A Traditional Chinese Medicine, Zhenqi Granule, Potentially Alleviates Dextran Sulfate Sodium-Induced Mouse Colitis Symptoms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Animal Treatment
2.4. Disease Activity Index (DAI) Calculation
2.5. Determination of the Levels of Pro-Inflammatory Cytokines in Colons
2.6. Histological Analysis
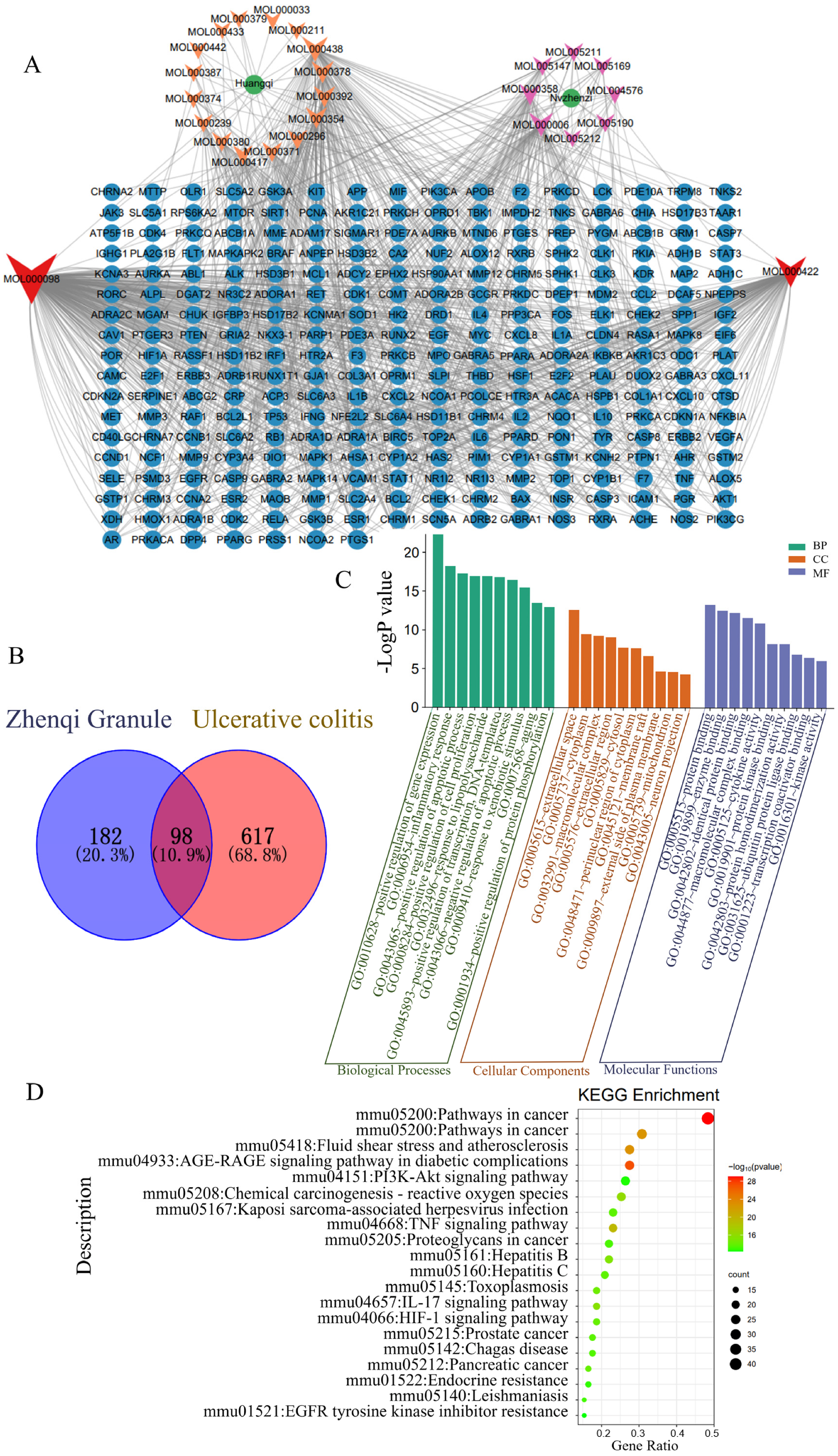
2.7. Network Pharmacological Analysis
2.8. Statistical Analysis
3. Results
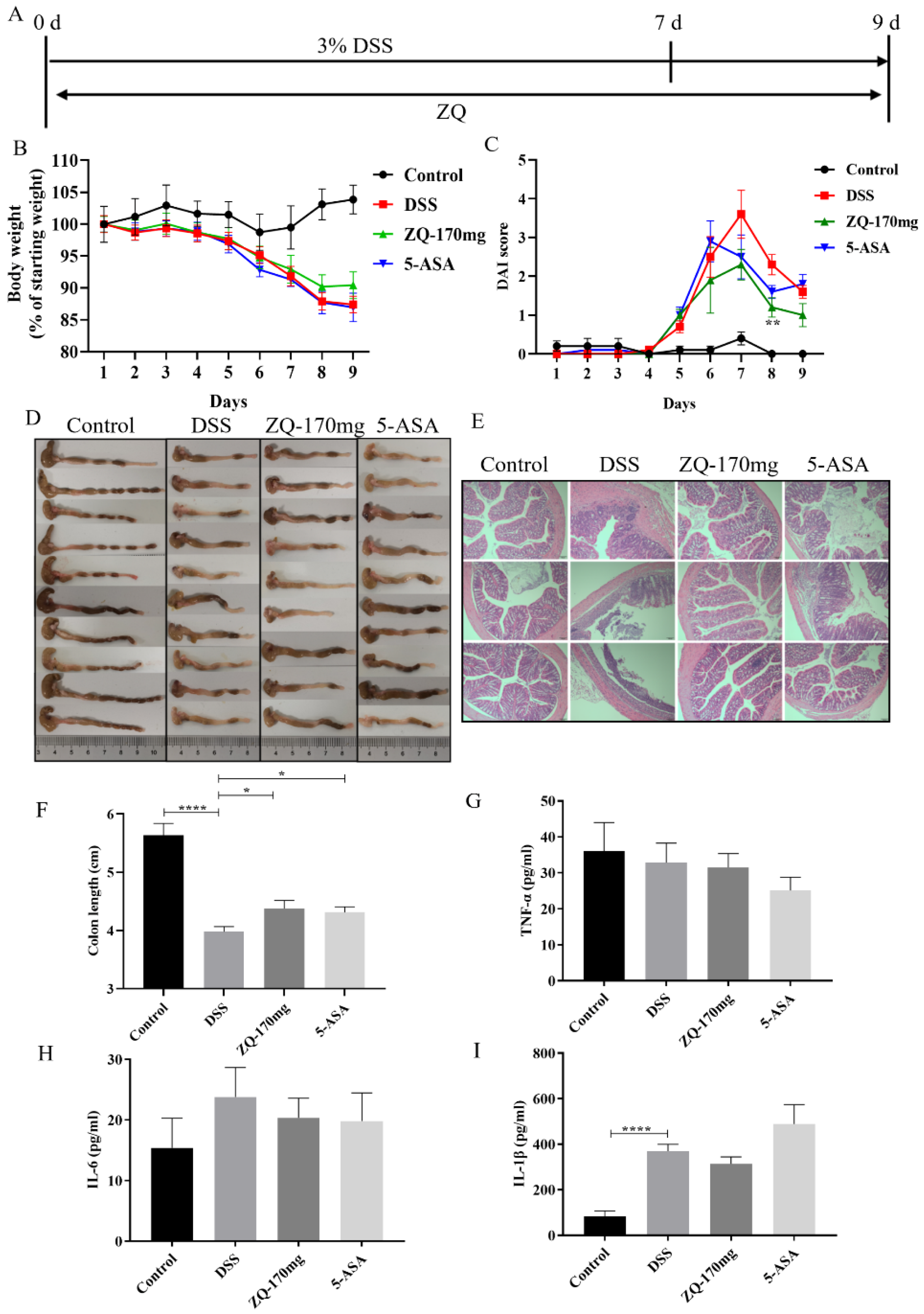
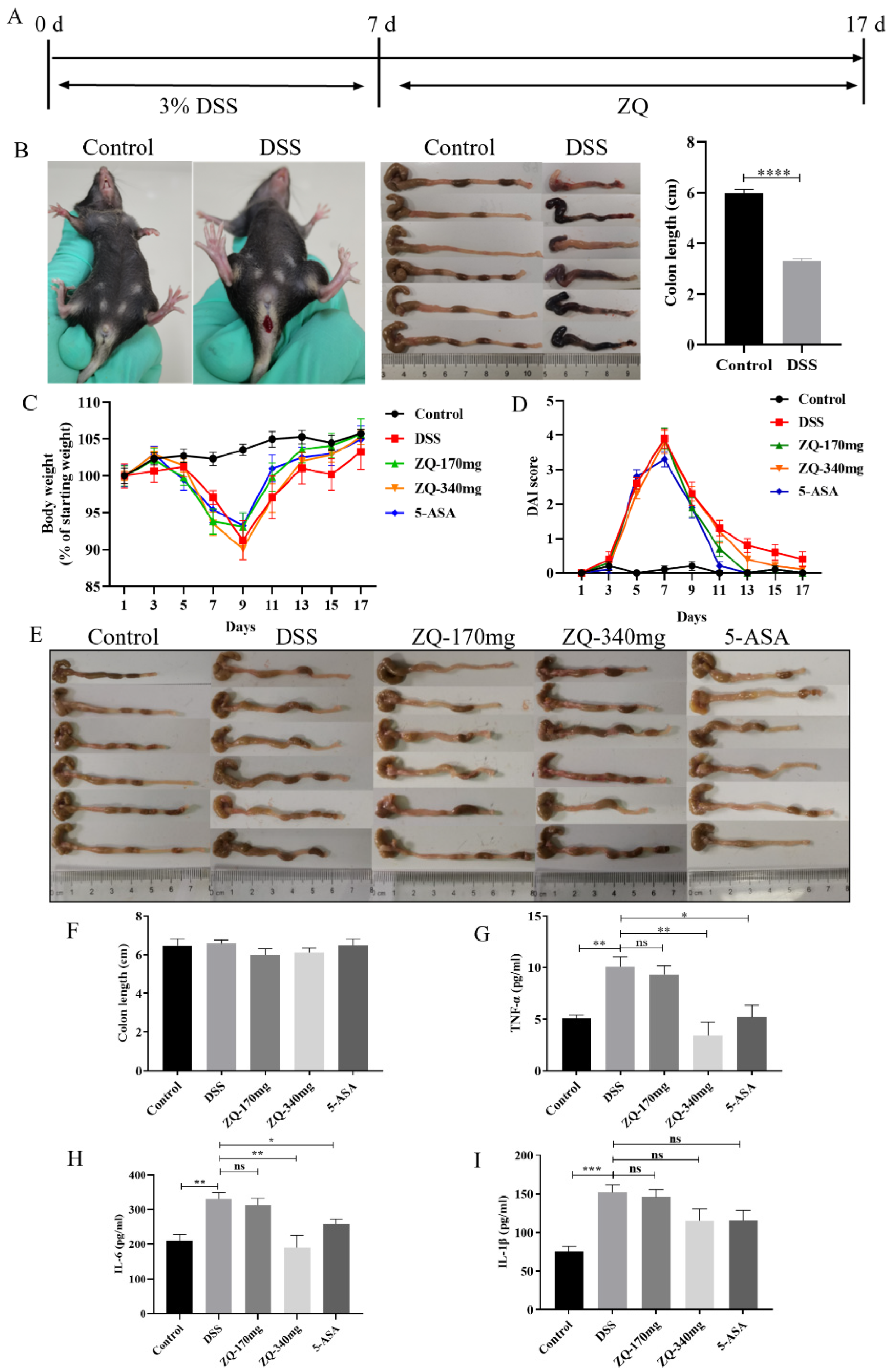
3.1. Establishment of the DSS-Induced Mouse Colitis Model

3.2. Preventive Effects of Zhenqi Granule on DSS-Induced Mouse Colitis
3.3. Therapeutic Effects of Zhenqi Granule on DSS-Induced Mouse Colitis
3.4. Network Pharmacological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sokic-Milutinovic, A.; Milosavljevic, T. IBD: From conventional immunosuppression to biological therapy. Dig. Dis. 2023; ahead of print. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Wong, W.-T.; Hsu, H.-T.; Cheng, Y.-H.; Yu, Y.-H.; Chen, W.-J.; Ho, C.-L.; Hsu, H.-C.; Hua, K.-F. Surfactin Containing Bacillus licheniformis-Fermented Products Alleviate Dextran Sulfate Sodium-Induced Colitis by Inhibiting Colonic Inflammation and the NLRP3 Inflammasome in Mice. Animals 2022, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, M.-F.; Liang, Y.-J.; Xu, J.; Xu, H.-M.; Nie, Y.-Q.; Wang, L.-S.; Yao, J.; Li, D.-F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Di, B.; Xu, L.-L. Recent advances in the treatment of IBD: Targets, mechanisms and related therapies. Cytokine Growth Factor Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Kamm, M.A. Therapeutic strategies for the management of ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, P.; Chen, W.; Chen, G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol. Lett. 2020, 225, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, S.K.; Signs, S.; Hu, B.; Yeu, Y.; Feng, H.; Ni, Y.; Hill, D.R.; Fisher, R.C.; Ferrandon, S.; DeHaan, R.K.; et al. Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity. Nat. Commun. 2021, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sui, W.; Yang, Y.; Liu, L.; Li, Q.; Guo, A. Establishment of an Enteric Inflammation Model in Broiler Chickens by Oral Administration with Dextran Sulfate Sodium. Animals 2022, 12, 3552. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.M.; Guney, E.; Egea, J.; López, M.G.; Baumbach, J.; et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Li, Z.; Zhang, Y.; Yang, A.; Wang, Y.; Zhou, Y.; Wu, W.; Qiu, Y.; Li, L. ZhenQi FuZheng formula inhibits the growth of colorectal tumors by modulating intestinal microflora-mediated immune function. Aging 2022, 14, 4769–4785. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother. 2022, 150, 113015. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wu, J.; Peng, Y.; Dong, B.; Jiang, Y.; Hu, C.; Yu, L.; Chen, Z. Ligustri Lucidi Fructus, a traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology, and toxicity. J. Ethnopharmacol. 2023, 301, 115789. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, C.; Wang, Z.; Liu, X.; You, Y.; Wei, H.; Guo, T. Ligustri lucidi fructus as a traditional Chinese medicine: A review of its phytochemistry and pharmacology. Nat. Prod. Res. 2015, 29, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, L.; Li, L.; Zhu, R.; Liu, H.; Liu, C.; Ma, R.; Jia, Q.; Zhao, D.; Niu, J.; et al. Fructus Ligustri Lucidi in Osteoporosis: A Review of its Pharmacology, Phytochemistry, Pharmacokinetics and Safety. Molecules 2017, 22, 1469. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xiao, Q.; Kang, Z.; Huang, J.; Ge, W.; Wan, Q.; Wang, H.; Zhou, W.; Zhao, H.; Liu, D. Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of Tfh/Treg cells. Int. Immunopharmacol. 2022, 111, 109108. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-C.; Zhang, W.; Xiong, P.-Y.; Song, L.; Jia, B.; Liu, X.-L. Anti-inflammatory and antioxidant activity of astragalus polysaccharide in ulcerative colitis: A systematic review and meta-analysis of animal studies. Front. Pharmacol. 