Thai Oakleaf Lettuce Phenocopies a Phytochrome B Mutant
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Light Sources
2.3. Early Photomorphogenesis Experiments
2.4. Shade Avoidance
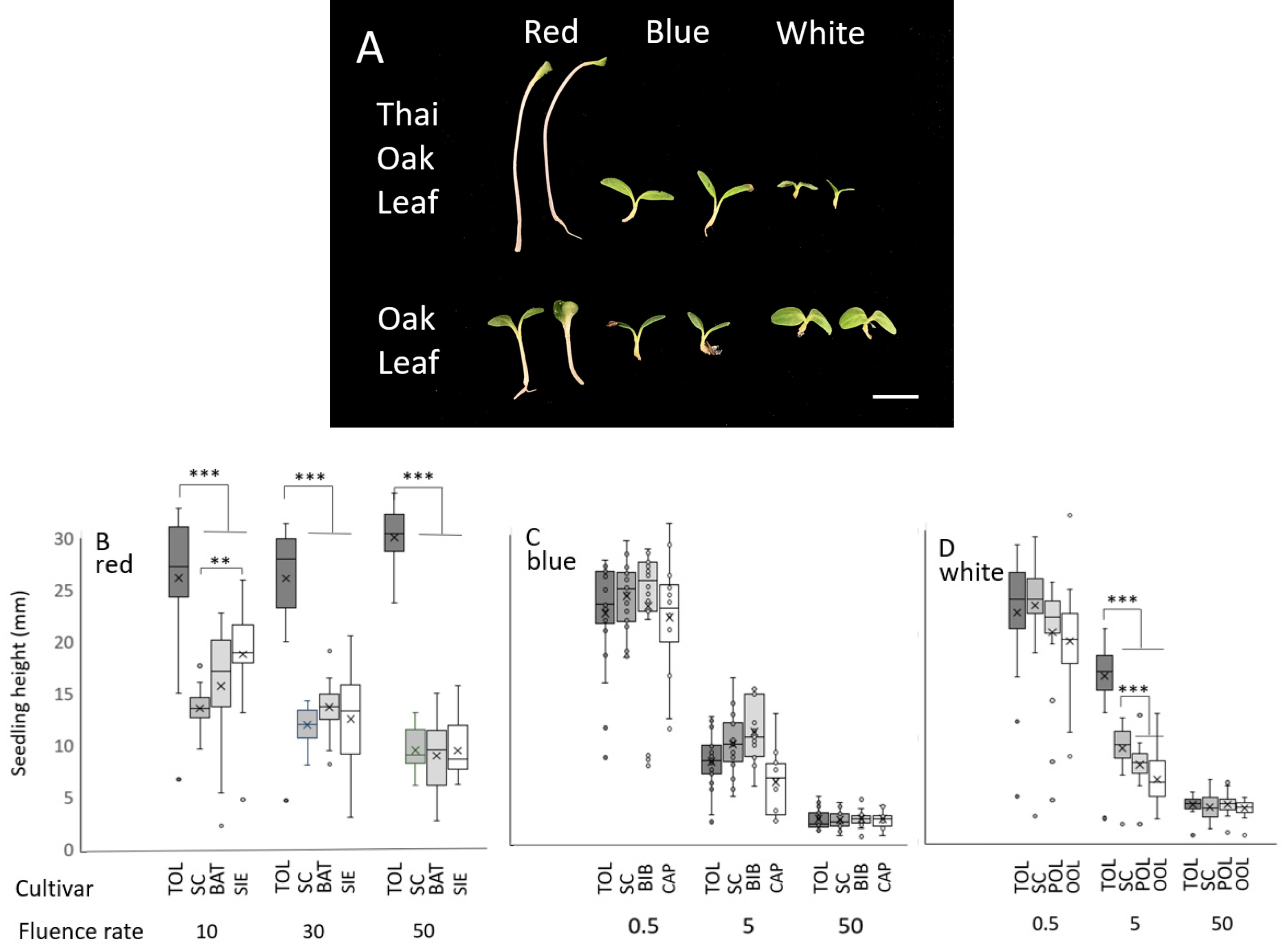
3. Results
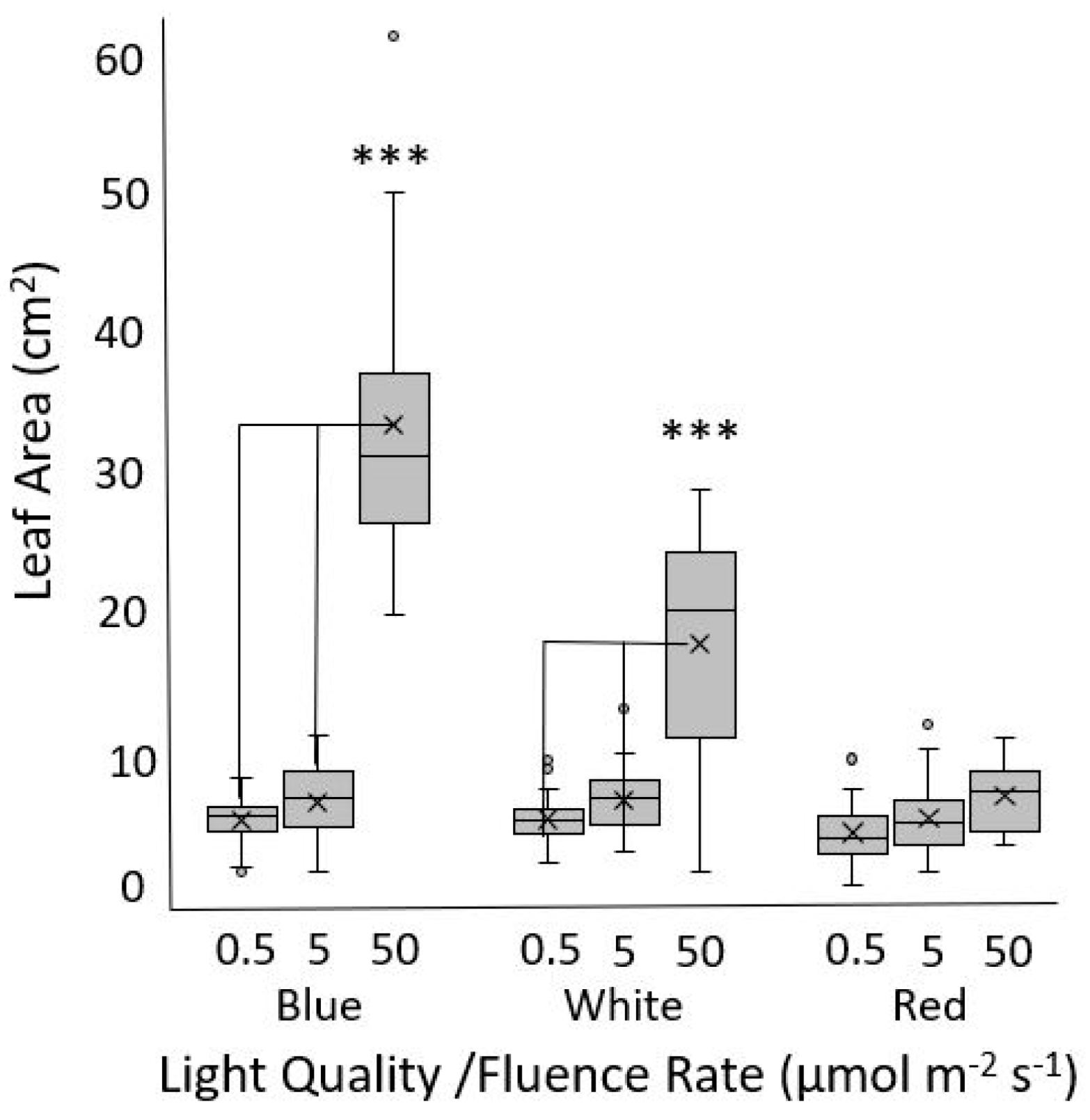
3.1. Fluence Rate/Response Experiments
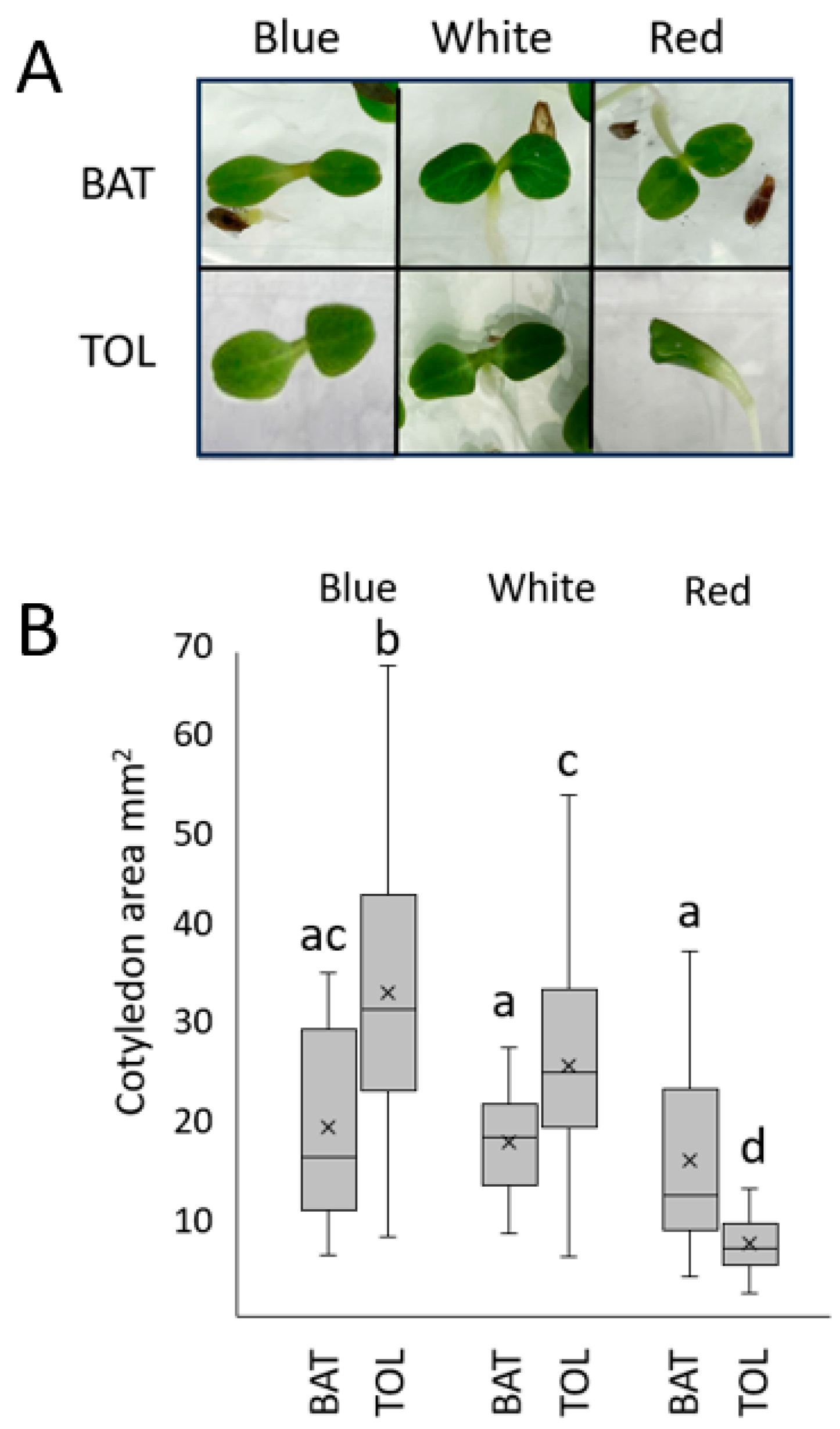
3.2. True Leaf Growth
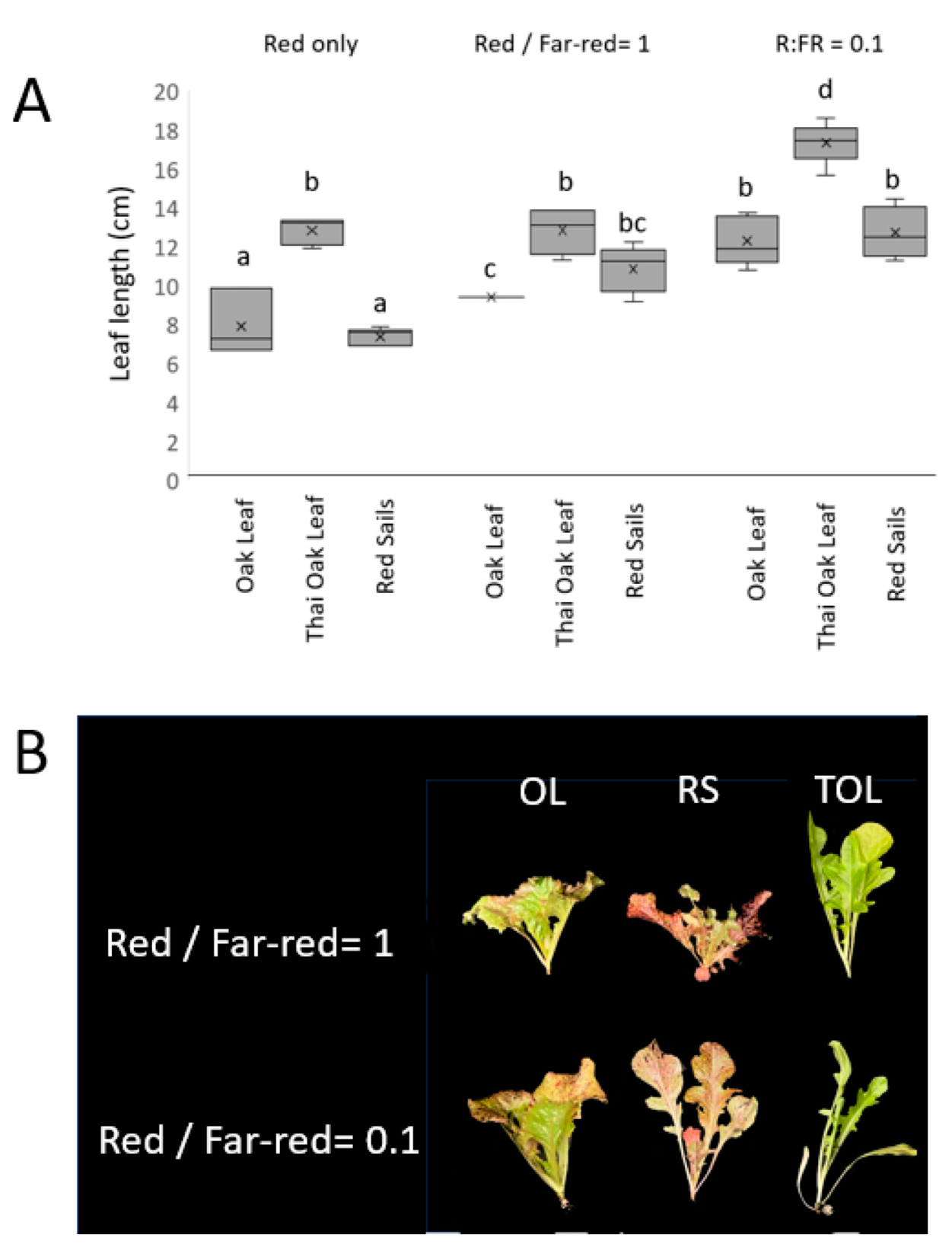
3.3. Responses to Variation in R/FR Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozai, T.; Niu, G. Chapter 2—Role of the plant factory with artificial lighting (pfal) in urban areas. In Plant Factory, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 7–34. [Google Scholar]
- Mawphlang, O.I.L.; Kharshiing, E.V. Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef]
- Folta, K.M. Breeding new varieties for controlled environments. Plant Biol. 2019, 21, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H. Phytochrome photosensory signalling networks. Nat. Rev. Mol. Cell Biol. 2002, 3, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, C. Blue light receptors and signal transduction. Plant Cell 2002, 14 (Suppl. S1), S207–S225. [Google Scholar] [CrossRef]
