Modification of Keratin Integrations and the Associated Morphogenesis in Frizzling Chicken Feathers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genotyping and Phenotyping of Frizzle Flight Feathers
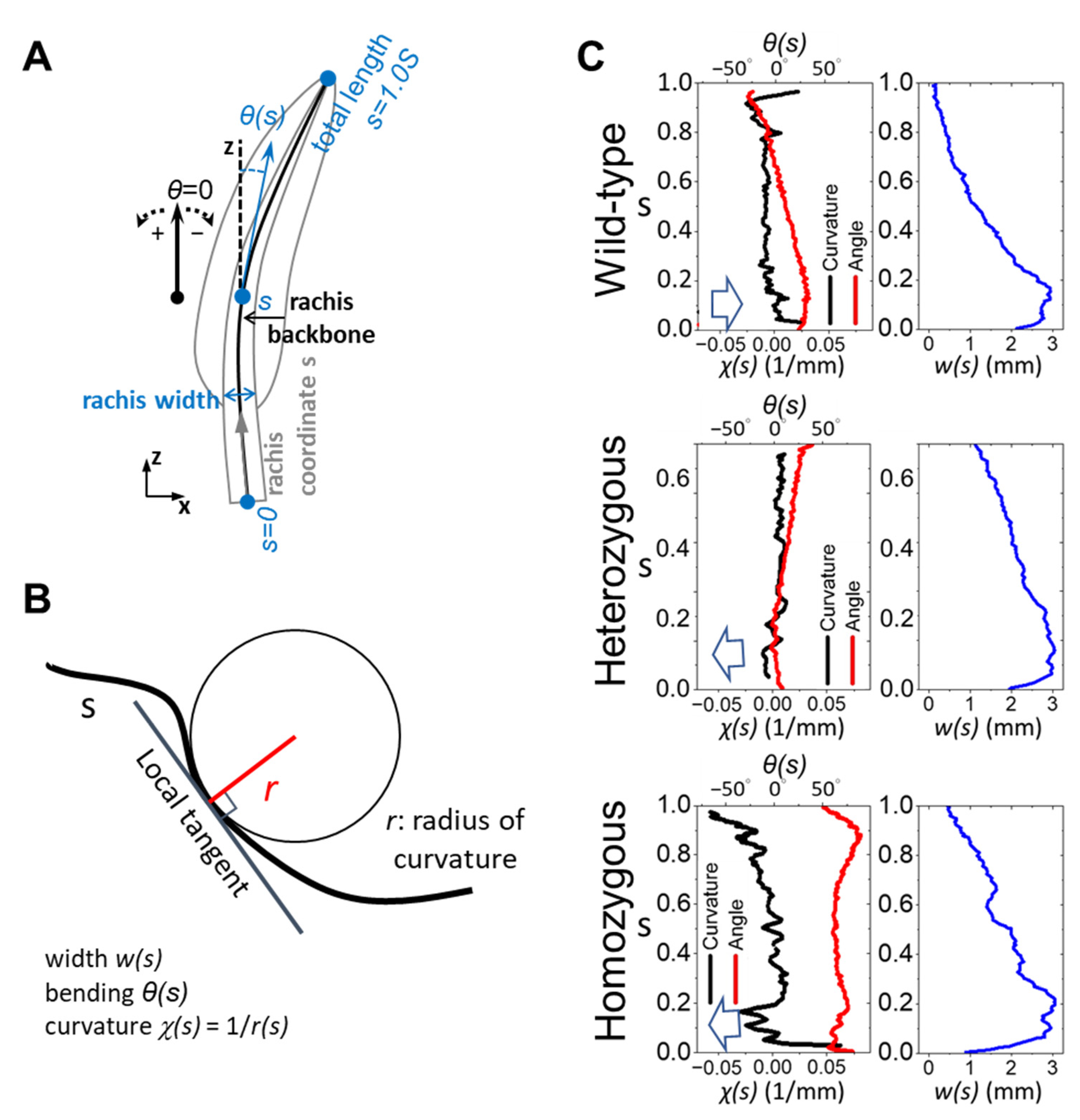
3.2. Full-Length Characterization of the Frizzling Feather Shaft
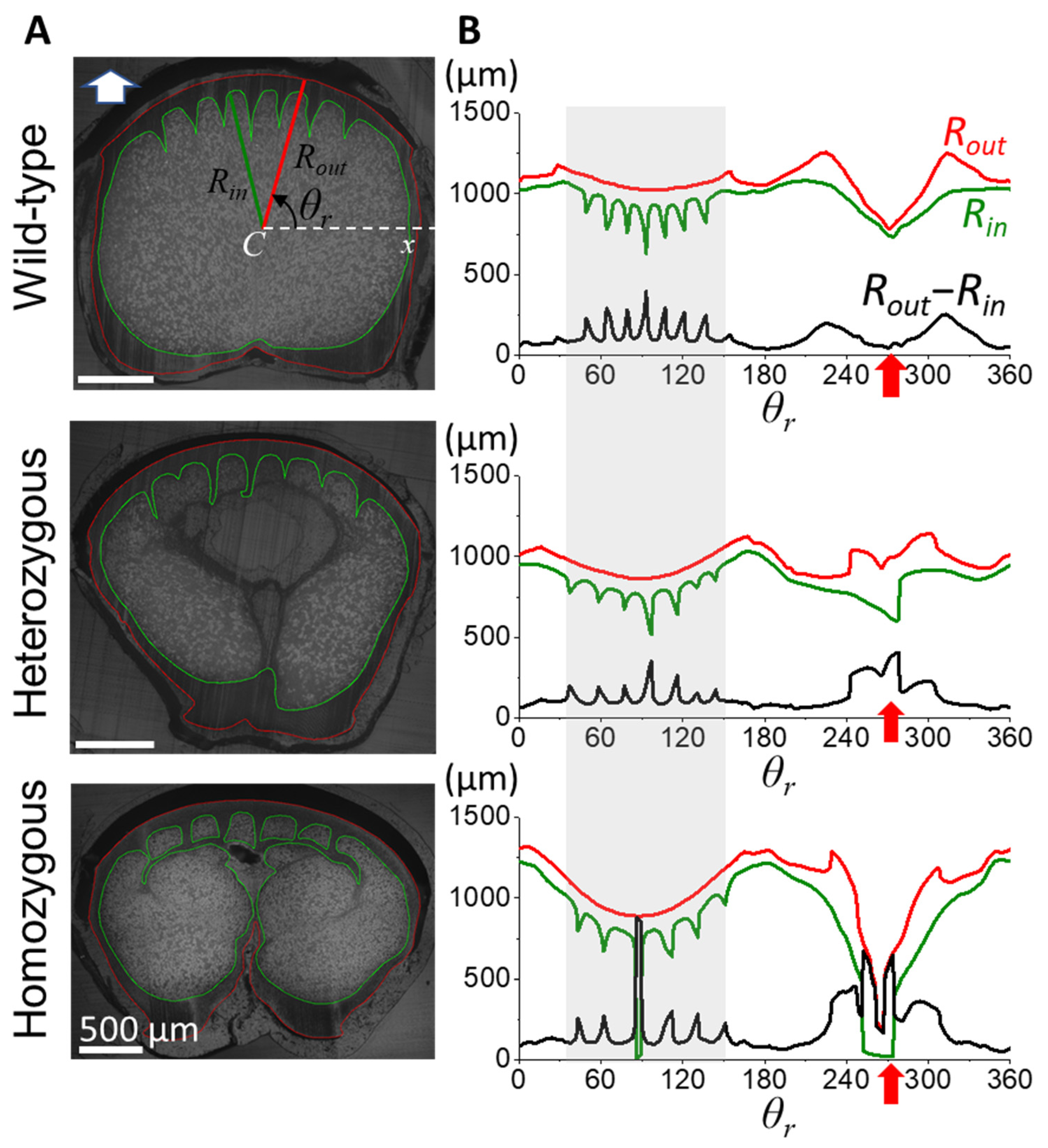
3.3. Morphological Characterization of Cortex from the Rachis Cross-Section
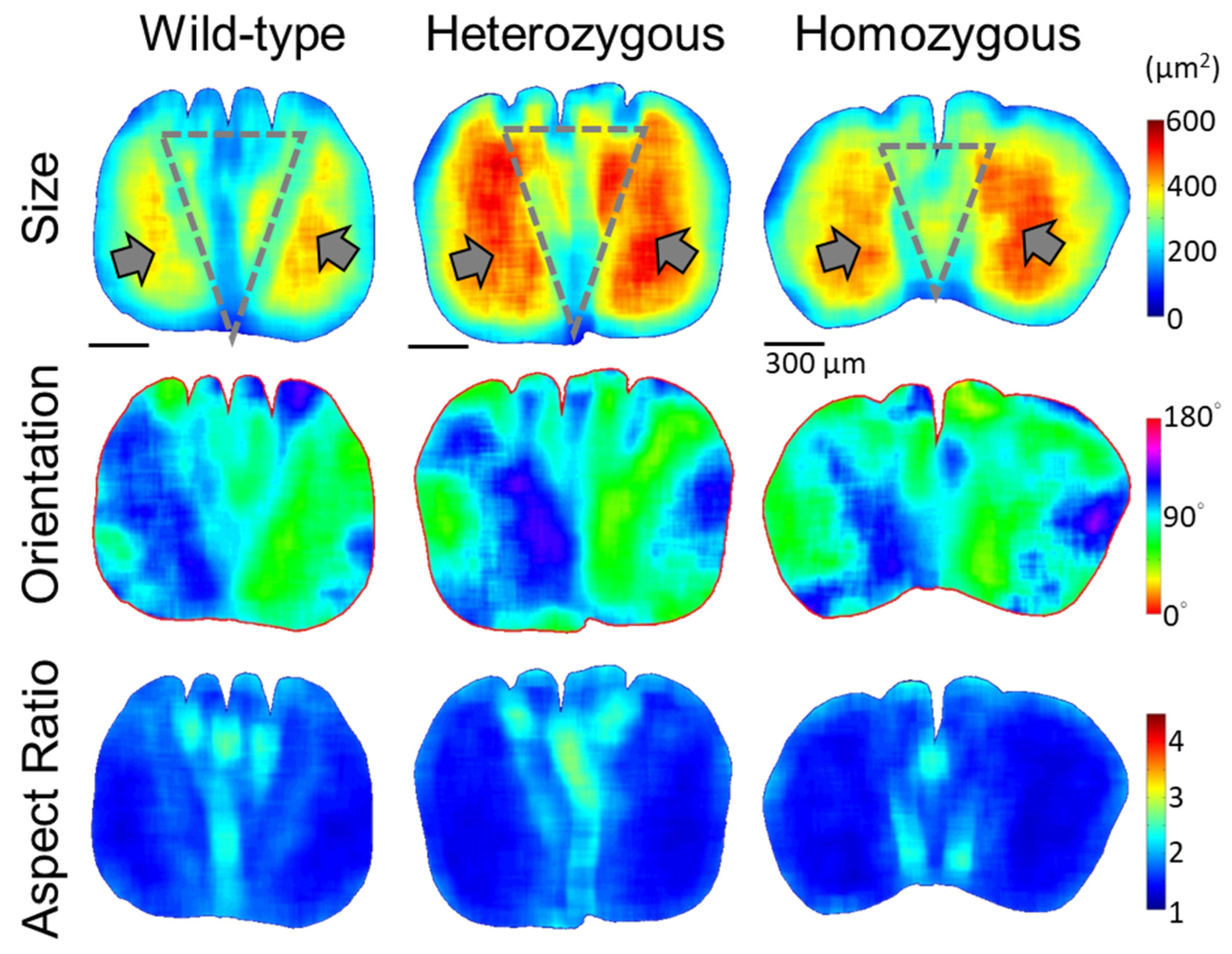
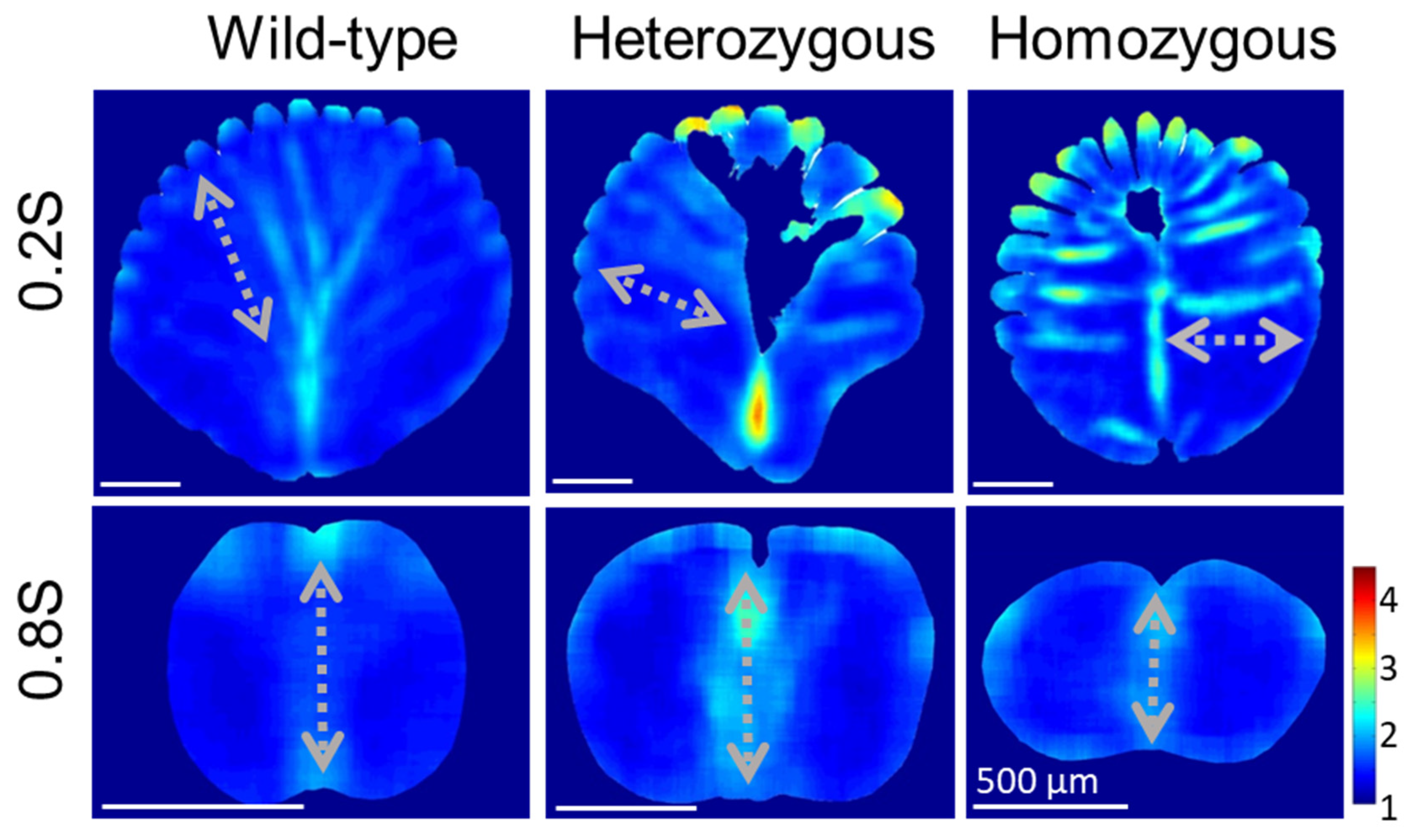
3.4. Quantitative Morphology of Cellular Structures in Rachis Medulla
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prum, R.O. Development and evolutionary origin of feathers. J. Exp. Zool. 1999, 285, 291–306. [Google Scholar] [CrossRef]
- Sullivan, T.N.; Wang, B.; Espinosa, H.D.; Meyers, M.A. Extreme lightweight structures: Avian feathers and bones. Mater. Today 2017, 20, 377–391. [Google Scholar] [CrossRef]
- Prum, R.O.; Brush, A.H. The Evolutionary Origin and Diversification of Feathers. Q. Rev. Biol. 2002, 77, 261–295. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L.; Wu, H.; Chiu, Y.-K.; Wang, S.; Jiang, T.-X.; Luo, Z.-L.; Lin, Y.-C.; Li, A.; Hsu, J.-T.; Huang, H.-L.; et al. The Making of a Flight Feather: Bio-architectural Principles and Adaptation. Cell 2019, 179, 1409–1423.e17. [Google Scholar] [CrossRef] [PubMed]
- Lingham-Soliar, T.; Bonser, R.H.C.; Wesley-Smith, J. Selective biodegradation of keratin matrix in feather rachis reveals classic bioengineering. Proc. R. Soc. B Biol. Sci. 2010, 277, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Han, B.; Xu, Y.; Guo, E.; Sun, B.; Zeng, Y.; Hou, H.; Wu, S. Anisotropic