2022, 13, 1043236. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Song, L.; Pang, W.; Zhang, S.; Yu, J.; Liang, P.; Li, T.; Sun, Y.; Wang, Y.; Yan, J.; et al. Astragalus polysaccharide protects experimental colitis through an aryl hydrocarbon receptor-dependent autophagy mechanism. Br. J. Pharmacol. 2024, 181, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Linghu, K.-G.; Ma, Q.; Xiong, S.-H.; Zhao, M.; Chen, Q.; Xu, W.; Chen, M.; Zhang, J.-Y.; Hu, Y.; Xu, W.; et al. The “whole ingredients extract” of Astragali Radix improves the symptoms of dextran sulfate sodium-induced ulcerative colitis in mice through systemic immunomodulation. Chin. Med. 2022, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, S.; Zhang, K.; Li, H.; Xin, M.; Liu, Y.; Yan, J. Fructus ligustri lucidi suppresses inflammation and restores the microbiome profile in murine colitis models. Phytomedicine 2022, 106, 154438. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Asif, M.; Ashfaq, U.A.; Qasim, M.; Qamar, M.T.U. Machine learning for synergistic network pharmacology: A comprehensive overview. Briefings Bioinform. 2023, 24, bbad120. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shen, Q.; Lyu, W.; Lv, L.; Wang, W.; Yu, M.; Yang, H.; Tao, S.; Xiao, Y. Clostridium butyricum and Its Derived Extracellular Vesicles Modulate Gut Homeostasis and Ameliorate Acute Experimental Colitis. Microbiol. Spectr. 2022, 10, e0136822. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, L.; Yang, T.; Mao, P.; Huang, X.; Luo, Y.; Jiang, Z.; Hu, L.; Zhao, Y.; Fu, Z.; et al. Preventive and Therapeutic Spermidine Treatment Attenuates Acute Colitis in Mice. J. Agric. Food Chem. 2021, 69, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Fan, Y.M.; Ga, Y.; Zhang, Y.N.; Han, J.C.; Hao, Z.H. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 2021, 92, 153743. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.C.; Lyra, A.C.; Rocha, R.; Santana, G.O. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J. Gastroenterol. 2014, 20, 9458–9467. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- El-Far, Y.M.; Elsherbiny, N.M.; El-Shafey, M.; Said, E. The interplay of the inhibitory effect of nifuroxazide on NF-κB/STAT3 signaling attenuates acetic acid-induced ulcerative colitis in rats. Environ. Toxicol. Pharmacol. 2020, 79, 103433. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Nestor, M.; Onyewadume, L.; de Silva, P.S.; Korzenik, J.R.; Aguilar, H.; Bailen, L.; Berman, A.; Bhaskar, S.K.; Brown, M.; et al. High Dietary Intake of Specific Fatty Acids Increases Risk of Flares in Patients with Ulcerative Colitis in Remission during Treatment with Aminosalicylates. Clin. Gastroenterol. Hepatol. 2017, 15, 1390–1396.e1. [Google Scholar] [CrossRef] [PubMed]
- Opstelten, J.L.; de Vries, J.H.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.Y.; Kim, S.; Son, M.J.; Son, C.Y.; Kim, J.Y.; Kronbichler, A.; Lee, K.H.; Shin, J.I. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun. Rev. 2019, 18, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Macbeth, A.E.; Holmes, S.; Thompson, A.R.; Chiu, W.S.; Gallardo, W.R.; Messenger, A.G.; Tziotzios, C.; De Lusignan, S. Epidemiology, management and the associated burden of mental health illness, atopic and autoimmune conditions, and common infections in alopecia areata: Protocol for an observational study series. BMJ Open 2021, 11, e045718. [Google Scholar] [CrossRef]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA 2017, 318, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fu, L.; Li, X.; Lu, C.; Su, Y.; Xiong, K.; Zhang, L. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: A prospective randomized pilot study. Microb. Cell Factories 2021, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Allegretti, J.R.; Siddique, S.M.; Terdiman, J.P. AGA Technical Review on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1465–1496.e17. [Google Scholar] [CrossRef]