- Briggs, W.R.; Christie, J.M. Phototropins 1 and 2: Versatile plant blue-light receptors. Trends. Plant Sci. 2002, 7, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, L.; Favory, J.-J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of uv-b by the arabidopsis uvr8 protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Folta, K.M.; Spalding, E.P. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001, 26, 471–478. [Google Scholar] [CrossRef]
- Pierik, R.; Whitelam, G.C.; Voesenek, L.A.; de Kroon, H.; Visser, E.J. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J. 2004, 38, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Botto, J.F.; Alonso-Blanco, C.; Garzarón, I.; Sánchez, R.A.; Casal, J.J. The cape verde islands allele of cryptochrome 2 enhances cotyledon unfolding in the absence of blue light in arabidopsis. Plant Physiol. 2003, 133, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- El-Assal, S.E.D.; Alonso-Blanco, C.; Hanhart, C.J.; Koornneef, M. Pleiotropic effects of the arabidopsis cryptochrome 2 allelic variation underlie fruit trait-related qtl. Plant Biol. 2004, 6, 370–374. [Google Scholar] [CrossRef]
- Sanchez-Bermejo, E.; Zhu, W.; Tasset, C.; Eimer, H.; Sureshkumar, S.; Singh, R.; Sundaramoorthi, V.; Colling, L.; Balasubramanian, S. Genetic architecture of natural variation in thermal responses of arabidopsis. Plant Physiol. 2015, 169, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Sawers, R.J.H.; Wang, H.; Kim, J.-K.; Walker, J.M.; Brutnell, T.P.; Parthasarathy, M.V.; Vierstra, R.D.; Wu, R.J. Light-regulated overexpression of an arabidopsis phytochrome a gene in rice alters plant architecture and increases grain yield. Planta 2006, 223, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of constans protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Seaton, D.D.; Krahmer, J.; Halliday, K.J. Photoreceptor effects on plant biomass, resource allocation, and metabolic state. Proc. Natl. Acad. Sci. USA 2016, 113, 7667–7672. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.W.; Nagpal, P.; Poole, D.S.; Furuya, M.; Chory, J. Mutations in the gene for the red/far-red light receptor phytochrome b alter cell elongation and physiological responses throughout arabidopsis development. Plant Cell 1993, 5, 147–157. [Google Scholar]
