Endolithic Fungal Diversity in Antarctic Oligocene Rock Samples Explored Using DNA Metabarcoding
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Strategy and Processing
2.2. Stratigraphy
2.3. Geochemical Analysis
2.4. Petrographic Analyses
2.5. DNA Extraction, Illumina Library Construction and Sequencing
2.6. Data Analysis and Fungal Identification
2.7. Fungal Diversity
3. Results
3.1. Fungal Identification
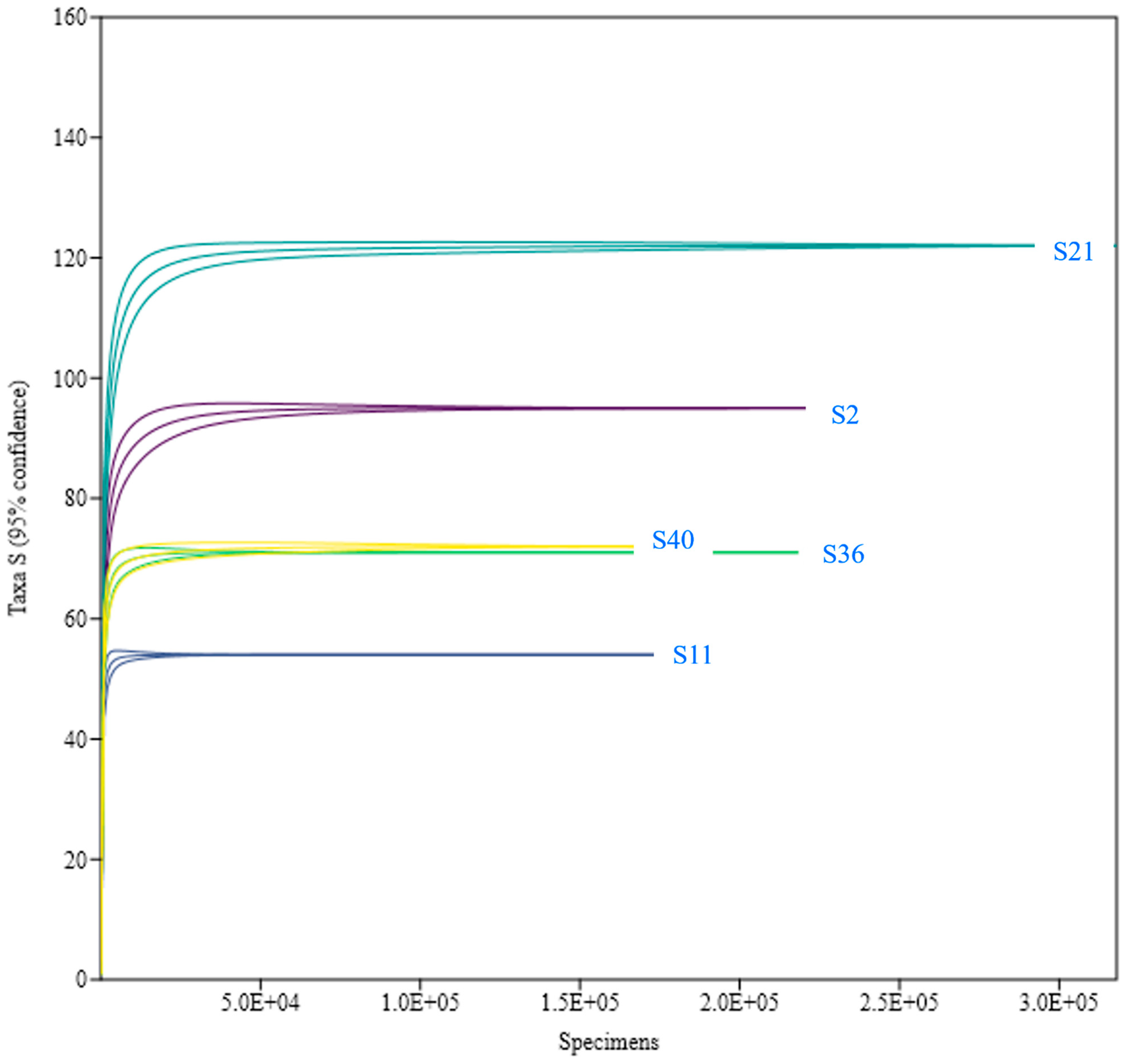
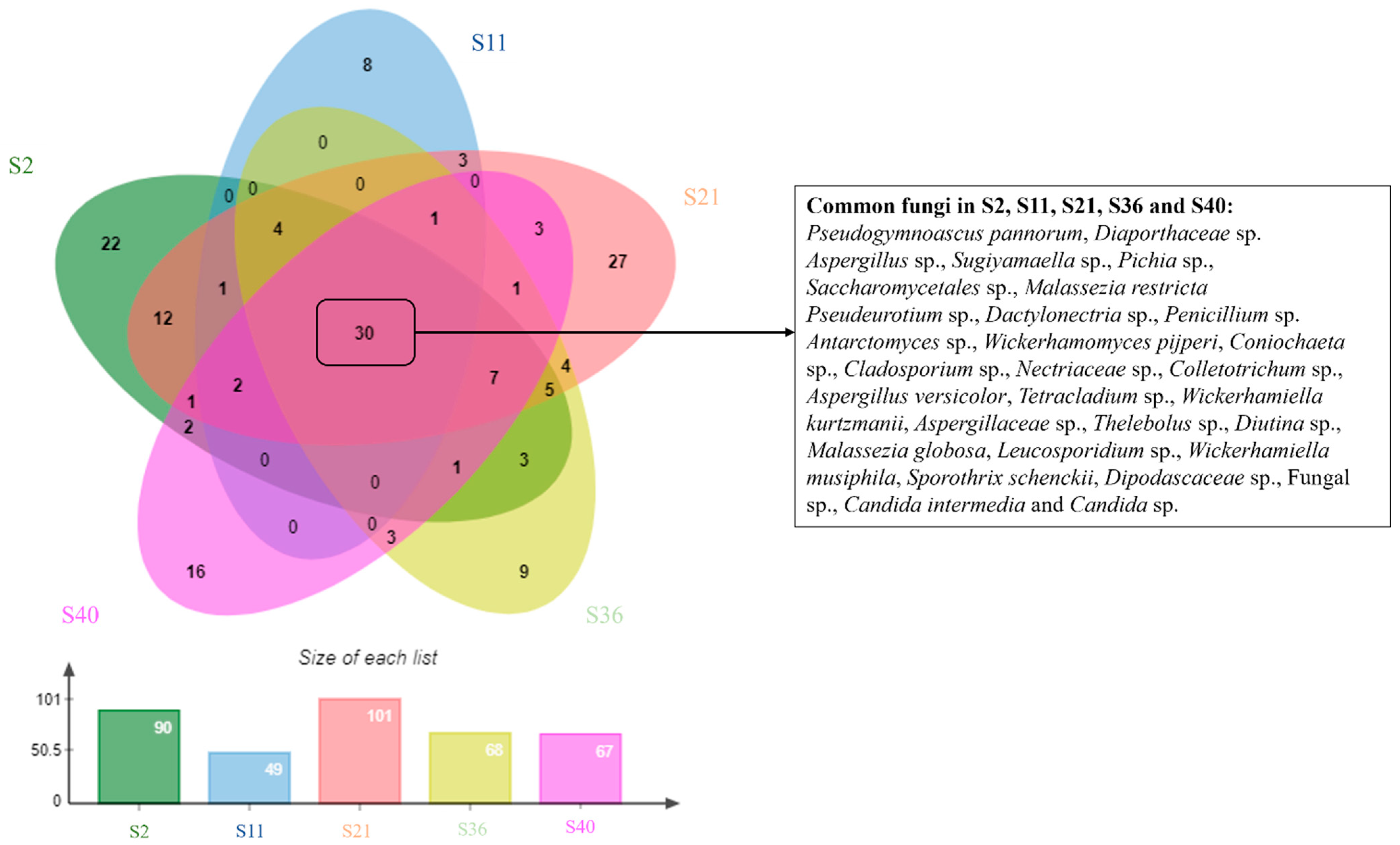
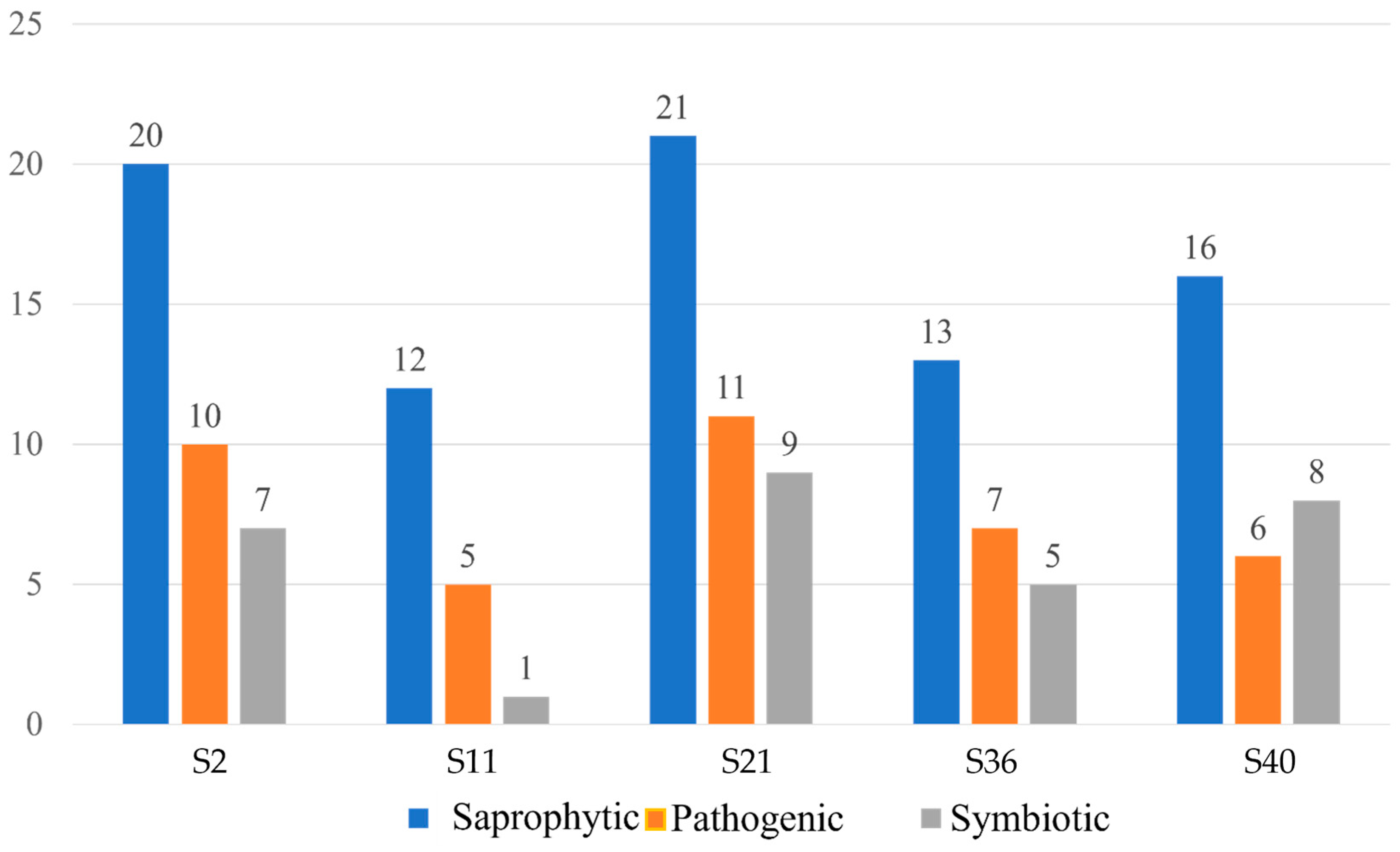
3.2. Fungal Diversity and Distribution
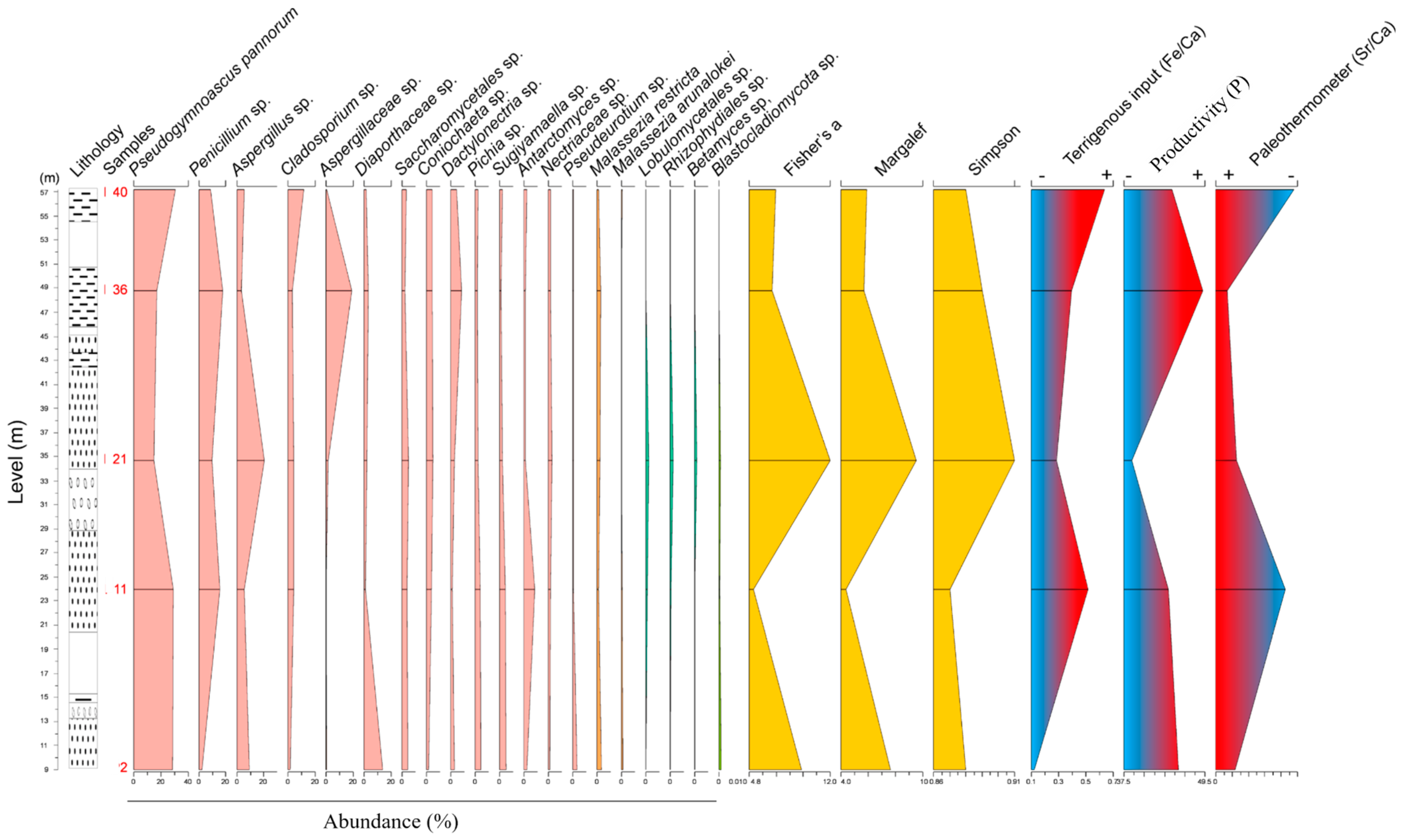
3.3. Geochemical Results
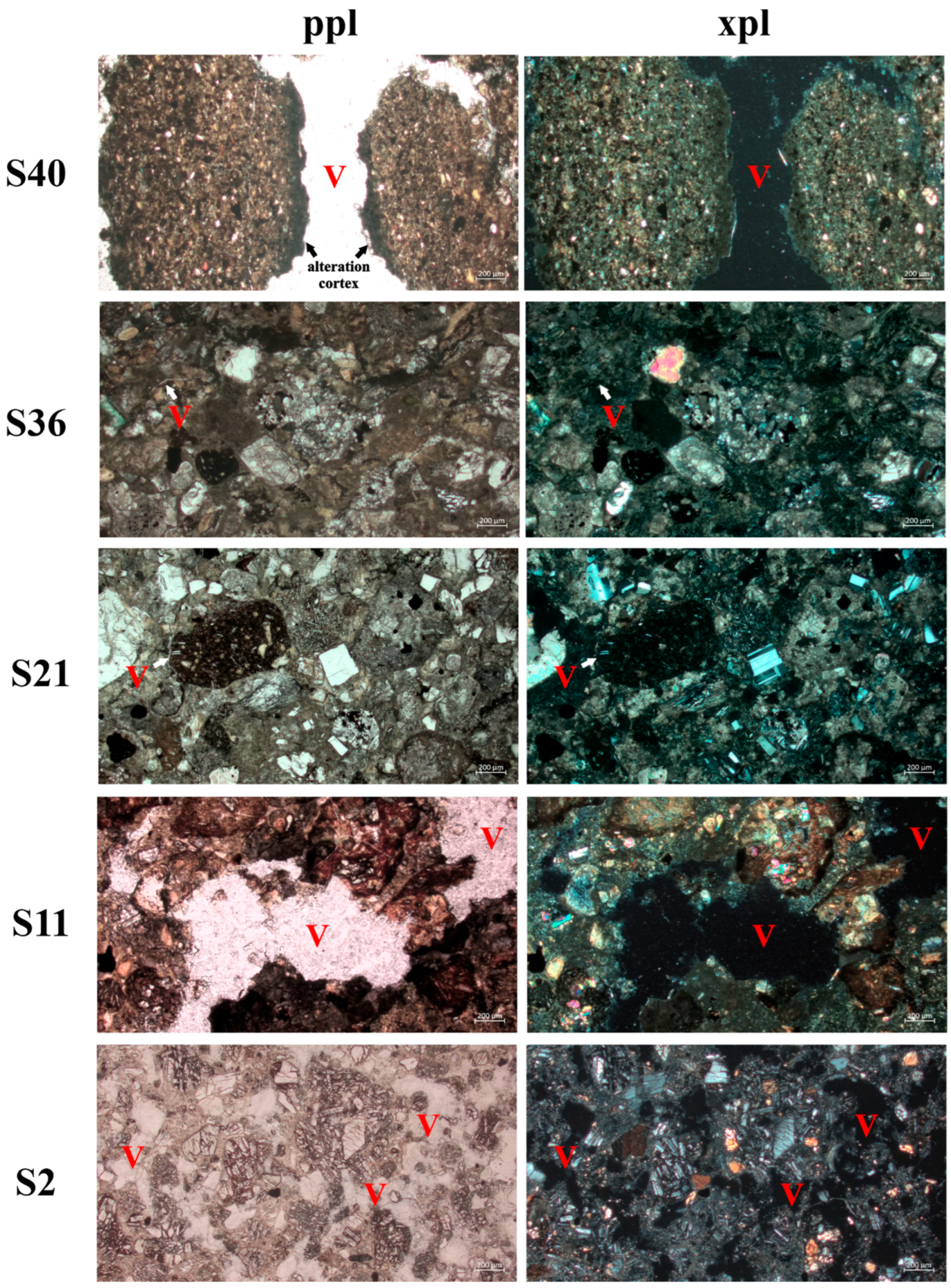
3.4. Petrographic Analyses
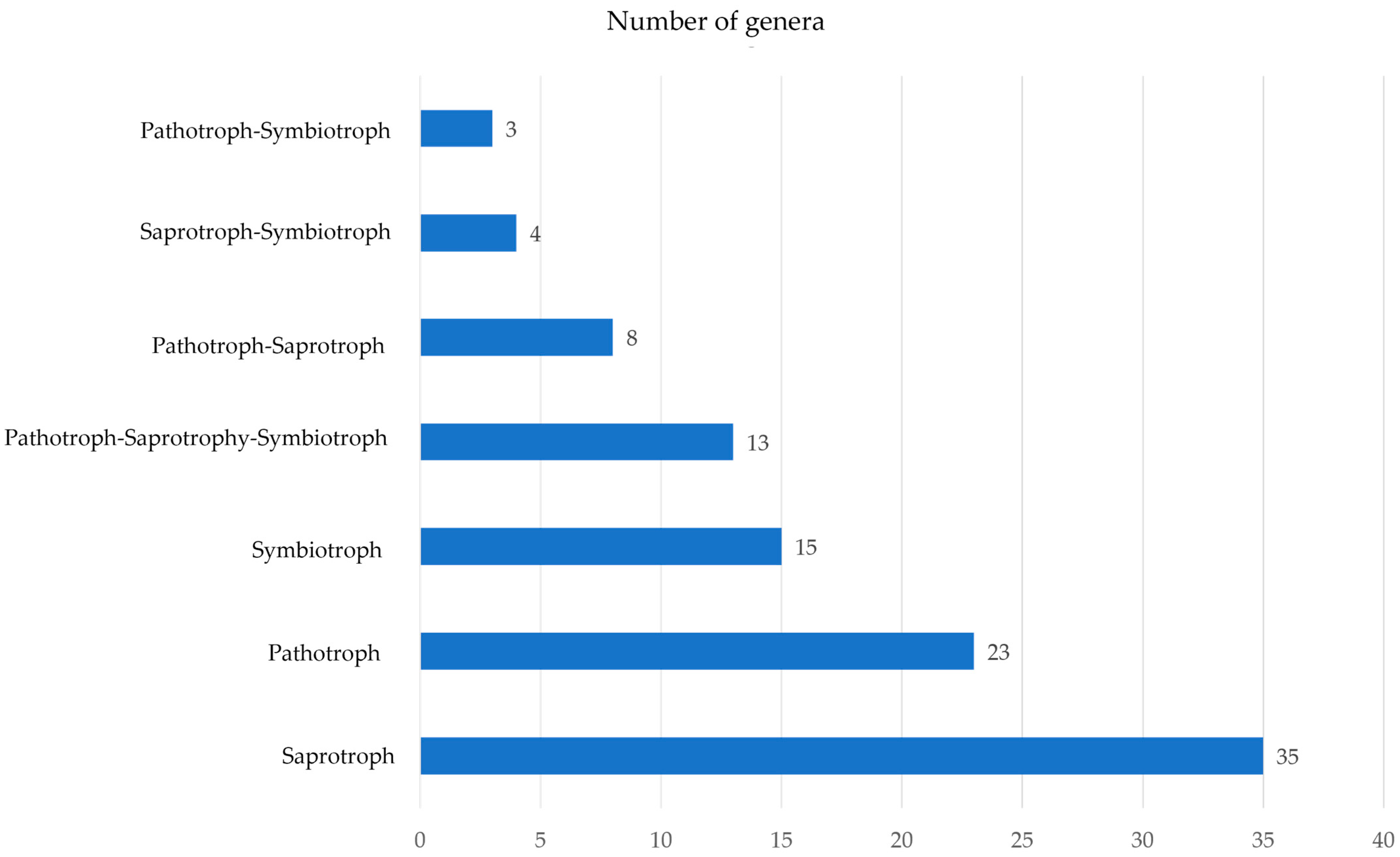
3.5. Assignment to Fungal Lifestyles
4. Discussion
Fungal Taxonomy, Fungal Diversity, and Fungal Lifestyle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, V.N.; Vaz, A.B.M.; Rosa, C.A.; Rosa, L.H. Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol. Ecol. 2012, 82, 459–471. [Google Scholar] [CrossRef]
- Convey, P. Antarctic ecosystems. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Cowan, D.A. Diverse hypolithic refuge communities in Antarctic Dry Valleys. Antarct. Sci. 2010, 22, 714–720. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Aimée, T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Rosa, L.H. Fungi in Antarctica: Diversity, Ecology, Effects of Climate Change, and Bioprospection for Bioactive Compounds. In Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications; Rosa, L.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–18. [Google Scholar]
- Vincent, W.F. Evolutionary origins of Antarctic microbiota: Invasion, selection and endemism. Antarct. Sci. 2000, 12, 374–385. [Google Scholar] [CrossRef]
- Onofri, S.; Fenice, M.; Cicalini, A.R.; Tosi, S.; Magrino, A.; Pagano, S.; Selbmann, L.; Zucconi, L.; Vishniac, H.S.; Ocampo-Friedmann, R.; et al. Ecology and biology of microfungi from Antarctic rocks and soil. Ital. J. Zool. 2000, 67, 163–168. [Google Scholar] [CrossRef]
- Arenz, B.E.; Blanchette, R.A. Distribution and abundance of soil fungi in Antarctica at sites on the Peninsula, Ross Sea Region and McMurdo Dry Valleys. Soil Biol. Biochem. 2011, 43, 308–315. [Google Scholar] [CrossRef]
- Stoops, G. Guidelines for the Analysis and Description of Soil and Regolith Thin Sections; SSSA: Madison, WI, USA, 2003. [Google Scholar]
- Friedmann, E.I. Endolithic microorganisms in the Antarctic cold desert. Science 1982, 215, 1045–1053. [Google Scholar] [CrossRef]
- Hughes, K.A.; Lawley, B. A novel Antarctic microbial endolithic community within gypsum crusts. Environ. Microbiol. 2003, 5, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Selbmann, L.; de Hoog, G.S.; Mazzaglia, A.; Friedmann, E.I.; Onofri, S. Fungi at the edge of life: Cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 2005, 51, 1–32. [Google Scholar]
- Gonçalves, V.N.; Oliveira, F.S.; Carvalho, C.R.; Schaefer, C.E.G.R.; Rosa, C.A.; Rosa, L.H. Antarctic rocks from continental Antarctica as source of potential human opportunistic fungi. Extremophiles 2017, 21, 851–860. [Google Scholar] [CrossRef]
- Alves, I.M.S.; Gonçalves, V.N.; Oliveira, F.S.; Schaefer, C.E.G.R.; Rosa, C.A.; Rosa, L.H. Diversity, distribution, and pathogenic potential of fungi present in rocks of the South Shetlands Archipelago, Maritime Antarctica. Extremophiles 2019, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Coleine, C.; Pombubpa, N.; Zucconi, L.; Onofri, S.; Turchetti, B.; Buzzini, P.; Stajich, J.E.; Selbmann, L. Uncovered microbial diversity in Antarctic cryptoendolithic communities sampling three representative locations of the Victoria Land. Microorganisms 2020, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.N.; Alves, I.M.S.; de Oliveira, F.S.; Schaefer, C.E.G.R.; Turbay, C.V.G.; Rosa, C.A.; Rosa, L.H. Rock-inhabiting fungi in Antarctica: New frontiers of the edge of life. In Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications, 1st ed.; Rosa, L.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 99–126. [Google Scholar]