- Burri, E.; Maillard, M.H.; Schoepfer, A.M.; Seibold, F.; Van Assche, G.; Rivière, P.; Laharie, D.; Manz, M. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion 2020, 101 (Suppl. S1), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Bressler, B.; Marshall, J.K.; Bernstein, C.N.; Bitton, A.; Jones, J.; Leontiadis, G.I.; Panaccione, R.; Steinhart, A.H.; Tse, F.; Feagan, B.; et al. Clinical Practice Guidelines for the Medical Management of Nonhospitalized Ulcerative Colitis: The Toronto Consensus. Gastroenterology 2015, 148, 1035–1058.e3. [Google Scholar] [CrossRef] [PubMed]
- Alsoud, D.; Verstockt, B.; Fiocchi, C.; Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 2021, 6, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.J.; Peppercorn, M.A. Ulcerative colitis. Lancet 2002, 359, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Piao, M.; Song, Y.; Liu, C. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. 2020, 2020, 9242601. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, S.; Deng, W.; Gong, Z.; Guo, Y.; Zeng, S.; Xu, Q. Kaempferol Attenuates Gouty Arthritis by Regulating the Balance of Th17/Treg Cells and Secretion of IL-17. Inflammation 2023, 46, 1901–1916. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Li, Z.; Xu, W.; Xiang, C.-H.; Ma, Y.-F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar] [PubMed]
- Liu, G.; Zhao, W.; Bai, J.; Cui, J.; Liang, H.; Lu, B. Formononetin protects against concanavalin-A-induced autoimmune hepatitis in mice through its anti-apoptotic and anti-inflammatory properties. Biochem. Cell Biol. 2021, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, J.; Lin, Z.; Liang, W.; Qin, L.; Ding, J.; Chen, S.; Zhou, L. Isorhamnetin Alleviates Airway Inflammation by Regulating the Nrf2/Keap1 Pathway in a Mouse Model of COPD. Front. Pharmacol. 2022, 13, 860362. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, J.; Shang, Y.; Zhao, Q.; Li, P.; Wu, B. Anti-inflammatory effects of hederagenin on diabetic cardiomyopathy via inhibiting NF-κB and Smads signaling pathways in a type-2 diabetic mice model. RSC Adv. 2019, 9, 26238–26247. