A New Source and Large Quantity of Resveratrol in Cratoxylum Species and Their Activities on Normal Human and Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phytochemical Extraction
2.3. Phytochemical Preparation for Phytochemical Composition and Identification Analysis
2.4. Phytochemical Composition Analysis Using Gas Chromatography–Mass Spectrometry (GC-MS)
2.5. α-Amyrin Detection by Gas Chromatography–Frame Ionization Detector (GC-FID)
2.6. Resveratrol Investigation Using Reverse-Phase-High Performance Liquid Chromatography (HPLC)
2.7. Phytochemical Preparation for Toxicity Evaluation
2.8. Melanoma Cell (CHL-1), Colon Cancer Cell (HCT-116) and Hepatoblastoma Cell (HepG2) Preparation
2.9. Human Peripheral Blood Mononuclear Cells (PBMCs) Isolation
2.10. Cytotoxicity Testing via (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Tetrazolium Reduction (MTT) Assay
2.11. Genotoxicity Testing via Comet Assay
2.12. Programmed Cell Death Evaluation by Flowcytometry Assay
2.13. Cell Cycle Arrest by Flowcytometry Assay
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Profiles Contained in the Three Cratoxylum Leaf Extracts
3.2. Quantitative Analysis of α-Amyrin and Resveratrol in the Cratoxylum Leaf Extracts
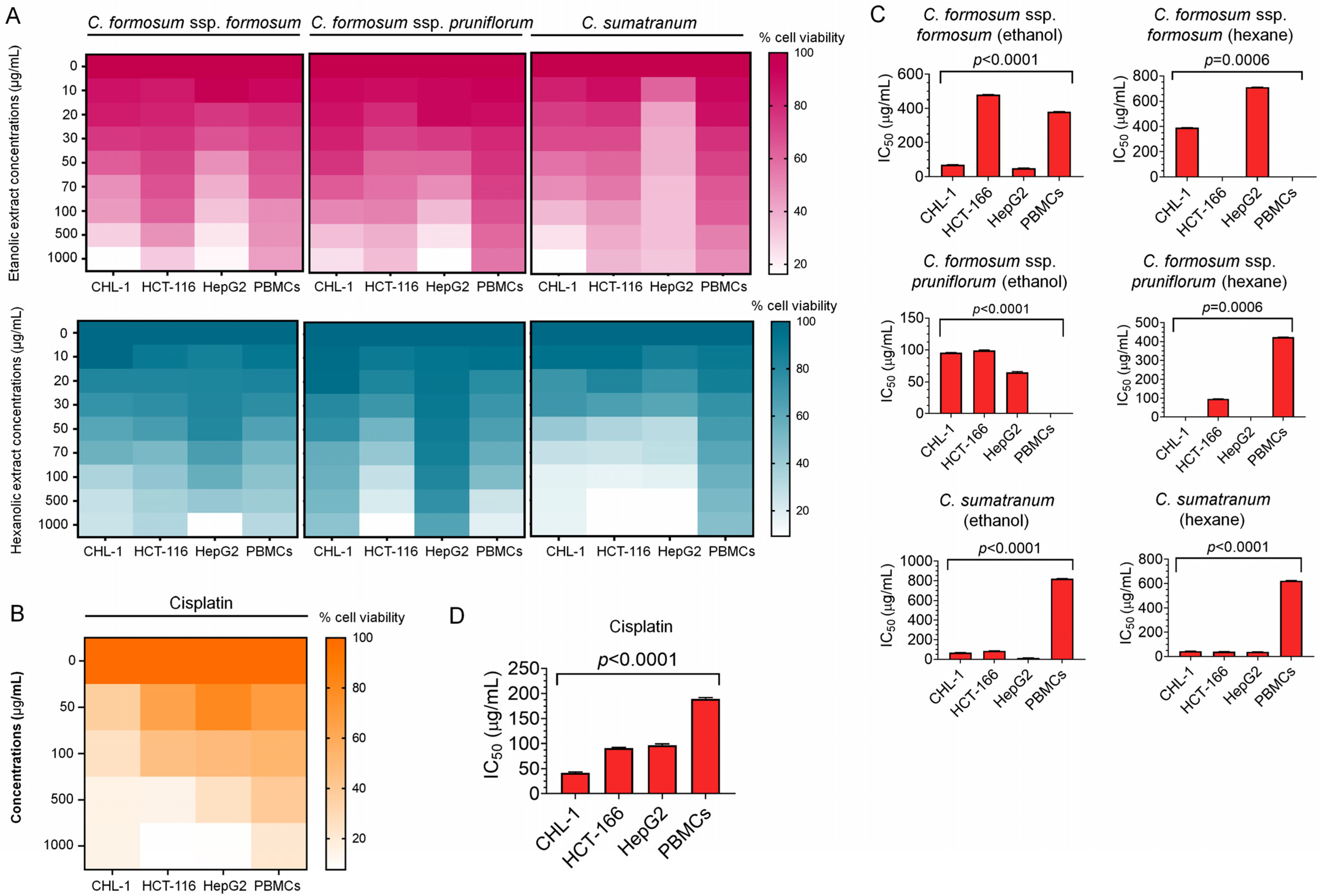
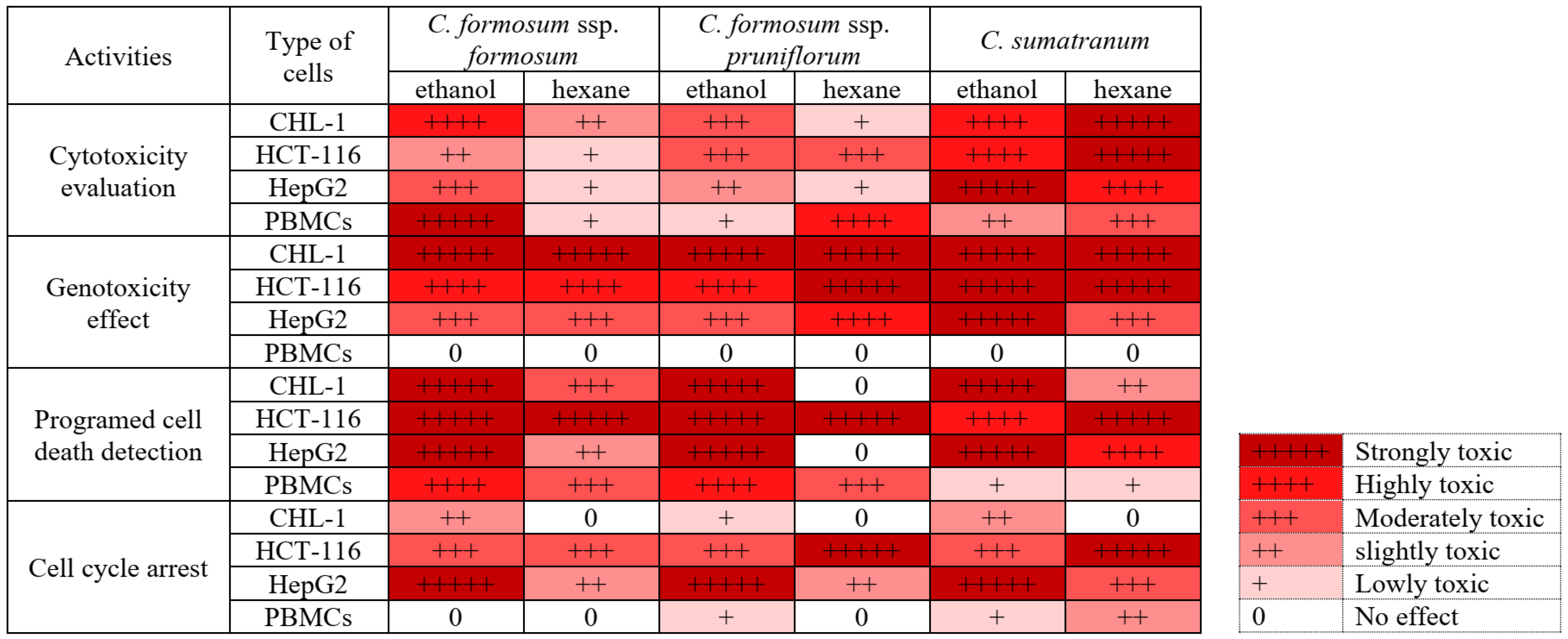