Composition and Mechanical Behavior of a Natural Thin-Walled Composite: Eagle Feather Shaft. Polymers 2022, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Prum, R.O.; Dyck, J. A hierarchical model of plumage: Morphology, development, and evolution. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298B, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.S.; Chadha, C.; Velasco-Hogan, A.; Barbosa, J.D.V.; Jasiuk, I.; Meyers, M.A. Engineering with keratin: A functional material and a source of bioinspiration. iScience 2021, 24, 102798. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Wu, P.; Fan, W.-L.; Yan, J.; Chen, C.-K.; Lai, Y.-T.; Wu, S.-M.; Mao, C.-T.; Chen, J.-J.; Lu, M.-Y.J.; et al. Genomic Organization, Transcriptomic Analysis, and Functional Characterization of Avian α- and β-Keratins in Diverse Feather Forms. Genome Biol. Evol. 2014, 6, 2258–2273. [Google Scholar] [CrossRef]
- Feduccia, A. Aerodynamic Model for the Early Evolution of Feathers Provided by Propithecus (Primates, Lemuridae). J. Theor. Biol. 1993, 160, 159–164. [Google Scholar] [CrossRef]
- Philip, J.R. The Evolutionary Origin of Feathers. Q. Rev. Biol. 1975, 50, 35–66. [Google Scholar]
- Dyck, J. The Evolution of Feathers*. Zool. Scr. 1985, 14, 137–154. [Google Scholar] [CrossRef]
- Osváth, G.; Vincze, O.; David, D.-C.; Nagy, L.J.; Lendvai, Á.Z.; Nudds, R.L.; Pap, P.L. Morphological characterization of flight feather shafts in four bird species with different flight styles. Biol. J. Linn. Soc. 2020, 131, 192–202. [Google Scholar] [CrossRef]