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, Y.; Li, S.; Lei, M. Predicting the anti-inflammatory mechanism of Radix Astragali using network pharmacology and molecular docking. Medicine 2023, 102, e34945. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Lin, Y.; Li, L.; Kong, M.; Lou, Y.; Wu, J.; Liu, Z. Integrating Network Pharmacology and Experimental Validation to Investigate the Effects and Mechanism of Astragalus Flavonoids against Hepatic Fibrosis. Front. Pharmacol. 2021, 11, 618262. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Y.; Zhong, R.-H.; Guo, X.-J.; Li, G.-T.; Zhou, J.-Y.; Yang, W.-J.; Ren, B.-T.; Zhu, Y. Jinfeng pills ameliorate premature ovarian insufficiency induced by cyclophosphamide in rats and correlate to modulating IL-17A/IL-6 axis and MEK/ERK signals. J. Ethnopharmacol. 2023, 307, 116242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Luo, W.; Li, H.; Li, T.; Huang, Y.; Huang, Q.; Zhou, R. A Traditional Chinese Medicine, Zhenqi Granule, Potentially Alleviates Dextran Sulfate Sodium-Induced Mouse Colitis Symptoms. Biology 2024, 13, 427. https://doi.org/10.3390/biology13060427
Qiu X, Luo W, Li H, Li T, Huang Y, Huang Q, Zhou R. A Traditional Chinese Medicine, Zhenqi Granule, Potentially Alleviates Dextran Sulfate Sodium-Induced Mouse Colitis Symptoms. Biology. 2024; 13(6):427. https://doi.org/10.3390/biology13060427
Chicago/Turabian StyleQiu, Xiuxiu, Wentao Luo, Haotian Li, Tingting Li, Yaxue Huang, Qi Huang, and Rui Zhou. 2024. "A Traditional Chinese Medicine, Zhenqi Granule, Potentially Alleviates Dextran Sulfate Sodium-Induced Mouse Colitis Symptoms" Biology 13, no. 6: 427. https://doi.org/10.3390/biology13060427
APA StyleQiu, X., Luo, W., Li, H., Li, T., Huang, Y., Huang, Q., & Zhou, R. (2024). A Traditional Chinese Medicine, Zhenqi Granule, Potentially Alleviates Dextran Sulfate Sodium-Induced Mouse Colitis Symptoms. Biology, 13(6), 427. https://doi.org/10.3390/biology13060427





