and temperature gradient in the eastern Inner Mongolia steppe. Catena 2017, 152, 47–56. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M.K.; Hu, G.; Weng, B. Seasonal Change in Microbial Diversity and Its Relationship with Soil Chemical Properties in an Orchard. PLoS ONE 2019, 14, e0215556. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Cai, X.H.; Liu, X.L.; Wang, J.X.; Cheng, J.X.; Cheng, S.; Zhang, X.; Li, D.; Li, M. Soil microbial population dynamics along a chronosequence of moist evergreen broad-leaved forest succession in southwestern China. J. Mount. Sci. 2010, 7, 327–338. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Kandeler, E.; Tscherko, D.; Hobbs, P.J.; Bezemer, T.M.; Jones, T.H.; Thompson, L.J. Below-ground microbial community development in a high temperature world. Oikos 1999, 85, 193. [Google Scholar] [CrossRef]
- Shishido, M.; Sakamoto, K.; Yokoyama, H.; Momma, N.; Miyashita, S. Changes in microbial communities in an apple orchard and its adjacent bush soil in response to season, land-use, and violet root rot infestation. Soil Biol. Biochem. 2008, 40, 1460–1473. [Google Scholar] [CrossRef]
- Kim, C.S.; Nam, J.W.; Jo, J.W.; Kim, S.Y.; Han, J.G.; Hyun, M.W.; Sung, G.H.; Han, S.K. Studies on seasonal dynamics of soilhigher fungal communities in Mongolian oak-dominant Gwangneung forest in Korea. J. Microbiol. 2016, 54, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Is vegetation composition or soil chemistry the best predictor of the soil microbial community? Plant Soil 2010, 3, 417–430. [Google Scholar] [CrossRef]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Thoms, C.; Gleixner, G. Seasonal differences in tree species’ influence on soil microbial communities. Soil Biol. Biochem. 2013, 6, 239–248. [Google Scholar] [CrossRef]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. A novel amidohydrolase gene from Bacillus subtilis cloning: DNA-sequence analysis and map position of amhX. FEMS Microbiol. Lett. 1996, 141, 129–137. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Whitely, A.S.; O’Donnell, A.G.; Bailey, M.J. Influence of depth and sampling time on bacterial community structure in an upland grassland soil. FEMS Microbiol. Ecol. 2003, 43, 35–43. [Google Scholar] [CrossRef]
- Jefferies, R.L.; Walker, N.A.; Edwards, K.A.; Dainty, J. Is the decline of soil microbial biomass in late winter coupled to changes in the physical state of cold soils? Soil Biol. Biochem. 2010, 42, 129–135. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, S.Y.; Zhang, J.F.; He, X.Y.; Liu, W.J.; Zhao, Q.; Zhao, L.; Tian, C.J. Depth-related responses of soil microbial communities to experimental warming in an alpine meadow on the Qinghai-Tibet Plateau. Eur. J. Soil Sci. 2015, 6, 496–504. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Bukar, M.; Sodipo, O.; Dawkins, K.; Ramirez, R.; Kaldapa, J.T.; Tarfa, M.; Esiobu, N. Microbiomes of Top and Sub-Layers of Semi-Arid Soils in North-Eastern Nigeria Are Rich in Firmicutes and Proteobacteria with Surprisingly High Diversity of Rare Species. Adv. Microbiol. 2019, 9, 102–118. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Berbel, N.; Ortega, R.; Lucas-Borja, M.E.; Solé-Benet, A.; Miralles, I. Long-Term Effects of Two Organic Amendments on Bacterial Communities of Calcareous Mediterranean Soils Degraded by Mining. J. Environ. Manag. 2020, 271, 110920. [Google Scholar] [CrossRef] [PubMed]
- Fontúrbel, M.T.; Barreiro, A.; Vega, J.A.; Martín, A.; Jiménez, E.; Carballas, T.; Fernández, C.; Díaz-Raviña, M. Effects of an Experimental Fire and Post-Fire Stabilization Treatments on Soil Microbial Communities. Geoderma 2012, 191, 51–60. [Google Scholar] [CrossRef]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aguirre, A.J.; Aznar, J.M.; González-Pérez, J.A.; De la Rosa, J.M.; León, J.; Ibarra, P.; Echeverría, T. Wildfire effects on nutrients and organic carbon of a Rendzic Phaeozem in NE Spain: Changes at cm-scale topsoil. CATENA 2014, 113, 267–275. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Farguell, J.; Úbeda, X. Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí massif, Catalonia, Spain). Sci. Total Environ. 2016, 572, 1329–1335. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Iwai, C.B.; Yuttitham, M.; Hatano, R. Post-fire recovery of soil organic carbon, soil total nitrogen, soil nutrients, and soil erodibility in rotational shifting cultivation in Northern Thailand. Front. Environ. Sci. 2023, 1, 1117427. [Google Scholar] [CrossRef]
- Giorgis, M.A.; Zeballos, S.R.; Carbone, L.; Zimmermann, H.; von Wehrden, H.; Aguilar, R.; Ferreras, A.E.; Tecco, P.A.; Kowaljow, E.; Barri, F.; et al. A review of fire effects across South American ecosystems: The role of climate and time since fire. Fire Ecol. 2021, 17, 11. [Google Scholar] [CrossRef]
- Doerr, S.H.; Santín, C.; Merino, A.; Belcher, C.M.; Baxter, G. Fire as a removal mechanism of pyrogenic carbon from the environment: Effects of fire and pyrogenic carbon characteristics. Front. Earth Sci. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C. Forest Fire Effects on Soil Color and Texture. Soil Sci. Soc. Am. J. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- DeLuca, T.; Nilsson, M.-C.; Zackrisson, O. Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 2002, 133, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Chambers, J.C.; Johnson, D.W.; Blank, R.R.; Board, D.I. Effect of repeated burning on plant and soil carbon and nitrogen in cheatgrass (Bromus tectorum) dominated ecosystems. Plant Soil 2015, 386, 47–64. [Google Scholar] [CrossRef]