3.3. Cytotoxicity Evaluation of the Studied Cratoxylum Samples on the Cancer Cells and PBMCs
3.4. Genotoxicity Effect of the Studied Cratoxylum Samples on the Studied Cells
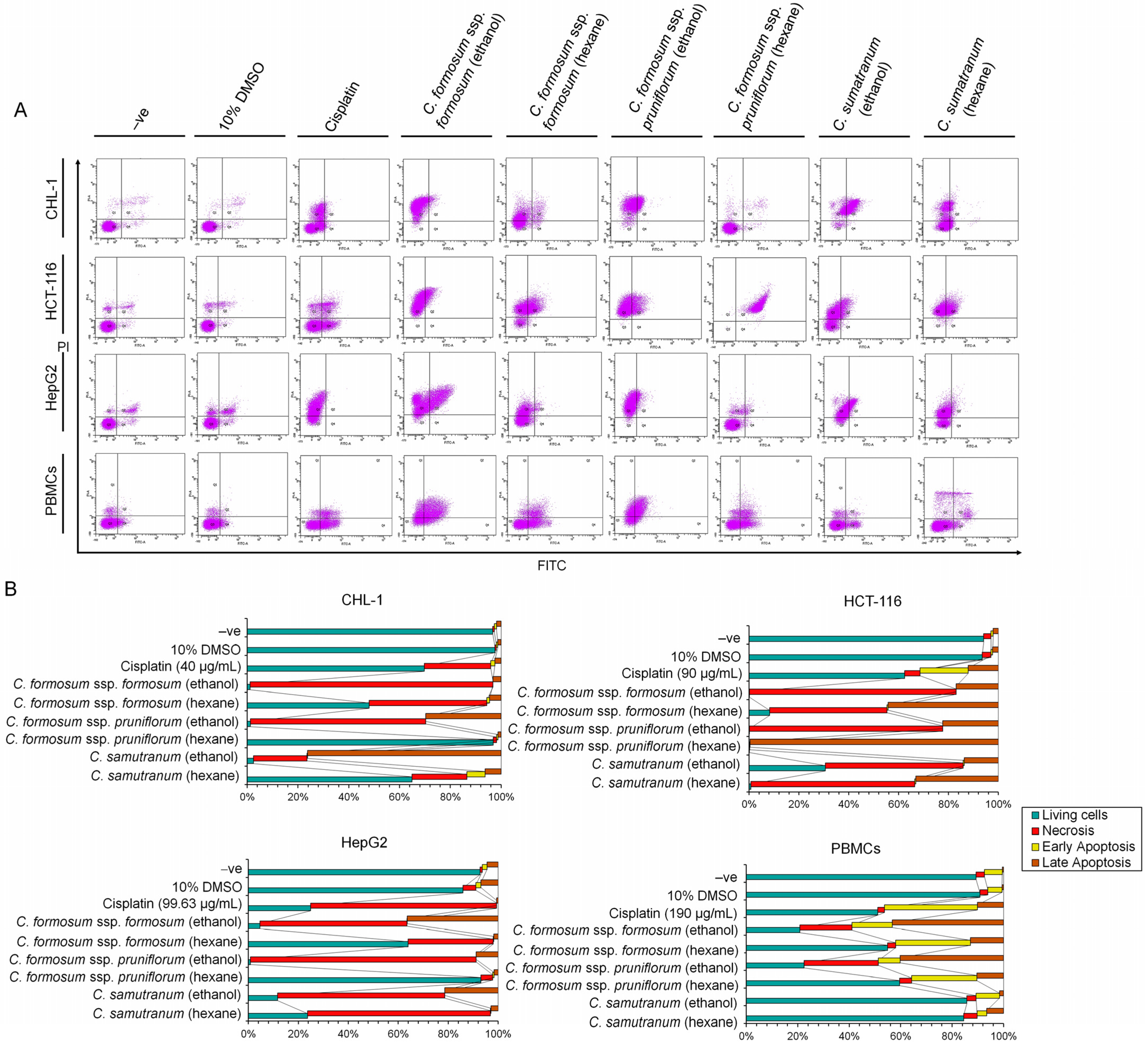
3.5. Programed Cell Death Detection in Cancer Cells and Normal Immune Cells (PBMCs) for Efficacy Testing at the IC50 or the Highest Concentration Value of the Cratoxylum Samples
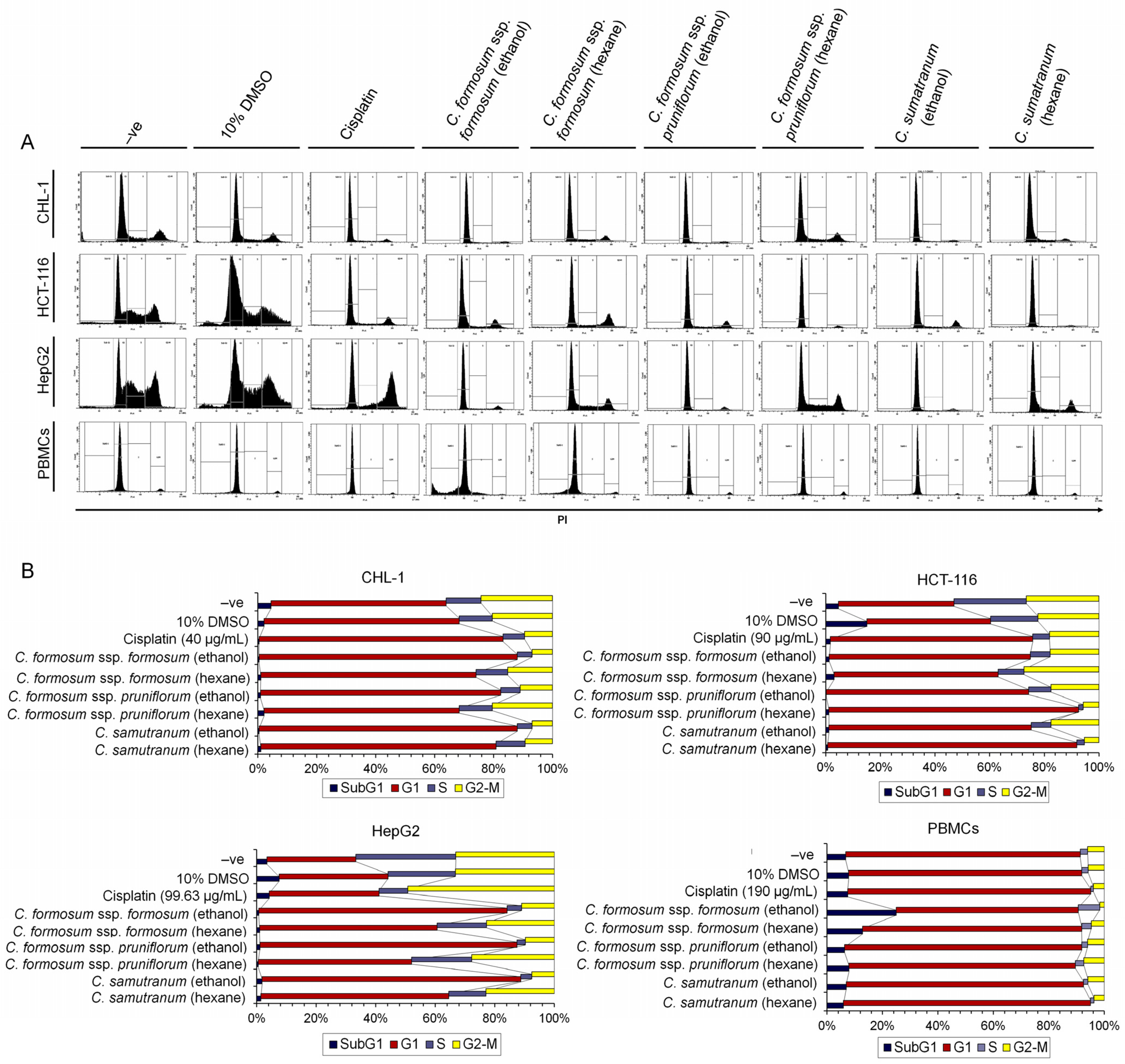
3.6. Cratoxylum Sample Activity Testing on Cell Cycle Distribution Using IC50 or the Highest Concentration Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smitinand, T. Thai Plants Names, revised ed.; Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 2014; Available online: https://botany.dnp.go.th/mplant/search.html?group=genus (accessed on 16 November 2023).
- Sudmoon, R.; Kaewdaungdee, S.; Tanee, T.; Siripiyasing, P.; Ameamsri, U.; Syazwan, S.A.; Lee, S.Y.; Chaveerach, A. Characterization of the plastid genome of Cratoxylum species (Hypericaceae) and new insights into phylogenetic relationships. Sci. Rep. 2022, 12, 18810. [Google Scholar] [CrossRef] [PubMed]
- Juanda, D.; Fidrianny, I.; Ruslan, K.; Insanu, M. Overview of phytochemical compounds and pharmacology activities of Cratoxylum genus. Rasayan J. Chem. 2019, 12, 2065–2073. [Google Scholar] [CrossRef]