- Terrill, R.S.; Shultz, A.J. Feather function and the evolution of birds. Biol. Rev. 2023, 98, 540–566. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.-M.; Homberger, D.G. Development and evolution of the amniote integument: Current landscape and future horizon. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298B, 1–11. [Google Scholar] [CrossRef]
- Chuong, C.-M.; Chodankar, R.; Widelitz, R.B.; Jiang, T.-X. Evo-Devo of feathers and scales: Building complex epithelial appendages: Commentary. Curr. Opin. Genet. Dev. 2000, 10, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.H.; Knapp, L.W. Avian skin development and the evolutionary origin of feathers. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298B, 57–72. [Google Scholar] [CrossRef]
- Lin, C.-M.; Jiang, T.X.; Widelitz, R.B.; Chuong, C.-M. Molecular signaling in feather morphogenesis. Curr. Opin. Cell Biol. 2006, 18, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Prum, R.O. Evolution of the morphological innovations of feathers. J. Exp. Zool. Part B Mol. Dev. Evol. 2005, 304B, 570–579. [Google Scholar] [CrossRef]
- Prum, R.O.; Williamson, S. Theory of the growth and evolution of feather shape. J. Exp. Zool. 2001, 291, 30–57. [Google Scholar] [CrossRef]
- Widelitz, R.B.; Jiang, T.X.; Yu, M.; Shen, T.; Shen, J.-Y.; Wu, P.; Yu, Z.; Chuong, C.-M. Molecular biology of feather morphogenesis: A testable model for evo-devo research. J. Exp. Zool. Part B Mol. Dev. Evol. 2003, 298B, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Widelitz, R.B.; Veltmaat, J.M.; Mayer, J.A.; Foley, J.; Chuong, C.