- Verma, S.; Jayakumar, S. Effect of recurrent fires on soil nutrient dynamics in a tropical dry deciduous forest of Western Ghats, India. J. Sustain. For. 2018, 37, 678–690. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Meharg, A.; Killham, K. Carbon distribution within the plant and rhizosphere in laboratory and field-grown Lolium perenne at different stages of development. Soil Biol. Biochem. 1990, 2, 471–477. [Google Scholar] [CrossRef]
- Ren, B.H.; Hu, Y.M.; Chen, B.D.; Zhang, Y.; Thiele, J.; Shi, R.J.; Liu, M.; Bu, R.C. Soil pH and plant diversity shape soil bacterial community structure in the active layer across the latitudinal gradients in continuous permafrost region of northeastern china. Sci. Rep. 2018, 8, 5619. [Google Scholar] [CrossRef]
- Giller, K.E.; Beare, M.H.; Lavalle, P.; Izac, A.M.N.; Swift, M.J. Agricultural intensification, soil biodiversity and agroecosystem function. Appl. Soil Ecol. 1997, 6, 3–16. [Google Scholar] [CrossRef]
- D’Ascoli, R.; Rutigliano, F.A.; De Pascale, R.A.; Gentile, A.; Virzo De Santo, A. Functional diversity of the microbial community in Mediterranean maquis soils as affected by fires. Int. J. Wildland Fire 2005, 14, 355–363. [Google Scholar] [CrossRef]



| Sites | Variables | Time Points | Fire Temperature in the Litter Top (°C) (min–max) | Flame Length (m) | Flame Residence Time (s) | Spread Rate (m/min) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Burning | 5 min after Burning | 3 Months after Burning | 6 Months after Burning | 9 Months after Burning | 12 Months after Burning | ||||||
| RSC-6Y | Soil moisture (%) | 34.2 ± 5.5 b | 31.7 ± 6.9 b | 41.3 ± 7.5 a | 43.5 ± 8.0 a | 33.8 ± 5.5 b | 32.5 ± 5.5 b | 253–612 | 3.6 ± 1.0 | 22.0 ± 19.0 | 12.5 ± 8.5 |
| Soil temperature (°C) | 23.3 ± 3.1 a | 46.0 ± 2.5 b | 25.2 ± 3.5 a | 23.4 ± 3.0 a | 26.5 ± 3.0 a | 27.5 ± 2.5 a | |||||
| RSC-12Y | Soil moisture (%) | 35.8 ± 4.4 b | 30.4 ± 5.8 b | 40.1 ± 6.0 a | 45.4 ± 5.8 a | 32.5 ± 6.0 b | 32.1 ± 6.2 b | 315–754 | 5.5 ± 2.0 | 33.0 ± 21.0 | 15.5 ± 9.0 |
| Soil temperature (°C) | 23.6 ± 2.2 a | 47.8 ± 1.0 b | 25.1 ± 2.0 a | 23.5 ± 2.5 a | 26.1 ± 3.5 a | 27.5 ± 3.0 a | |||||
| Site | Time Point | pH (1:1) | ECe (dS m−1) | BD (Mg m−3) | SOM (%) | SOC (%) | TN (%) | %Sand | %Silt | %Clay | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| RSC-6Y | pre-burning | 5.69 a | 0.03 | 0.14 a | 0.01 | 1.35 a | 0.02 | 7.03 a | 0.31 | 4.08 a | 0.18 | 0.26 a | 0.02 | 24.18 a | 0.71 | 48.30 a | 0.57 | 27.52 a | 0.74 |
| 5 min after burning | 6.72 b | 0.05 | 1.22 b | 0.01 | 1.29 a | 0.01 | 5.74 b | 0.11 | 3.33 b | 0.06 | 0.19 b | 0.01 | 34.46 b | 0.48 | 45.47 a | 0.04 | 20.07 b | 0.52 | |
| 3 months after burning | 6.93 b | 0.13 | 1.60 b | 0.01 | 1.31 a | 0.02 | 5.21 b | 0.11 | 3.02 b | 0.06 | 0.14 b | 0.01 | 22.16 a | 0.05 | 54.12 b | 0.05 | 23.72 b | 0.09 | |
| 6 months after burning | 6.97 b | 0.12 | 1.11 b | 0.02 | 1.32 a | 0.01 | 5.33 b | 0.09 | 3.09 b | 0.06 | 0.19 b | 0.02 | 22.38 a | 0.05 | 53.04 b | 0.05 | 24.58 a | 0.09 | |