- Ruan, J.; Zheng, C.; Liu, Y.; Qu, L.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Chemical and biological research on herbal medicines rich in xanthones. Molecules 2017, 22, 1698. [Google Scholar] [CrossRef] [PubMed]
- Chailap, B.; Nuanyai, T.; Puthong, S.; Buakeaw, A. Chemical constituents of fruits and leaves of Cratoxylum cochinchinense and their cytotoxic activities. Naresuan Univ. J. Sci. Technol. 2017, 25, 22–30. [Google Scholar]
- Thu, Z.M.; Aung, H.T.; Seinb, M.M.; Maggiolinic, M.; Lappanoc, R.; Vidari, G. Highly cytotoxic xanthones from Cratoxylum cochinchinense collected in Myanmar. Nat. Prod. Commun. 2017, 12, 1759–1762. [Google Scholar] [CrossRef]
- Mardhiyyah, S.; Zakiyah, M.; Renata, E.D.; Tjahjandarie, T.S.; Supratman, U.; Retnowati, R.; Saputri, R.D.; Tanjung, M. Cytotoxic activity of xanthones from the stem bark of Cratoxylum sumatranum. Trop. J. Nat. Prod. Res. 2023, 7, 3908–3910. [Google Scholar]
- Bok, C.Y.; Low, E.K.J.; Augundhooa, D.; Ariffin, H.; Mok, Y.B.; Lim, K.Q.; Chew, S.L.; Salvamani, S.; Loh, K.E.; Loke, C.F.; et al. Comprehensive review of Cratoxylum genus: Ethnomedical uses, phytochemistry, and pharmacological properties. Pertanika J. Trop. Agric. Sci. 2023, 46, 213–241. [Google Scholar] [CrossRef]