- de Menezes, G.C.A.; Câmara, P.E.A.S.; Pinto, O.H.B.; Carvalho-Silva, M.; Oliveira, F.O.; Souza, C.D.; Schaefer, C.E.R.; Convey, P.; Rosa, C.A.; Rosa, L.H. Fungal diversity present on rocks from a polar desert in continental Antarctica assessed using DNA metabarcoding. Extremophiles 2021, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Smelie, J.L.; McIntosh, W.C.; Whittle, R.; Troedson, A.; Hunt, R.J. A lithostratigraphical and chronological study of Oligocene-Miocene sequences on eastern King George Island, South Shetland Islands (Antarctica), and correlation of glacial episodes with global isotope events. Antarct. Sci. 2021, 33, 502–532. [Google Scholar] [CrossRef]
- Birkenmajer, K. A guide to Tertiary geochronology of King George Island, West Antarctica. Pol. Polar Res. 1989, 10, 555–579. [Google Scholar]
- Zachos, J.C.; Berggren, W.A.; Aubry, M.P.; Mackensen, A. Stable isotope record and trace element ratios of Eocene and Oligocene foraminifers from ODP Site 120-748, Pangea, Kerguelen Plateau. In Ocean Drilling Program; Wise, S.W., Schlich, R., Eds.; PANGAEA: College Station, TX, USA, 1992; Volume 120, pp. 839–854. [Google Scholar]
- Cooper, A.; Cooper, R.A. The Oligocene Bottleneck and New Zealand Biota: Genetic record of a past environmental crisis. PRS Lond. B 1995, 261, 293–302. [Google Scholar]
- Convey, P.; Bowman, V.; Chown, S.; Francis, J.; Fraser, C.; Smellie, J.L.; Storey, B.; Terauds, A. Ice bound Antarctica: Biotic consequences of the shift from a temperate to a polar climate. In Mountains, Climate, and Biodiversity; Hoorn, C., Perrigo, A., Antonelli, A., Eds.; Wiley: Oxford, UK, 2018; ISBN 978-1-119-15987-2. [Google Scholar]
- Warny, S.; Kymes, C.M.; Askin, R.A.; Krajewski, K. Terrestrial and marine floral response to latest Eocene and Oligocene events on the Antarctic Peninsula. Palynology 2019, 43, 1–18. [Google Scholar] [CrossRef]
- Smellie, J.L.; Caça, R.J.; Mcintosh, W.C.; Esser, R.P. Lithostratigraphy, age and distribution of Eocene volcanic sequences on eastern King George Island, South Shetland Islands, Antarctica. Antarc. Sci. 2021, 33, 373–401. [Google Scholar] [CrossRef]
- Light, J.J.; Passchier, S. Eocene to Oligocene cooling and ice growth based on the geochemistry of interglacial mudstones from the East Antarctic continental shelf. Antarct. Sci. 2023, 35, 270–282. [Google Scholar] [CrossRef]
- Rosa, L.H.; Pinto, O.H.B.; Santl-Temkiv, T.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Câmara, P.E.A.S. DNA metabarcoding of fungal diversity in air and snow of Livingston Island, South Shetland Islands, Antarctica. Sci. Rep. 2020, 10, 21793. [Google Scholar] [CrossRef]
- Rosa, L.H.; Pinto, O.H.B.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Câmara, P.E.A.S. DNA metabarcoding to assess the diversity of airborne fungi present over Keller Peninsula, King George Island, Antarctica. Microb. Ecol. 2021, 82, 165–172. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.M.D.; Ogaki, M.B.; Câmara, P.E.A.S.; Pinto, O.H.B.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Rosa, L.H. Assessment of fungal diversity present in lakes of maritime Antarctica using DNA metabarcoding: A temporal microcosm experiment. Extremophiles 2021, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.M.D.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; Carvalho-Silva, M.; Oliveira, F.S.; Rosa, C.A.; Câmara, P.E.A.S.; Rosa, L.H. Diversity, distribution and ecology of fungal communities present in Antarctic lake sediments uncovered by DNA metabarcoding. Sci. Rep. 2022, 12, 8407. [Google Scholar] [CrossRef] [PubMed]