| 9 months after burning | 7.02 b | 0.11 | 1.14 b | 0.01 | 1.39 a | 0.01 | 5.38 b | 0.06 | 3.12 b | 0.04 | 0.13 b | 0.01 | 22.83 a | 0.05 | 50.91 a | 0.04 | 26.26 a | 0.08 | |
| 12 months after burning | 6.37 b | 0.15 | 1.11 b | 0.01 | 1.42 a | 0.01 | 5.40 b | 0.02 | 3.13 b | 0.01 | 0.21 a | 0.02 | 27.06 a | 1.09 | 45.62 a | 0.75 | 27.32 a | 0.84 | |
| RSC-12Y | pre-burning | 5.31 a | 0.03 | 0.13 a | 0.01 | 1.28 a | 0.02 | 7.45 a | 0.21 | 4.32 a | 0.12 | 0.31 a | 0.02 | 35.19 a | 0.90 | 45.66 a | 1.14 | 19.15 a | 1.99 |
| 5 min after burning | 6.26 b | 0.03 | 0.56 b | 0.02 | 1.25 a | 0.01 | 6.14 b | 0.21 | 3.56 a | 0.12 | 0.21 b | 0.03 | 33.43 a | 2.77 | 51.29 b | 0.36 | 15.28 a | 3.00 | |
| 3 months after burning | 6.23 b | 0.02 | 0.67 b | 0.01 | 1.27 a | 0.02 | 5.48 b | 0.08 | 3.18 a | 0.05 | 0.23 b | 0.02 | 22.31 b | 0.16 | 54.19 b | 0.09 | 23.50 b | 0.18 | |
| 6 months after burning | 6.03 b | 0.07 | 1.03 b | 0.07 | 1.28 a | 0.02 | 6.03 b | 0.07 | 3.50 a | 0.04 | 0.24 b | 0.02 | 22.53 b | 0.16 | 53.10 b | 0.08 | 24.37 b | 0.18 | |
| 9 months after burning | 6.12 b | 0.03 | 1.20 b | 0.03 | 1.35 a | 0.02 | 6.22 b | 0.09 | 3.61 a | 0.05 | 0.16 b | 0.02 | 22.98 b | 0.17 | 50.98 b | 0.08 | 26.04 b | 0.19 | |
| 12 months after burning | 6.04 b | 0.09 | 1.20 b | 0.06 | 1.36 a | 0.02 | 6.28 b | 0.11 | 3.64 a | 0.06 | 0.20 b | 0.02 | 26.70 b | 0.99 | 50.64 b | 2.35 | 22.66 b | 1.53 | |
| Site | Time Point | CEC (cmol kg−1) | Avail. P (mg kg−1) | Exch. K (mg kg−1) | Exch. Ca (mg kg−1) | Exch. Mg (mg kg−1) | NH4-N (mg kg−1) | NO3-N (mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| RSC-6Y | pre-burning | 12.52 a | 0.00 | 2.49 a | 0.09 | 147.66 a | 3.38 | 455.21 a | 16.26 | 175.99 a | 4.40 | 7.11 a | 0.00 | 14.21 a | 0.00 |
| 5 min after burning | 13.99 a | 0.36 | 50.31 b | 1.92 | 398.92 b | 9.72 | 1024.87 b | 46.60 | 278.51 b | 9.27 | 68.68 b | 4.10 | 0.00 b | 0.00 | |
| 3 months after burning | 11.27 a | 0.00 | 30.75 b | 0.37 | 256.20 b | 7.70 | 1244.45 b | 70.04 | 265.38 b | 5.77 | 28.42 c | 0.00 | 14.21 a | 0.00 | |
| 6 months after burning | 10.39 a | 0.07 | 25.82 b | 0.31 | 215.20 b | 6.47 | 1045.33 b | 58.83 | 222.92 b | 4.84 | 35.53 c | 0.00 | 28.42 b | 0.00 | |
| 9 months after burning | 10.72 a | 0.37 | 16.52 c | 0.20 | 137.72 a | 4.14 | 669.01 a | 37.65 | 142.66 a | 3.10 | 48.35 c | 0.00 | 35.53 b | 0.00 | |
| 12 months after burning | 11.68 a | 0.16 | 11.01 c | 0.29 | 135.38 a | 23.61 | 634.73 a | 23.90 | 139.54 a | 10.51 | 35.53 c | 0.00 | 14.21 a | 0.00 | |
| RSC-12Y | pre-burning | 12.52 a | 0.00 | 2.62 a | 0.12 | 93.94 a | 1.02 | 174.54 a | 6.77 | 87.43 a | 0.60 | 35.53 a | 0.00 | 132.63 a | 4.10 |
| 5 min after burning | 10.65 a | 0.63 | 50.31 b | 6.74 | 334.23 b | 4.93 | 546.43 b | 11.81 | 165.03 b | 8.39 | 90.00 b | 4.10 | 7.11 b | 0.00 | |