-M. Mammary glands and feathers: Comparing two skin appendages which help define novel classes during vertebrate evolution. Semin. Cell Dev. Biol. 2007, 18, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hou, L.; Plikus, M.; Hughes, M.; Scehnet, J.; Suksaweang, S.; Widelitz, R.; Jiang, T.X.; Chuong, C.M. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004, 48, 249–270. [Google Scholar] [PubMed]
- Yu, M.; Wu, P.; Widelitz, R.B.; Chuong, C.M. The morphogenesis of feathers. Nature 2002, 420, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yue, Z.; Wu, P.; Wu, D.Y.; Mayer, J.A.; Medina, M.; Widelitz, R.B.; Jiang, T.X.; Chuong, C.M. The developmental biology of feather follicles. Int. J. Dev. Biol. 2004, 48, 181–191. [Google Scholar]
- Sawyer, R.H.; Rogers, L.; Washington, L.; Glenn, T.C.; Knapp, L.W. Evolutionary origin of the feather epidermis. Dev. Dyn. 2005, 232, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Wu, P.; Foley, J.; Foley, A.; McDonald, M.L.; Juan, W.T.; Huang, C.J.; Lai, Y.T.; Lo, W.S.; Chen, C.F.; et al. The chicken frizzle feather is due to an α-keratin (KRT75) mutation that causes a defective rachis. PLoS Genet. 2012, 8, e1002748. [Google Scholar] [CrossRef]
- Chen, C.-F.; Foley, J.; Tang, P.-C.; Li, A.; Jiang, T.X.; Wu, P.; Widelitz, R.B.; Chuong, C.M. Development, Regeneration, and Evolution of Feathers. Annu. Rev. Anim. Biosci. 2015, 3, 169–195. [Google Scholar] [CrossRef]
- Filshie, B.K.; Rogers, G.E. An electron microscope study of the fine structure of feather keratin. J. Cell Biol. 1962, 13, 1–12. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; MacRae, T.P.; Parry, D.A.D.; Suzuki, E. The structure of feather keratin. Polymer 1971, 12, 35–56. [Google Scholar] [CrossRef]