- Troedson, A.L.; Smellie, J.L. The Polonez Cove Formation of King George Island, Antarctic: Stratigraphy, facies and implications for mid-Cenozoic cryosphere development. Sedimentology 2002, 49, 277–301. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Cantrell, C.L.; Wedge, D.E.; Ferreira, M.C.; Soares, M.A.; Jacob, M.R.; Fabio, S.O.; Galante, D.; Rodrigues, F.; Alves, T.M.A.; et al. Fungi associated with rocks of the Atacama Desert: Taxonomy, distribution, diversity, ecology and bioprospection for bioactive compounds. Environ. Microbiol. 2016, 18, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Purvis, O.W.; Convey, P.; Flowerdew, M.J.; Peat, H.J.; Najorka, J.; Kearsley, A. Iron localization in Acarospora colonizing schist following glacial retreat on Signy Island. Antarct. Sci. 2013, 25, 24–30. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed]
- Câmara, P.; Convey, P.; Rangel, S.B.; Konrath, M.; Barreto, C.C.; Pinto, O.H.B.; Silva, M.C.; Henriques, D.D.; Oliveira, H.C.; Rosa, L.H. The largest moss carpet transplant in Antarctica and its bryosphere cryptic biodiversity. Extremophiles 2021, 25, 369–384. [Google Scholar] [CrossRef]
- Câmara, P.; Souza, L.M.D.; Pinto, O.H.B.; Convey, P.; Amorim, E.T.; Carvalho-Silva, M.; Rosa, L.H. Periphyton diversity in two different Antarctic lakes assessed using metabarcoding. Antarct. Sci. 2021, 33, 596–604. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2014. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN community edition-interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, 1004957. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]
- Abarenkov, K.; Allan, Z.; Timo, P.; Raivo, P.; Filipp, I.; Nilsson, H.R.; Urmas, K. UNITE QIIME Release for Fungi, Version 04.02.2020; UNITE Community: Salt Lake City, UT, USA, 2020. [Google Scholar]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D. Heatmapper: Web-enabled heat mapping for all. Nucl. Acids Res. 2016, 44, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Medinger, R.; Nolte, V.; Pandey, R.V.; Jost, S.; Ottenwalder, B.; Schlotterer, C.; Boenigk, J. Diversity in a hidden world: Potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Mol. Ecol. 2010, 19, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.A.; Pawlowski, J. Can abundance of protists be inferred from sequence data: A case study of Foraminifera. PLoS ONE 2013, 8, 56739. [Google Scholar] [CrossRef] [PubMed]
- Giner, C.R.; Forn, I.; Romac, S.; Logares, R.; De Vargas, C.; Massana, R. Environmental sequencing provides reasonable estimates of the relative abundance of specific picoeukaryotes. Appl. Environ. Microbiol. 2016, 82, 4757–4766. [Google Scholar] [CrossRef]
- Deiner, K.; Bik, H.M.; Machler, E.; Seymour, M.; Lacoursière-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; de Vere, N.; et al. Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Jones, J.I.; Pont, D.; Boets, P.; Bouchez, A.; Bruce, K.; Drakare, S.; Hanfling, B.; Kahlert, M.; et al. Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive. Water Res. 2018, 138, 192–205. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.; Stalpers, J.; Minter, D.W. Dictionary of the Fung; CAB International: Wallingford, UK, 2011. [Google Scholar]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Doring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Div. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, G.C.A.; Camara, P.; Pinto, O.; Convey, P.; Carvalho-Silva, M.; Simoes, J.C.; Rosa, C.A.; Rosa, L.H. Fungi in the Antarctic Cryosphere: Using DNA Metabarcoding to Reveal Fungal Diversity in Glacial Ice from the Antarctic Peninsula Region. Microb. Ecol. 2022, 83, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.N.; de Souza, L.M.D.