- Neff, M.M.; Chory, J. Genetic interactions between phytochrome a, phytochrome b, and cryptochrome 1 during arabidopsis development. Plant Physiol. 1998, 118, 27–35. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (leds). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Devlin, P.F.; Yanovsky, M.J.; Kay, S.A. A genomic analysis of the shade avoidance response in arabidopsis. Plant Physiol. 2003, 133, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes b, d, and e act redundantly to control multiple physiological responses in arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Parks, B.M.; Quail, P.H. Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell 1991, 3, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.J.; Bhoo, S.H.; Durski, A.M.; Walker, J.M.; Vierstra, R.D. The heme-oxygenase family required for phytochrome chromophore biosynthesis is necessary for proper photomorphogenesis in higher plants. Plant Physiol. 2001, 126, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.-Y.; Zhao, X.-G.; Chen, P.-Y.; Li, Y.-L.; Wen, X.-Z. Cloning of alternative splice variants of lsphyb in lettuce and its expression patterns under heat stress. Sci. Agric. Sin. 2022, 55, 1822–1830. [Google Scholar]
- Southern Exposure Seed Exchange. Thai Oakleaf Looseleaf Lettuce; Southern Exposure Seed Exchange: Mineral, VA, USA, 2020. [Google Scholar]
- Jung, J.-H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors in arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.R.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome b integrates light and temperature signals in arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, C.; Folta, K.M. Thai Oakleaf Lettuce Phenocopies a Phytochrome B Mutant. Biology 2024, 13, 390. https://doi.org/10.3390/biology13060390
Cooper C, Folta KM. Thai Oakleaf Lettuce Phenocopies a Phytochrome B Mutant. Biology. 2024; 13(6):390. https://doi.org/10.3390/biology13060390
Chicago/Turabian StyleCooper, Cade, and Kevin M. Folta. 2024. "Thai Oakleaf Lettuce Phenocopies a Phytochrome B Mutant" Biology 13, no. 6: 390. https://doi.org/10.3390/biology13060390
APA StyleCooper, C., & Folta, K. M. (2024). Thai Oakleaf Lettuce Phenocopies a Phytochrome B Mutant. Biology, 13(6), 390. https://doi.org/10.3390/biology13060390