- Huong, N.T.; Hop, N.Q.; Son, N.T. The genus Cratoxylum: Traditional use, phytochemistry and pharmacology. J. Pharm. Pharmacol. 2023, 75, 1259–1293. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.C.; Xu, X.-F.; Yan, H.; Zhang, Y. Xanthone derivatives from Cratoxylum formosum (Jack) Dyer subsp. pruniflorum (Kurz) Gogelin and their chemotaxonomic significance. Biochem. Syst. Ecol. 2024, 112, 104758. [Google Scholar] [CrossRef]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC-MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 16438. [Google Scholar] [CrossRef]
- Arimilli, S.; Damratoski, B.E.; Prasad, G.L. Methods to evaluate cytotoxicity and immunosuppression of combustible tobacco product preparations. J. Vis. Exp. 2015, 95, e52351. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Louis, K.S.; Siegel, A.C. Cell Viability Analysis Using Trypan Blue: Manual and Automated Methods. In Mammalian Cell Viability. Methods in Molecular Biology; Stoddart, M., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 7–12. [Google Scholar] [CrossRef]
- Sudmoon, R.; Kaewdaungdee, S.; Ameamsri, U.; Tanee, T.; Siripiyasing, P.; Wonok, W.; Chaveerach, A. Investigation of Morinda citrifolia activities through pinoresinol and α-EG related gene expression. Plants 2022, 11, 1985. [Google Scholar] [CrossRef]
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. 2009. Available online: http://www.who.int/ipcs/publications/pesticideshazard2009.pdf/ (accessed on 12 February 2023).