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; Carvalho-Silva, M.; de Oliveira, F.S.; Câmara, P.E.A.S.; Rosa, L.H. Diversity and ecology of fungal assemblages present in lake sediments at Clearwater Mesa, James Ross Island, Antarctica, assessed using metabarcoding of environmental DNA. Fungal Biol. 2022, 126, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.N.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; de Oliveira, F.S.; Carvalho-Silva, M.; Câmara, P.E.A.S.; Rosa, L.H. Soil fungal diversity and ecology assessed using DNA metabarcoding along a deglaciated chronosequence at Clearwater Mesa, James Ross Island, Antarctic Peninsula. Biology 2023, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Wierzchos, J.; de Los Ríos, A.; Sancho, L.G.; Ascaso, C. Viability of endolithic microorganisms in rocks from the McMurdo Dry Valleys of Antarctica established by confocal and fluorescence microscopy. J. Microsc. 2004, 216, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.; Godinho, V.M.; Silva, D.A.S.; de Paula, M.T.R.; Vitoreli, G.A.; Zani, C.L.; Alves, T.M.A.; Junior, P.A.S.; Murta, S.M.F.; Barbosa, E.C.; et al. Cultivable fungi present in Antarctic soils: Taxonomy, phylogeny, diversity, and bioprospecting of antiparasitic and herbicidal metabolites. Extremophiles 2018, 22, 381–393. [Google Scholar] [CrossRef]
- Villanueva, P. Description of the first four species of the genus Pseudogymnoascus from Antarctica. Front. Microbiol. 2021, 12, 713189. [Google Scholar] [CrossRef]
- Mercantini, R. Keratinophilic fungi isolated from Antarctic soil. Mycopathologia 1989, 106, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Godinho, V.M.; Gonçalves, V.N.; Santiago, I.F.; Figueredo, H.M.; Vitoeli, G.A.; Schaefer, C.E.R.; Barbosa, E.C.; Oliveira, J.G.; Alves, T.M.A.; Zani, C.L.; et al. Diversity and bioprospection of fungal community present in oligotrophic soil of continental Antarctica. Extremophiles 2015, 19, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I.F.; Alvez, T.M.A.; Rabello, A.; Junior, P.A.S.; Romanha, A.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Leishmanicidal and antitumoral activities of endophytic fungi associated with the Antarctic angiosperms Deschampsia antarctica Desv. and Colobanthus quitensis (kunth) Bartl. Extremophiles 2012, 16, 95–103. [Google Scholar]
- Godinho, V.M.; Furbino, L.E.; Santiago, I.F.; Pellizzari, F.M.; Yokoya, N.S.; Pupo, D.; Alves, T.M.A.; Junior, P.A.S.; Romanha, A.J.; Zani, C.L.; et al. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013, 7, 1434–1451. [Google Scholar] [CrossRef] [PubMed]
- Furbino, L.E.; Godinho, V.M.; Santiago, I.F.; Pellizari, F.M.; Alves, T.M.A.; Zani, C.L.; Junior, P.A.S.; Romanha, A.J.; Carvalho, A.G.O.; Gil, L.H.V.G.; et al. Diversity patterns, ecology and biological activities of fungal communities associated with the endemic macroalgae across the Antarctic Peninsula. Microb. Ecol. 2014, 67, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I.F.; Soares, M.A.; Rosa, C.A.; Rosa, L.H. Lichenosphere: A protected natural microhabitat of the non-lichenized fungal communities living in extreme environments of Antarctica. Extremophiles 2015, 19, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, M.; Vergana, K.; Norambuena, J.; Beiza, A.; Maza, F.; Ubilla, P.; Araya, I.; Chávez, R.; San-Martín, A.; Darias, J.; et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J. Microbiol. Biotechnol. 2014, 30, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B.; Teixeira, D.R.; Vieira, R.; Lírio, J.M.; Felizardo, J.P.S.; Abuchacra, R.C.; Cardoso, R.P.; Zani, C.L.; Alves, T.M.A.; Junior, P.A.S.; et al. Diversity and bioprospecting of cultivable fungal assemblages in sediments of lakes in the Antarctic Peninsula. Fungal Biol. 2020, 124, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Furhan, J. Adaptation, production, and biotechnological potential of cold-adapted proteases from psychrophiles and psy-chrotrophs: Recent overview. J. Genet. Eng. Biotechnol. 2020, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- McRae, C.F.; Hocking, A.D.; Seppelt, R.D. Penicillium species from terrestrial habitats in the Windmill Islands, east Antarctica, including a new species, Penicillium antarcticum. Polar Biol. 1999, 21, 97–111. [Google Scholar] [CrossRef]