| 3 months after burning | 14.40 a | 0.00 | 19.79 c | 0.91 | 248.57 b | 2.45 | 311.13 b | 11.51 | 174.38 b | 10.82 | 78.16 c | 0.00 | 35.53 c | 0.00 | |
| 6 months after burning | 11.26 a | 0.33 | 16.62 c | 0.76 | 208.80 b | 2.06 | 261.34 b | 9.67 | 146.47 b | 9.09 | 78.16 c | 0.00 | 59.21 d | 4.10 | |
| 9 months after burning | 11.92 a | 0.42 | 10.63 c | 0.49 | 133.63 a | 1.32 | 167.25 a | 6.18 | 93.73 a | 5.82 | 78.72 c | 0.15 | 60.89 d | 0.19 | |
| 12 months after burning | 12.30 a | 0.15 | 8.39 c | 0.27 | 106.27 a | 6.63 | 139.66 a | 1.40 | 83.16 a | 8.17 | 78.16 c | 0.00 | 35.53 c | 0.00 | |
| Factors | Alpha Diversity | Beta Diversity | |||
|---|---|---|---|---|---|
| Richness | Diversity Index | Bray–Curtis | |||
| Observed | ACE | Shannon | Simpson | ||
| Site | 0.44 | 0.54 | 0.13 | 0.41 | 3.10 * |
| Sampling time | 14.28 * | 14.03 * | 15.98 * | 7.96 * | 4.10 * |
| Site:Sampling time | 0.22 | 0.17 | 0.13 | 0.3 | 2.16 * |
| Soil Properties | RSC-6Y | RSC-12Y | ||
|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| pH | 0.184 | 0.054 | 0.257 | 0.008 * |
| SOM | 0.339 | 0.004 * | 0.302 | 0.009 * |
| SOC | 0.341 | 0.004 * | 0.300 | 0.010 * |
| ECe | 0.296 | 0.005 * | 0.404 | 0.001 * |
| CEC | 0.354 | 0.002 * | 0.298 | 0.003 * |
| NH4-N | 0.410 | 0.001 * | 0.304 | 0.001 * |
| NO3-N | 0.131 | 0.119 | 0.442 | 0.001 * |
| Avail. P | 0.424 | 0.001 * | 0.320 | 0.002 * |
| Exch. K | 0.340 | 0.001 * | 0.303 | 0.003 * |
| Exch. Ca | 0.235 | 0.013 * | 0.296 | 0.006 * |
| Exch. Mg | 0.183 | 0.030 * | 0.218 | 0.026 * |
| Total. N | 0.138 | 0.100 | 0.155 | 0.086 |
| BD | 0.237 | 0.012 * | 0.228 | 0.012 * |
| Sand | 0.301 | 0.011 * | 0.360 | 0.001 * |
| Silt | 0.218 | 0.019 * | 0.221 | 0.034 * |
| Clay | 0.333 | 0.004 * | 0.255 | 0.023 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunrat, N.; Sansupa, C.; Sereenonchai, S.; Hatano, R.; Lal, R. Fire-Induced Changes in Soil Properties and Bacterial Communities in Rotational Shifting Cultivation Fields in Northern Thailand. Biology 2024, 13, 383. https://doi.org/10.3390/biology13060383
Arunrat N, Sansupa C, Sereenonchai S, Hatano R, Lal R. Fire-Induced Changes in Soil Properties and Bacterial Communities in Rotational Shifting Cultivation Fields in Northern Thailand. Biology. 2024; 13(6):383. https://doi.org/10.3390/biology13060383
Chicago/Turabian StyleArunrat, Noppol, Chakriya Sansupa, Sukanya Sereenonchai, Ryusuke Hatano, and Rattan Lal. 2024. "Fire-Induced Changes in Soil Properties and Bacterial Communities in Rotational Shifting Cultivation Fields in Northern Thailand" Biology 13, no. 6: 383. https://doi.org/10.3390/biology13060383
APA StyleArunrat, N., Sansupa, C., Sereenonchai, S., Hatano, R., & Lal, R. (2024). Fire-Induced Changes in Soil Properties and Bacterial Communities in Rotational Shifting Cultivation Fields in Northern Thailand. Biology, 13(6), 383. https://doi.org/10.3390/biology13060383