- Chuang, T.C.; Cheng, J.-W.; Chuong, C.-M.; Juan, W.-T. Autofluorescence microscopy as a non-invasive probe to characterize the complex mechanical properties of keratin-based integumentary organs: A feather paradigm. Chin. J. Phys. 2023, 86, 561–571. [Google Scholar] [CrossRef]
- Dong, J.; He, C.; Wang, Z.; Li, Y.; Li, S.; Tao, L.; Chen, J.; Li, D.; Yang, F.; Li, N.; et al. A novel deletion in KRT75L4 mediates the frizzle trait in a Chinese indigenous chicken. Genet. Sel. Evol. 2018, 50, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xi, S.; El-Senousey, H.K.; Zhou, M.; Cheng, D.; Chen, K.; Wan, L.; Xiong, T.; Liao, M.; Liu, S.; et al. Deletion in KRT75L4 linked to frizzle feather in Xiushui Yellow Chickens. Anim. Genet. 2022, 53, 101–107. [Google Scholar] [CrossRef]
- Wu, P.; Ng, C.S.; Yan, J.; Lai, Y.-C.; Chen, C.-K.; Lai, Y.-T.; Wu, S.-M.; Chen, J.-J.; Luo, W.; Widelitz, R.B.; et al. Topographical mapping of α- and β-keratins on developing chicken skin integuments: Functional interaction and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2015, 112, E6770–E6779. [Google Scholar] [CrossRef]
- Aerts, J.; Crooijmans, R.; Cornelissen, S.; Hemmatian, K.; Veenendaal, T.; Jaadar, A.; van der Poel, J.; Fillon, V.; Vignal, A.; Groenen, M. Integration of chicken genomic resources to enable whole-genome sequencing. Cytogenet. Genome Res. 2004, 102, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.W. The chicken genome and the developmental biologist. Mech. Dev. 2004, 121, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.W.; White, S.J. Avian genomics in the 21st century. Cytogenet. Genome Res. 2007, 117, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, J.B. Chicken genome sequence: A centennial gift to poultry genetics. Cytogenet. Genome Res. 2004, 102, 291–296. [Google Scholar] [CrossRef]
- Dequéant, M.-L.; Pourquié, O. Chicken genome: New tools and concepts. Dev. Dyn. 2005, 232, 883–886. [Google Scholar] [CrossRef]
- Burt, D.; Pourquie, O. Chicken Genome–Science Nuggets to Come Soon. Science 2003, 300, 1669. [Google Scholar] [CrossRef]
- Antin, P.B.; Konieczka, J.H. Genomic resources for chicken. Dev. Dyn. 2005, 232, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.W. Chicken genomics charts a path to the genome sequence. Brief. Funct. Genom. Proteomic 2004, 3, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.W. Comparative mapping in farm animals. Brief. Funct. Genom. Proteomic 2002, 1, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Cogburn, L.A.; Porter, T.E.; Duclos, M.J.; Simon, J.; Burgess, S.C.; Zhu, J.J.; Cheng, H.H.; Dodgson, J.B.; Burnside, J. Functional Genomics of the Chicken—A Model Organism. Poult. Sci. 2007, 86, 2059–2094. [Google Scholar] [CrossRef] [PubMed]
- de Koning, D.J.; Cabrera, C.P.; Haley, C.S. Genetical Genomics: Combining Gene Expression with Marker Genotypes in Poultry. Poult. Sci. 2007, 86, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chiu, Y.K.; Tsai, J.C.; Chuong, C.M.; Juan, W.T. A quantitative image-based protocol for morphological characterization of cellular solids in feather shafts. STAR Protoc. 2021, 2, 100661. [Google Scholar] [CrossRef]