- Zucconi, L.; Selbmann, L.; Buzzini, P.; Turchetti, B.; Guglielmin, M.; Frisvad, J.C.; Onofri, S. Searching for eukaryotic life preserved in Antarctic permafrost. Polar Biol. 2012, 35, 749–757. [Google Scholar] [CrossRef]
- da Silva, T.H.; Silva, D.A.S.; Oliveira, F.S.; Schaefer, C.E.G.; Rosa, C.A.; Rosa, L.H. Diversity, distribution, and ecology of viable fungi in permafrost and active layer of Maritime Antarctica. Extremophiles 2020, 24, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Canini, F.; Borruso, L.; Newsham, K.K.; D’Alò, F.; D’Acqui, L.P.; Zucconi, L. Wide divergence of fungal communities inhabiting rocks and soils in a hyper-arid Antarctic desert. Environ. Microbiol. 2023, 25, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; Da Silva, T.H.; Ogaki, M.B.; Pinto, O.H.B.; Stech, M.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Câmara, E.A.S. DNA metabarcoding uncovers fungal diversity in soils of protected and non-protected areas on Deception Island, Antarctica. Sci. Rep. 2020, 10, 21986. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.H.; de Menezes, G.C.A.; Pinto, O.H.B.; Convey, P.; Carvalho-Silva, M.; Simoes, J.C.; Rosa, C.A.; Cama, P. Fungal diversity in seasonal snow of Martel Inlet, King George Island, South Shetland Islands, assessed using DNA metabarcoding. Polar Biol. 2022, 45, 627–636. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Pinto, O.H.B.; Vieira, R.; Neto, A.A.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Câmara, P.E.A.S.; Rosa, L.H. Fungi present in Antarctic deep-sea sediments assessed using DNA metabarcoding. Microb. Ecol. 2021, 82, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bockheim, J.G.; Tarnocai, C. Recognition of cryoturbation for classifying permafrost-affected soils. Geoderma 1998, 81, 281–293. [Google Scholar] [CrossRef]
- Tarnocai, C.; Broll, G.; Blume, H.P. Classification of permafrost-affected soils in the WRB. In Cryosols: Permafrost-Affected Soils; Kimble, J.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 637–656. [Google Scholar]
- Campbell, I.B.; Claridge, G.G.C. Antarctica: Soils, Weathering Processes an Environment; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; 368p. [Google Scholar]
- Bockheim, J.G. Properties and classification of cold desert soils from Antarctica. Soil Sci. Soc. Am. J. 1997, 61, 224–231. [Google Scholar] [CrossRef]
- Ugolini, F.C.; Bockheim, J.G. Antartic soil and soil formation in a changing environment: A review. Geoderma 2008, 144, 1–8. [Google Scholar] [CrossRef]
- Lopes, D.; Oliveira, F.S.; Pereira, T.T.C.; Schaefer, C.E.G.R. Pedogeomorphology and weathering at Snow Island, Maritime Antarctica. Catena 2022, 217, 106515. [Google Scholar] [CrossRef]
- Schaefer, C.E.G.R.; Simas, F.N.B.; Gilkes, R.J.; Mathison, C.; da Costa, L.M.; Albuquerque, M.A. Micromorphology and microchemistry of selected cryosols from maritime Antarctica. Geoderma 2008, 144, 104–115. [Google Scholar] [CrossRef]
- Pereira, T.T.C.; Schaefer, C.E.G.R.; Ker, J.C.; Almeida, C.C.; Almeida, I.C.C.; Pereira, A.B. Genesis, mineralogy and ecological significance of ornithogenic soils from a semi-desert polar landscape at Hope Bay, Antarctic Peninsula. Geoderma 2013, 209–210, 98–109. [Google Scholar] [CrossRef]
- Fortes, W.; Oliveira, F.S.; Schaefer, C.E.G.R.; Leite, M.G.P.; Pavinato, P. Phosphatization under birds’ activity: Ornithogenesis at different scales on Antarctic Soilscapes. Geoderma 2021, 391, 114950. [Google Scholar]