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin v/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar]
- Zhang, S.; Qi, J.; Li, X.; Wang, H.-L.; Britt, J.P.; Hoffman, A.F.; Bonci, A.; Lupica, C.R.; Morales, M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci. 2015, 18, 386–392. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Li, Y.; Li, C.; Liu, T.; Xine, Y.; Li, Y.; Zhang, D. Ginkgolic acid inhibits proliferation and migration of human hepatocellular carcinoma cells by inducing G0/G1 cell cycle arrest. Sci. Asia 2021, 47, 11–18. [Google Scholar] [CrossRef]
- Yu, Y.; Chang, P.; Yu, H.; Ren, H.; Hong, D.; Li, Z.; Wang, Y.; Song, H.; Huo, Y.; Li, C. Productive amyrin synthases for efficient α-amyrin synthesis in engineered Saccharomyces cerevisiae. SCS Synth. Biol. 2018, 7, 2391–2402. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.V.; Meyre-Silva, C.; Malheiros, A.; Muller, L.A.; Cani, G.S.; Santos, A.R.S.; Yunes, R.A.; Calixto, J.B. Antinociceptive properties of mixture of alpha-amyrin and beta-amyrin triterpenes: Evidence for participation of protein kinase C and protein kinase A pathways. J. Pharmacol. Exp. Ther. 2005, 313, 310–318. [Google Scholar] [CrossRef]
- Cardoso, B.K.; de Oliveira, H.L.M.; Melo, U.Z.; Fernandez, C.M.M.; Franco de Araújo Almeida Campo, C.; Gonçalves, J.E.; Laverde, A., Jr.; Romagnolo, M.B.; Linde, G.A.; Gazim, Z.C. Antioxidant activity of α and β-amyrin isolated from Myrcianthes pungens leaves. Nat. Prod. Res. 2018, 32, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Sangsawang, J.; Amroong, P.; Pratum, C.; Puakpong, S. Concentration of α-amyrin and antibacterial activity against Propionibacterium acnes of ethanolic extracts from Siam weed (Chromolaena odorata L.) leaves. UDRU Sci. Tech. J. 2022, 10, 91–105. [Google Scholar]
- Singh, C.K.; Liu, X.; Ahmad, M. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef]
- Hasan, M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Paula-Freire, L.I.G.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L.A. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine 2014, 21, 356–362. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; de Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K.; et al. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, L.; Xu, Z.; Xiong, H.; Zhou, D.; Huo, M. Contrasting roles of phenol and pyrocatechol on the degradation of 4-chlorophenol in a photocatalytic–biological reactor. Environ. Sci. Pollut. Res. 2017, 24, 24725–24731. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Sudmoon, R.; Kaewdaungdee, S.; Ameamsri, U.; Wonok, W.; Tanee, T.; Siripiyasing, P.; Chaveerach, A. High inhibition efficacy of pancreatic cholesterol esterase and porcine pancreatic lipase from natural products. Asian J. Agric. Biol. 2023, 2023041. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.; Almeida, A.A.C.; Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2023, 949452. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ayatollahi, S.A.; Zihad, S.M.N.K.; Sifat, N.; Khan, R.; Paul, A.; Salehi, B.; Islam, T.; Mubarak, M.S.; Martins, N.; et al. Phytol anti-inflammatory activity: Pre-clinical assessment and possible mechanism of action elucidation. Cell Mol. Biol. (Noisy-Le-Grand) 2020, 66, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Heimfarth, A.M.S.C.; Pereira, L.E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antoniolli, A.R.; Quintans, J.S.S.; Quintans-Júnior, L.J. Phytol, a chlorophyll component, produces antihyperalgesic, anti-inflammatory, and antiarthritic effects: Possible NF-κB pathway involvement and reduced levels of the proinflammatory cytokines TNF α and IL 6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Arrozi, A.P.; Shukri, S.N.S.; Ngah, W.Z.W.; Yusof, Y.A.M.; Damanhuri, M.H.A.; Jaafar, F.; Makpol, S. Comparative effects of alpha- and gamma-tocopherol on mitochondrial functions in Alzheimer’s disease in vitro model. Sci. Rep. 2020, 10, 8962. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; Omari, N.E.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Sirikhansaeng, P.; Tanee, T.; Sudmoon, R.; Chaveerach, A. Major phytochemical as γ-sitosterol disclosing and toxicity testing in Lagerstroemia species. Evid. Based Complement. Alternat. Med. 2017, 2017, 7209851. [Google Scholar] [CrossRef]
- Guo, F.; Tang, X.; Zhang, W.; Wei, J.; Tang, S.; Wu, H.; Yang, H. Exploration of the mechanism of traditional Chinese medicine by AI approach using unsupervised machine learning for cellular functional similarity of compounds in heterogeneous networks, XiaoErFuPi granules as an example. Pharmacol. Res. 2020, 160, 105077. [Google Scholar] [CrossRef] [PubMed]
- Laphookhieo, S.; Maneerat, W.; Koysomboon, S. Antimalarial and cytotoxic phenolic compounds from Cratoxylum maingayi and Cratoxylum cochinchinense. Molecules 2009, 14, 1389–1395. [Google Scholar] [CrossRef]
- Sudmoon, R.; Kaewdaungdee, S.; Tanee, T.; Siripiyasing, P.; Ang, A.; Lee, L.T.; Wong, X.J.; Lee, S.Y.; Chaveerach, A. Biological activity of leaves of three Morinda species detected by stimulation and suppression of gene expression of collagen, elastin, melanin and other related genes. Asian J. Agric. Biol. 2023, 2023117. [Google Scholar] [CrossRef]


| Rt (min) | Phytochemical Name | Molecular Formula | % Relative Content | |||||
|---|---|---|---|---|---|---|---|---|
| C. formosum ssp. formosum | C. formosum ssp. pruniflorum | C. sumatranum | ||||||
| Ethanol | Hexane | Ethanol | Hexane | Ethanol | Hexane | |||
| 4.28 | Catechol | C6H6O2 | - | - | 12.57 | - | 3.86 | - |
| 4.63 | 5-Hydroxymethylfurfural | C6H6O3 | - | - | 2.2 | - | 0.38 | - |
| 6.73 | Tetracosane | C24H50 | - | 12.9 | - | - | - | - |
| 7.18 | Caryophyllene | C15H24 | 17.57 | 0.9 | 3.68 | 1.83 | 0.66 | 0.93 |
| 7.29 | α-Bergamotene | C15H24 | - | - | - | - | - | 0.05 |
| 7.42 | Geranyl acetone | C13H22O | - | - | - | - | - | 0.06 |
| 7.60 | Humulene | C15H24 | 0.6 | - | 1.16 | 0.56 | 0.31 | 0.42 |
| 7.66 | α-Farnesene | C15H24 | 1.12 | - | - | - | - | - |
| 7.85 | α-Cubebene | C15H24 | 1.41 | - | - | - | - | - |
| 7.99 | β-Selinene | C15H24 | 4.38 | - | - | - | - | - |
| 8.22 | α-Curcumene | C15H22 | 1.19 | - | - | - | - | 0.05 |
| 8.73 | Benzoic acid, 4-ethoxy-, ethyl ester | C11H14O3 | 1.09 | 0.66 | - | - | - | - |
| 8.79 | trans-Nerolidol | C15H26O | - | - | - | - | - | 0.07 |
| 9.17 | Hexadecane | C16H34 | - | - | 1.36 | - | 0.16 | - |
| 9.18 | Caryophyllene oxide | C15H24O | 2.17 | 0.41 | - | 0.43 | - | - |
| 10.98 | Tetradecanoic acid | C14H28O2 | - | - | - | - | 0.1 | - |
| 11.40 | Octadecane | C18H38 | - | - | 0.76 | - | 0.11 | - |
| 11.91 | Phytol, acetate | C22H42O2 | 1.19 | - | 6.13 | 0.36 | 0.91 | 0.49 |