- Lucas, A.M. Avian Anatomy Integument; Avian Anatomy Project, Poultry Research Branch, Animal Science Research Division, Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1972. [Google Scholar]
- Harland, D.P.; Vernon, J.A.; Woods, J.L.; Nagase, S.; Itou, T.; Koike, K.; Scobie, D.A.; Grosvenor, A.J.; Dyer, J.M.; Clerens, S. Intrinsic curvature in wool fibres is determined by the relative length of orthocortical and paracortical cells. J. Exp. Biol. 2018, 221, jeb172312. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Chuang, T.-C.; Liao, W.-C.; Chi, K.-J.; Ng, C.-S.; Cheng, H.-C.; Juan, W.-T. Modification of Keratin Integrations and the Associated Morphogenesis in Frizzling Chicken Feathers. Biology 2024, 13, 464. https://doi.org/10.3390/biology13070464
Wu H, Chuang T-C, Liao W-C, Chi K-J, Ng C-S, Cheng H-C, Juan W-T. Modification of Keratin Integrations and the Associated Morphogenesis in Frizzling Chicken Feathers. Biology. 2024; 13(7):464. https://doi.org/10.3390/biology13070464
Chicago/Turabian StyleWu, Hao, Tsao-Chi Chuang, Wan-Chi Liao, Kai-Jung Chi, Chen-Siang Ng, Hsu-Cheng Cheng, and Wen-Tau Juan. 2024. "Modification of Keratin Integrations and the Associated Morphogenesis in Frizzling Chicken Feathers" Biology 13, no. 7: 464. https://doi.org/10.3390/biology13070464
APA StyleWu, H., Chuang, T.-C., Liao, W.-C., Chi, K.-J., Ng, C.-S., Cheng, H.-C., & Juan, W.-T. (2024). Modification of Keratin Integrations and the Associated Morphogenesis in Frizzling Chicken Feathers. Biology, 13(7), 464. https://doi.org/10.3390/biology13070464