- Rosa, L.H.; Coelho, L.C.; Pinto, O.H.B.; Carvalho-Silva, M.; Convey, P.; Rosa, C.A.; Câmara, P.E.A.S. Ecological succession of fungal and bacterial communities in Antarctic mosses affected by a fairy ring disease. Extremophiles 2021, 25, 471–481. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.K.; de Souza, L.M.D.; Vieira, R.; Neto, A.A.; Lopes, F.A.C.; de Oliveira, F.S.; Convey, P.; Carvalho-Silva, M.; Duarte, A.W.F.; Câmara, P.E.A.S.; et al. Fungal and fungal-like diversity in marine sediments from the maritime Antarctic assessed using DNA Metabarcoding. Sci. Rep. 2022, 6, 21044. [Google Scholar] [CrossRef] [PubMed]
- Schutte, C.A.; Samarkin, V.A.; Peters, B.; Madigan, M.T.; Boeles, M.; Morgan-Kiss, R.; Casciotti, K.; Joye, S. Vertical stratification and stability of biogeochemical processes in the deep saline waters of Lake Vanda, Antarctic. Limnol. Oceanogr. 2020, 65, 569–581. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Guarro, J.; Gen JéFigueras, M.J. Atlas of Clinical Fungi; Centraalbureau voor Schimmel-cultures: Utrecht, The Netherlands, 2000; p. 1126. [Google Scholar]
- Sung, G.-H.; Hywel-Jones, N.L.; Sung, J.-M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Schoutteten, N.; Yurkov, A.; Leroux, O.; Haelewaters, D.; Straeten, D.V.D.; Miettinen, O.; Boekhout, T.; Begerow, D.; Verbeken, A. Diversity of colacosome-interacting mycoparasites expands the understanding of the evolution and ecology of Microbotryomycetes. Stud. Mycol. 2023, 106, 41–94. [Google Scholar]
- Ludwig, A.; de Jesus, F.; Dutra, V.; Cândido, S.; Alves, S.; Santurio, J. Susceptibility profile of Candida rugosa (Diutina rugosa) against antifungals and compounds of essential oils. J. Med Mycol. 2019, 29, 154–157. [Google Scholar] [CrossRef]
- Bisby, F.A.; Roskov, Y.R.; Orrell, T.M.; Nicolson, D.; Paglinawan, L.E.; Bailly, N.; Kirk, P.M.; Bourgoin, T.; Baillargeon, G.; Ouvrard, D. Species 2000 & ITIS Catalogue of Life: 2011 Annual Checklis; 2011 Annual Checklist, DVD; Species 2000: Reading, UK, 2011. [Google Scholar]
- Zhang, J.; Huang, X.; Zhang, X.; Zhu, Y.; Liao, K.; Ma, J.; Wang, G.; Guo, Y.; Xie, C. Coinfection of disseminated Talaromyces marneffei and Mycobacteria kansasii in a patient with papillary thyroid cancer. Medicine 2017, 96, e9072. [Google Scholar] [CrossRef]
- De Andrade, G.A.K.; Cañón, E.R.P.; Alves, R.P.; Schmitz, D.; Schünemann, A.L.; De Albuquerque, M.P.; Putzke, J.; Pereira, A.B.; Victoria, F.D.C. First Record of Juncaceicola as Endophytic Fungi Associated with Deschampsia antarctica Desv. Diversity 2018, 10, 107. [Google Scholar] [CrossRef]
- Lachance, M.-A.; Starmer, W.T. Kurtzmaniella gen. nov. and description of the heterothallic, haplontic yeast species Kurtzmaniella cleridarum sp. nov., the teleomorph of Candida cleridarum. Int. J. Syst. Evol. Microbiol. 2008, 58, 520–524. [Google Scholar] [CrossRef]
- Targino, H.M.d.L.; Silva, V.S.L.; Escobar, I.E.C.; Ribeiro, P.R.d.A.; Gava, C.A.T.; Fernandes-Júnior, P.I. Maize-associated Meyerozyma from the Brazilian semiarid region are effective plant growth-promoting yeasts. Rhizosphere 2022, 22. [Google Scholar] [CrossRef]
- Huang, S.; Xia, J.; Zhang, X.; Sun, W.; Li, Z. Two new species of Microdochium from Indocalamus longiauritus in south-western China. MycoKeys 2020, 72, 93–108. [Google Scholar] [CrossRef]
- Rocha, F.P.; Machado, J.L. O gênero Myzocytiopsis (Oomycota) no estado do Piauí: Novos registros para o Brasil. Gaia Sci. 2017, 11. [Google Scholar] [CrossRef]
- Corsaro, D.; Walochnik, J.; Venditti, D.; Steinmann, J.; Müller, K.-D.; Michel, R. Microsporidia-like parasites of amoebae belong to the early fungal lineage Rozellomycota. Parasitol. Res. 2014, 113, 1909–1918. [Google Scholar] [CrossRef]