| 12.35 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 0.51 | - | 3.65 | - | 0.51 | 0.22 |
| 12.60 | 4,4,8-Trimethyltricyclo [6.3.1.0(1,5)]dodecane-2,9-diol | C15H26O2 | - | - | 0.98 | - | - | - |
| 13.04 | Farnesyl acetone | C18H30O | - | - | - | - | 0.36 | 0.3 |
| 13.75 | n-Hexadecanoic acid | C16H32O2 | 0.55 | - | 4.51 | - | 1.4 | 0.15 |
| 14.24 | Hexadecanoic acid, ethyl ester | C18H36O2 | - | - | 0.65 | - | 0.11 | - |
| 14.83 | β-Elemene | C15H24 | 0.83 | - | - | - | - | - |
| 16.42 | Phytol | C20H40O | 2.53 | 3.44 | 0.27 | 0.17 | 0.21 | |
| 17.00 | Linolenic acid | C18H30O2 | - | - | - | - | 0.49 | - |
| 17.34 | Octadecanoic acid | C18H36O2 | - | - | - | - | 0.09 | - |
| 17.43 | Linolenic acid, ethyl ester | C20H34O2 | - | - | - | - | 0.09 | - |
| 18.21 | Trolox | C14H18O4 | - | - | 2.2 | - | - | |
| 23.17 | Glycerol β-palmitate | C19H38O4 | - | - | 0.56 | - | 0.08 | - |
| 25.25 | 5a,9,9-Trimethyloctahydro-2H,4H-cyclopropa[e][3] benzoxepine-2,4-dione | C14H20O3 | - | - | - | - | 2.07 | 2.27 |
| 26.09 | Heptacosane | C27H56 | - | 7.87 | - | 1.24 | ||
| 27.95 | Squalene | C30H50 | 0.82 | - | 0.68 | 0.35 | 0.72 | 0.78 |
| 28.92 | Nonacosane | C29H60 | - | 3.45 | - | 15.5 | 0.22 | |
| 31.04 | ᵞ-Tocopherol | C28H48O2 | - | 6.06 | - | - | 0.24 | 0.23 |
| 31.75 | Hentriacontane | C31H64 | - | 1.01 | - | 12.54 | - | 0.84 |
| 31.98 | Cycloartenol acetate | C32H52O2 | - | - | - | - | 0.33 | - |
| 32.36 | dl-α-Tocopherol | C29H50O2 | 2.46 | - | - | - | 0.74 | 0.67 |
| 32.59 | 2,6-Bis(3,4-methylenedioxyphenyl)-3,7-dioxabicyclo(3.3.0)octane | C20H18O6 | - | - | - | - | 0.5 | - |
| 33.07 | Serratene | C30H50 | - | - | - | - | 1.9 | 2.01 |
| 34.44 | 1,30-Triacontanediol | C30H62O2 | - | - | - | 8.93 | - | - |
| 34.61 | Stigmasterol | C29H48O | - | - | 5.12 | 1.66 | 0.47 | 0.53 |
| 35.83 | ᵞ-Sitosterol | C29H50O | 2.18 | - | 8.33 | 5.98 | - | - |
| 36.67 | Lupeol | C30H50O | 49.79 | 21.28 | - | 2.52 | 28.58 | 30.88 |
| 36.86 | β-Amyrin | C30H50O | - | - | - | 5.48 | 4.52 | 4.71 |
| 37.16 | Lupenone | C30H48O | - | - | 6.99 | 8.94 | - | - |
| 38.20 | α-Amyrin | C30H50O | 8.29 | - | 33.33 | 33.42 | 48.49 | 51.92 |
| 40.04 | Bauerenol | C30H50O | - | - | - | - | 0.9 | 1.16 |
| 40.89 | Friedelanol | C30H52O | - | - | - | - | 0.34 | 0.54 |
| 41.62 | Friedelan-3-one | C30H50O | - | - | - | - | 0.27 | 0.29 |
| Extracted Solvents | C. formosum ssp. formosum | C. formosum ssp. pruniflorum | C. sumatranum | |||
|---|---|---|---|---|---|---|
| Concentration (mg/mL) | Quantity (mg/g-Dried Leaf) | Concentration (mg/mL) | Quantity (mg/g-Dried Leaf) | Concentration (mg/mL) | Quantity (mg/g Dried Leaf) | |
| α-Amyrin | ||||||
| Ethanol | 0.027 | 0.001 | 0.076 | 0.003 | 0.601 | 0.024 |
| Hexane | 0.046 | 0.002 | 0.075 | 0.003 | 0.955 | 0.038 |
| Resveratrol | ||||||
| 80% Ethanol | - | - | - | - | 0.640 | 0.064 |
| Experiments | % Cell Viability (mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| Cisplatin | C. formosum ssp. formosum | C. formosum ssp. pruniflorum | C. sumatranum | ||||
| Ethanol | Hexane | Ethanol | Hexane | Ethanol | Hexane | ||
| CHL-1 | 14.96 ± 0.01–37.18 ± 0.02 | 17.77 ± 0.03–88.51 ± 0.10 | 47.80 ± 0.06–99.77 ± 0.08 | 20.45 ± 0.07–90.91 ± 0.20 | 60.97 ± 0.08–97.83 ± 0.16 | 16.24 ± 0.04–83.50 ± 0.07 | 16.57 ± 0.01–94.36 ± 0.07 |
| IC50 (µg/mL) | 40 | 70 | 390 | 95 | >1000 | 70 | 43.75 |