- Thomé, P.C.; Wolinska, J.; Wyngaert, S.V.D.; Reñé, A.; Ilicic, D.; Agha, R.; Grossart, H.-P.; Garcés, E.; Monaghan, M.T.; Strassert, J.F.H. Phylogenomics including new sequence data of phytoplankton-infecting chytrids reveals multiple independent lifestyle transitions across the phylum. bioRxiv 2023. [Google Scholar] [CrossRef]
- Crous, P.W.; Luangsa-Ard, J.J.; Wingfield, M.J.; Carnegie, A.J.; Hernández-Restrepo, M.; Lombard, L.; Roux, J.; Barreto, R.W.; Baseia, I.G.; Cano-Lira, J.F.; et al. Fungal Planet description sheets: 785–867. Persoonia 2018, 41, 238–417. [Google Scholar] [CrossRef]
- de Errasti, A.; Novas, M.V.; Carmarán, C.C. Plant-fungal association in trees: Insights into changes in ecological strategies of Peroneutypa scoparia (Diatrypaceae). Flora 2014, 209, 704–710. [Google Scholar] [CrossRef]
- Ferreira, E.M.S.; de Sousa, F.M.P.; Rosa, L.H.; Pimenta, R.S. Taxonomy and richness of yeasts associated with angiosperms, bryophytes, and meltwater biofilms collected in the Antarctic Peninsula. Extremophiles 2018, 23, 151–159. [Google Scholar] [CrossRef]
- Martinez, D.; Challacombe, J.; Morgenstern, I.; Hibbett, D.; Schmoll, M.; Kubicek, C.P.; Ferreira, P.; Ruiz-Duenas, F.J.; Martinez, A.T.; Kersten, P.; et al. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc. Natl. Acad. Sci. USA 2009, 106, 1954–1959. [Google Scholar] [CrossRef]
- Hawker, L.E. Fungi: Na Introduction; Hutchinson University Library: London, UK, 1966; p. 216. [Google Scholar]
- Adhikari, B.N.; Hamilton, J.P.; Zerillo, M.M.; Tisserat, N.; Lévesque, C.A.; Buell, C.R. Comparative Genomics Reveals Insight into Virulence Strategies of Plant Pathogenic Oomycetes. PLoS ONE 2013, 8, e75072. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, D.; Chen, X.; Gérard, F.; Li, M.; Wu, J.; Gao, J. Compartments of roots and mature leaves are key hubs in the connectivity of tea-plant mycobiomes and are influenced by environmental factors and host age. Sci. Total Environ. 2023, 893, 164827. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.-R.; Lv, S.-L.; Chai, C.-Y.; Hui, F.-L. Three new Scheffersomyces species associated with insects and rotting wood in China. MycoKeys 2020, 71, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.D.; Cibulski, S.P.; Mayer, F.Q.; Siqueira, F.M.; Rosa, C.A.; Hector, R.E.; Ayub, M.A.Z. Draft Genome Sequence of the d -Xylose-Fermenting Yeast Spathaspora xylofermentans UFMG-HMD23.3. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Péter, G.; Dlauchy, D.; Tornai-Lehoczki, J.; Suzuki, M.; Kurtzman, C.P. Spencermartinsiella europaea gen. nov., sp. nov., a new member of the family Trichomonascaceae. Int. J. Syst. Evol. Microbiol. 2011, 61, 993–1000. [Google Scholar] [CrossRef]
- Rosa, C.A.; Lachance, M.-A. The yeast genus Starmerella gen. nov. and Starmerella bombicola sp. nov., the teleomorph of Candida bombicola (Spencer, Gorin & Tullock) Meyer & Yarrow. Int. J. Syst. Evol. Microbiol. 1998, 48, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.F.L.; Barros, K.O.; Alvarenga, F.B.M.; Santos, A.R.O.; Fonseca, C.R.V.; Abegg, M.A.; Lachance, M.-A.; Rosa, C.A. Sugiyamaella bielyi f. a., sp. nov. and Sugiyamaella amazoniana f. a., sp. nov., two yeast species isolated from passalid beetles and rotting wood in Amazonia. Int. J. Syst. Evol. Microbiol. 2023, 73, 005839. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-F.; Zhang, K.-H.; Chai, C.-Y.; Yan, Z.-L.; Hui, F.-L. Diversity of the genus Sugiyamaella and description of two new species from rotting wood in China. MycoKeys 2021, 77, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, X.; Zhang, S.; Yao, Y.; Zhang, Y.; Liu, Y.; Peng, X.; Huang, J.; Peng, F. Identification and functional studies of microbial volatile organic compounds produced by Arctic flower yeasts. Front. Plant Sci. 2023, 13, 941929. [Google Scholar] [CrossRef]