| LD50 (µg/50Kg rat) | 416.84 | 513.31 | 972.47 | 575.06 | - | 513.31 | 430.97 |
| HCT-116 | 7.83 ± 0.02–64.31 ± 0.24 | 34.05 ± 0.09–85.56 ± 0.13 | 53.07 ± 0.07–92.83 ± 0.17 | 30.87 ± 0.11–89.05 ± 0.09 | 35.58 ± 0.11–92.32 ± 0.13 | 37.01 ± 0.21–89.23 ± 0.13 | 9.75 ± 0.00–93.93 ± 0.12 |
| IC50 (µg/mL) | 90 | 480 | >1000 | 98 | 96 | 87 | 40 |
| LD50 (µg/50Kg rat) | 563.61 | 1050.56 | - | 581.75 | 577.30 | 556.55 | 416.84 |
| HepG2 | 8.86 ± 0.01–80.10 ± 0.04 | 22.31 ± 0.05–97.89 ± 0.11 | 36.73 ± 0.13–89.56 ± 0.10 | 10.94 ± 0.04–91.75 ± 0.11 | 74.05 ± 0.06–94.87 ± 0.23 | 32.28 ± 0.05–61.040.05 | 9.83 ± 0.01–85.65 ± 0.08 |
| IC50 (µg/mL) | 99.63 | 50 | 710 | 63.50 | >1000 | 16 | 39 |
| LD50 (µg/50Kg rat) | 585.33 | 452.92 | 1215.26 | 495.03 | - | 296.44 | 412.93 |
| PBMCs | 22.16 ± 0.03–68.27 ± 0.11 | 45.92 ± 0.01–91.81 ± 0.01 | 52.26 ± 0.01–93.94 ± 0.01 | 53.27 ± 0.00–94.49 ± 0.00 | 41.79 ± 0.02–95.96 ± 0.02 | 48.70 ± 0.03–91.82 ± 0.02 | 46.71 ± 0.04–89.96 ± 0.10 |
| IC50 (µg/mL) | 190 | 380 | >1000 | >1000 | 423 | 820 | 620 |
| LD50 (µg/50Kg rat) | 744.21 | 963.12 | - | - | 1002.30 | 1282.15 | 1155.50 |
| Experiments | OTM Values (mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| -ve | 10% DMSO | Cisplatin | C. formosum ssp. formosum | C. formosum ssp. pruniflorum | C. sumatranum | ||||
| Ethanol | Hexane | Ethanol | Hexane | Ethanol | Hexane | ||||
| CHL-1 | 0.0414 ± 0.03 | 0.0399 ± 0.08 | 4.4950 ± 2.48 | 3.2875 ± 2.21 | 2.9309 ± 2.05 | 2.7148 ± 1.93 | 3.5335 ± 2.34 | 3.3423 ± 2.32 | 3.1684 ± 2.27 |
| p-values | - | 0.1929 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| HCT-116 | 0.0016 ± 0.00 | 0.0018 ± 0.01 | 2.7995 ± 1.96 | 2.1438 ± 1.25 | 2.2089 ± 1.23 | 2.8119 ± 1.88 | 3.5543 ± 2.13 | 4.2624 ± 2.32 | 4.2671 ± 2.71 |
| p-values | - | 0.6437 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| HepG2 | 0.4954 ± 0.25 | 0.4540 ± 0.28 | 4.1957 ± 2.42 | 3.1422 ± 2.14 | 2.7325 ± 1.85 | 3.1656 ± 2.12 | 3.9542 ± 2.42 | 5.2199 ± 2.53 | 2.6430 ± 1.87 |
| p-values | - | 0.1426 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| PBMCs | 0.5327 ± 0.26 | 0.5314 ± 0.29 | 1.5705 ± 0.32 | 0.5150 ± 0.21 | 0.5401 ± 0.27 | 0.5255 ± 0.25 | 0.6009 ± 0.25 | 0.5141 ± 0.25 | 0.5338 ± 0.26 |
| p-values | - | 0.9591 | <0.0001 | 0.7468 | 0.4350 | 0.7218 | 0.0247 | 0.4834 | 0.9819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewdaungdee, S.; Banlue, T.; Youngsanbhu, N.; Naeklang, M.; Lee, S.Y.; Ang, A.; Sudmoon, R.; Tanee, T.; Daduang, S.; Chaveerach, A. A New Source and Large Quantity of Resveratrol in Cratoxylum Species and Their Activities on Normal Human and Cancer Cells. Biology 2024, 13, 402. https://doi.org/10.3390/biology13060402
Kaewdaungdee S, Banlue T, Youngsanbhu N, Naeklang M, Lee SY, Ang A, Sudmoon R, Tanee T, Daduang S, Chaveerach A. A New Source and Large Quantity of Resveratrol in Cratoxylum Species and Their Activities on Normal Human and Cancer Cells. Biology. 2024; 13(6):402. https://doi.org/10.3390/biology13060402
Chicago/Turabian StyleKaewdaungdee, Sanit, Tankun Banlue, Napatsakon Youngsanbhu, Mallika Naeklang, Shiou Yih Lee, Arnold Ang, Runglawan Sudmoon, Tawatchai Tanee, Sakda Daduang, and Arunrat Chaveerach. 2024. "A New Source and Large Quantity of Resveratrol in Cratoxylum Species and Their Activities on Normal Human and Cancer Cells" Biology 13, no. 6: 402. https://doi.org/10.3390/biology13060402