- Rosa, M.M.; Tauk-Tornisielo, S.M.; Rampazzo, P.E.; Ceccato-Antonini, S.R. Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World J. Microbiol. Biotechnol. 2010, 26, 1491–1502. [Google Scholar] [CrossRef]
- Chang, C.-F.; Liu, Y.-R.; Naumov, G.I.; Naumova, E.S.; Lee, C.-F. Taxonomy of the yeast genus Vanderwaltozyma and proposal of Vanderwaltozyma meishanica sp. nov., Vanderwaltozyma huisunica sp. nov., and Vanderwaltozyma molinica sp. nov. Antonie Van Leeuwenhoek 2020, 113, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.B.; Moe, R.L.; Ascaso, C. The intertidal marine lichen formed by the pyrenomycete fungus Verrucaria tavaresiae (Ascomycotina) and the brown alga Petroderma maculiforme (Phaeophyceae): Thallus organization and symbiont interaction. Am. J. Bot. 2004, 91, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Lumbsch, H.T.; Huhndorf, S.M. Myconet Volume 14. Part One. Outline of Ascomycota—2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life Earth Sci. 2010, 1, 1–64. [Google Scholar] [CrossRef]

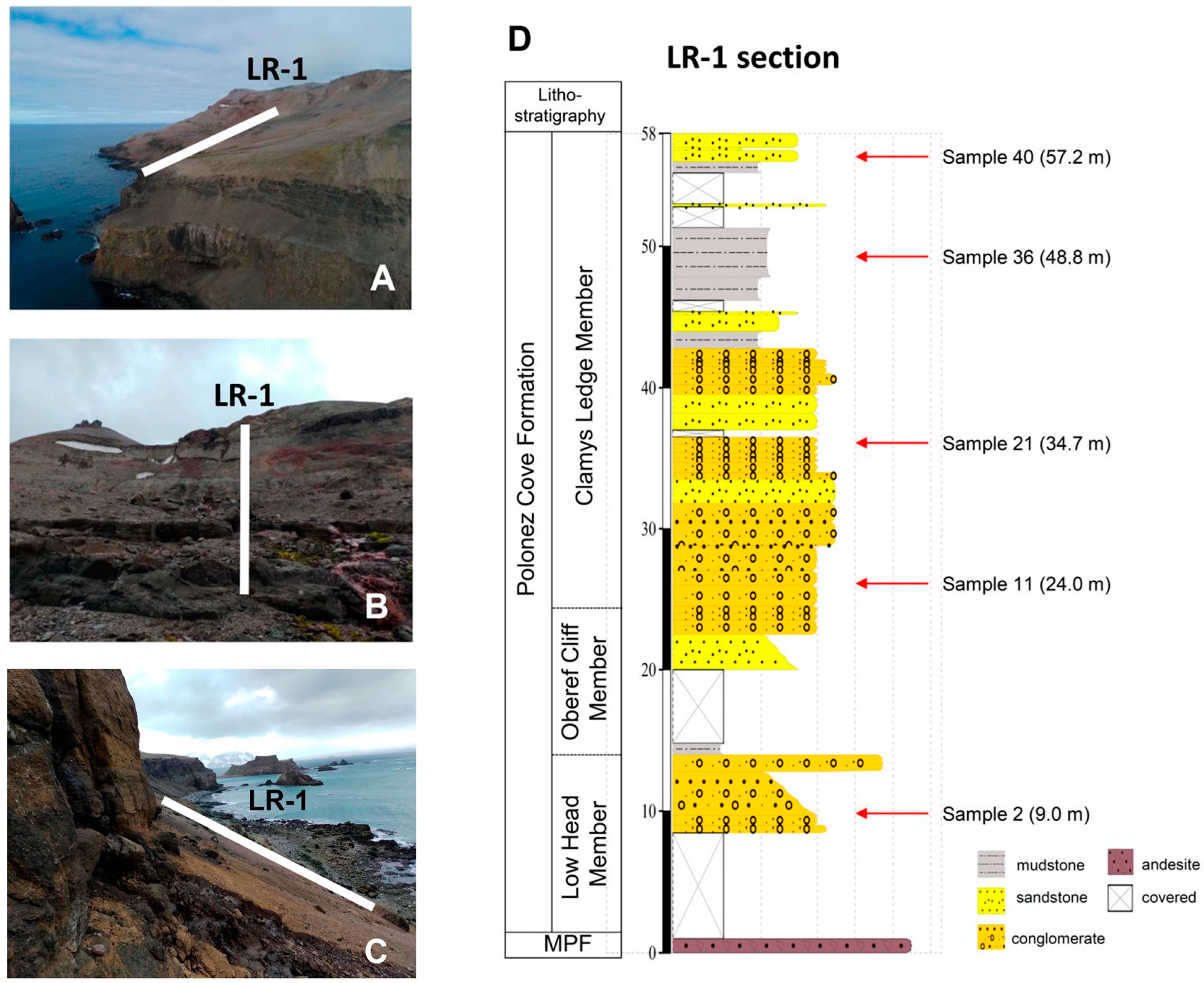
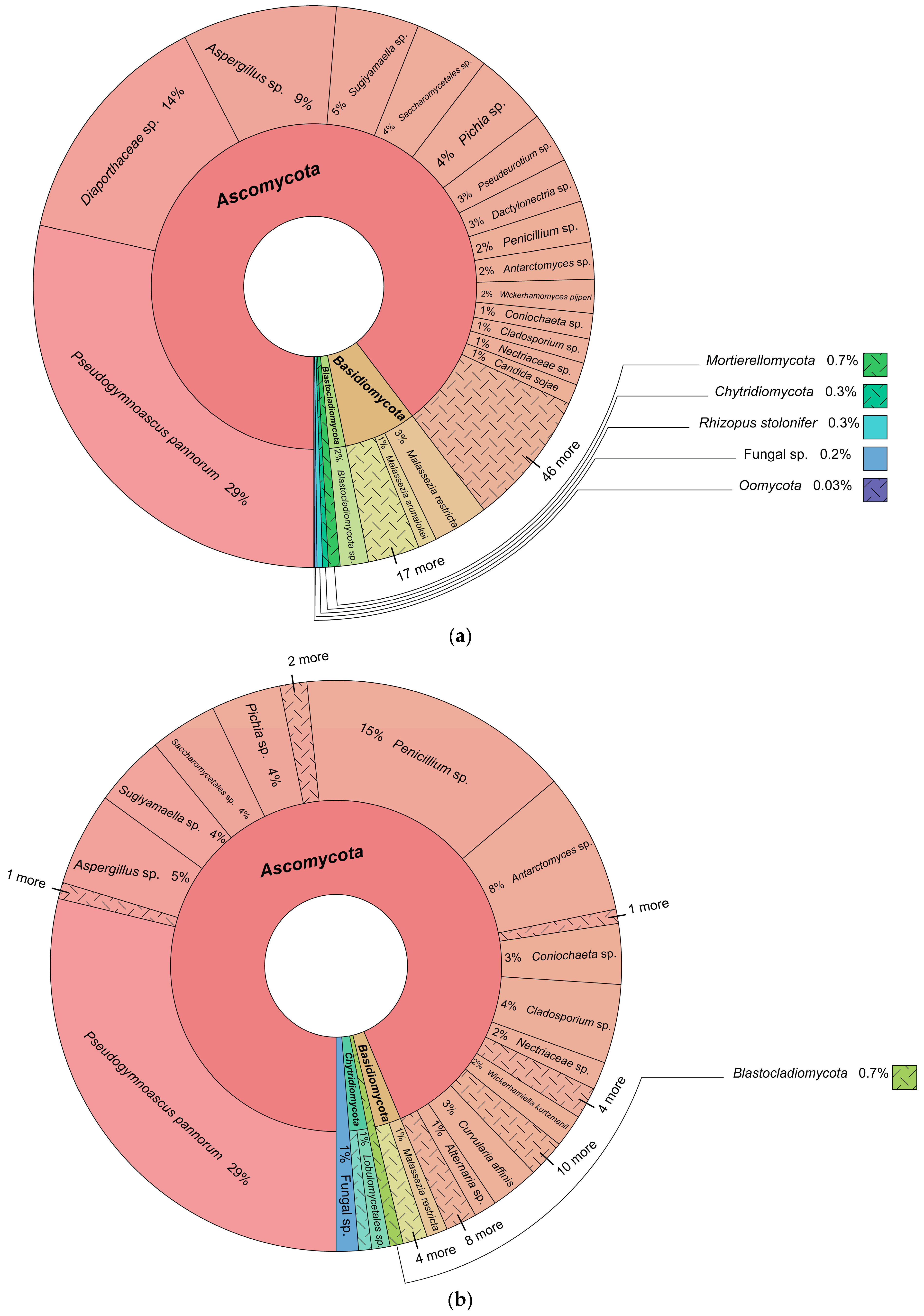
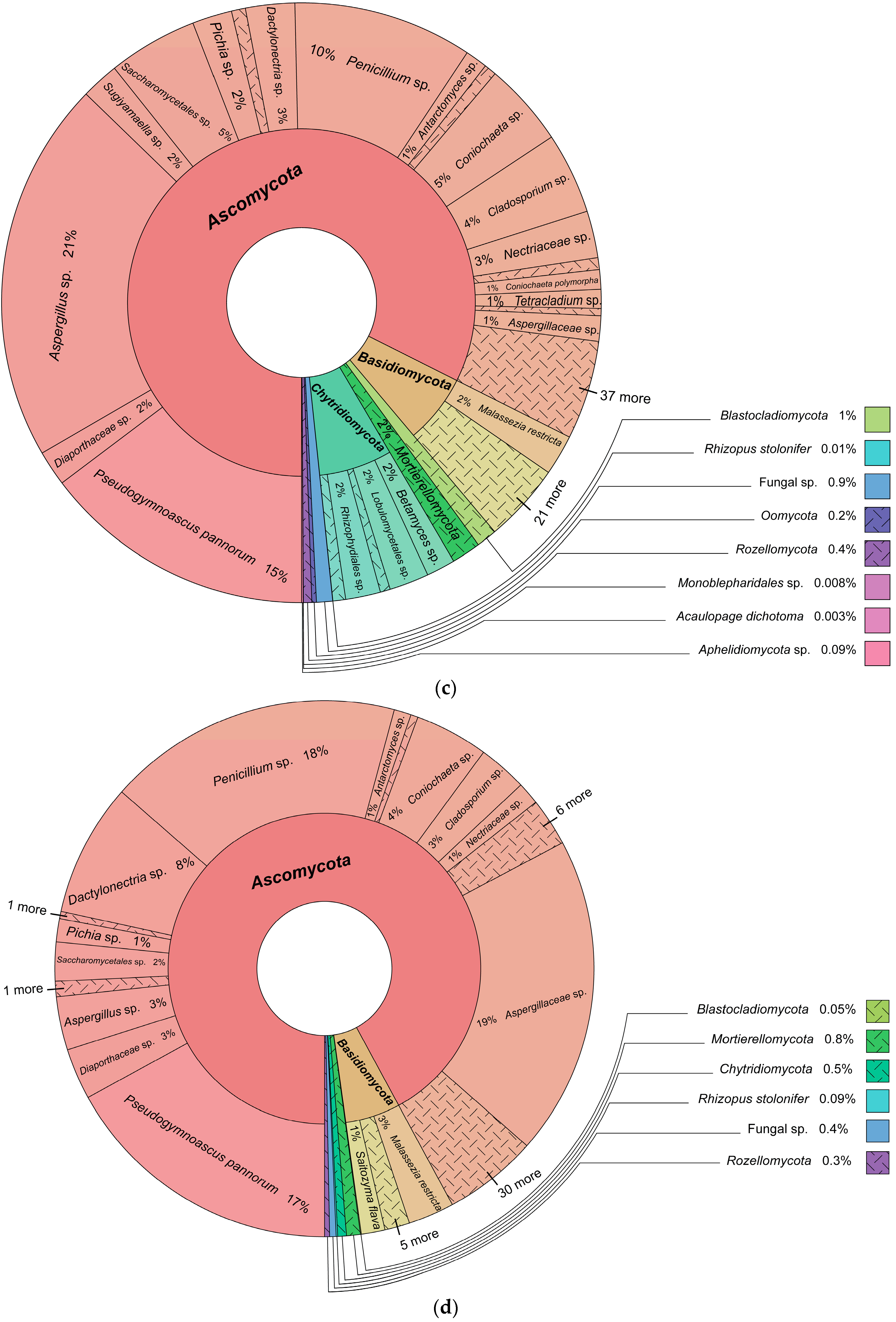
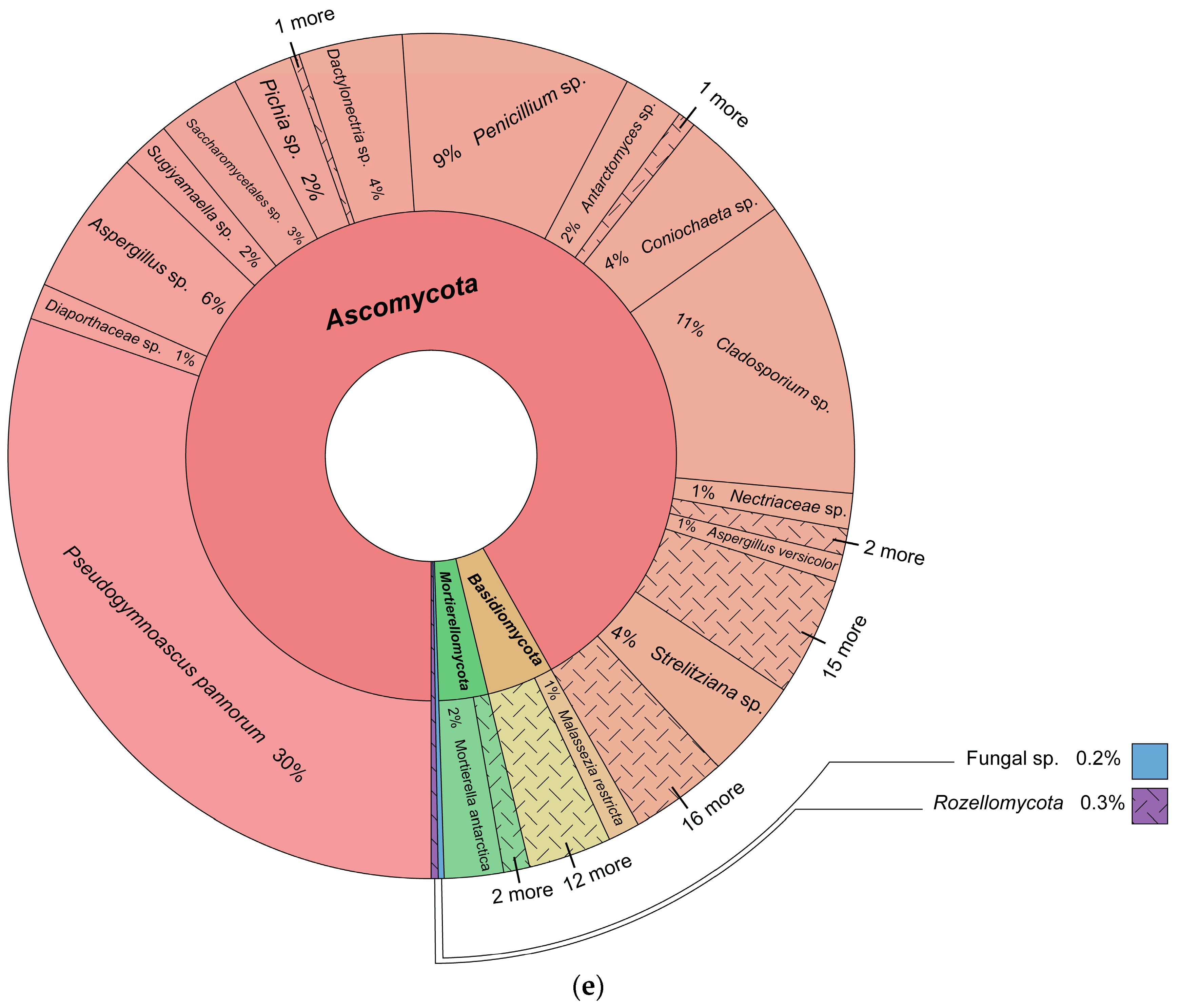
| Sample | |||||
|---|---|---|---|---|---|
| Chemical Elements * | S2 | S11 | S21 | S36 | S40 |
| Mg | 4.13 | 4.89 | 3.40 | <LOD | <LOD |
| Ca | 4.14 | 18.07 | 14.92 | 7.97 | 2.76 |
| Al | 5.87 | 7.89 | 8.16 | 8.83 | 7.88 |
| Si | 44.06 | 45.62 | 38.68 | 49.14 | 44.61 |
| P | 0.14 | 2.54 | 1.13 | 0.45 | 0.13 |
| S | 0.75 | 2.90 | 1.64 | 0.66 | 0.704 |
| Ti | 1.81 | 0.79 | 2.15 | 1.96 | 2.43 |
| V | 0.05 | <LOD | 0.05 | <LOD | 0.0858 |
| Cr | 0.21 | 0.15 | 0.215 | 0.27 | 0.11 |
| Mn | 0.64 | 2.23 | 0.59 | 1.17 | 2.15 |
| Fe | 40.03 | 29.72 | 41.83 | 34.27 | 38.25 |
| Co | 0.12 | <LOD | 0.14 | <LOD | 0.0794 |
| Ni | 0.52 | 0.31 | 0.22 | 0.33 | 0.21 |
| Cu | 0.1 | 0.1 | 0.11 | 0.02 | 0.09 |
| Zn | 0.05 | 0.05 | 0.06 | 0.04 | 0.06 |
| Zr | 0.34 | 0.58 | 0.45 | 0.19 | 0.38 |
| Pd | 1.11 | 2.06 | 1.16 | 0.87 | 1.08 |
| Altitude above sea level (m) | 9.0 | 24.0 | 34.7 | 48.8 | 57.2 |
| Diversity indices | |||||
| Number of amplicon sequence variants (ASVs) | 90 | 49 | 101 | 68 | 67 |
| Number of assigned ASVs | 225 | 177,098 | 319,448 | 219,426 | 172,971 |
| Fisher’s α | 9.42 | 5.17 | 11.97 | 6.84 | 7.13 |
| Margalef | 7.63 | 4.39 | 9.55 | 5.69 | 5.89 |
| Simpson | 0.88 | 0.87 | 0.91 | 0.89 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, N.G.; Gonçalves, V.N.; Carvalho, M.A.; Scheffler, S.M.; Santiago, G.; Sucerquia, P.A.; Oliveira, F.S.; Campos, L.P.; Lopes, F.A.C.; Santos, K.C.R.; et al. Endolithic Fungal Diversity in Antarctic Oligocene Rock Samples Explored Using DNA Metabarcoding. Biology 2024, 13, 414. https://doi.org/10.3390/biology13060414
Rabelo NG, Gonçalves VN, Carvalho MA, Scheffler SM, Santiago G, Sucerquia PA, Oliveira FS, Campos LP, Lopes FAC, Santos KCR, et al. Endolithic Fungal Diversity in Antarctic Oligocene Rock Samples Explored Using DNA Metabarcoding. Biology. 2024; 13(6):414. https://doi.org/10.3390/biology13060414
Chicago/Turabian StyleRabelo, Natana G., Vívian N. Gonçalves, Marcelo A. Carvalho, Sandro M. Scheffler, Gustavo Santiago, Paula A. Sucerquia, Fabio S. Oliveira, Larissa P. Campos, Fabyano A. C. Lopes, Karita C. R. Santos, and et al. 2024. "Endolithic Fungal Diversity in Antarctic Oligocene Rock Samples Explored Using DNA Metabarcoding" Biology 13, no. 6: 414. https://doi.org/10.3390/biology13060414
APA StyleRabelo, N. G., Gonçalves, V. N., Carvalho, M. A., Scheffler, S. M., Santiago, G., Sucerquia, P. A., Oliveira, F. S., Campos, L. P., Lopes, F. A. C., Santos, K. C. R., Silva, M. C., Convey, P., Câmara, P. E. A. S., & Rosa, L. H. (2024). Endolithic Fungal Diversity in Antarctic Oligocene Rock Samples Explored Using DNA Metabarcoding. Biology, 13(6), 414. https://doi.org/10.3390/biology13060414









