Bone Incorporation of a Poly (L-Lactide-Co-D, L-Lactide) Internal Fixation Device in a Rat’s Tibia: Microtomographic, Confocal LASER, and Histomorphometric Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. PLDLLA Device
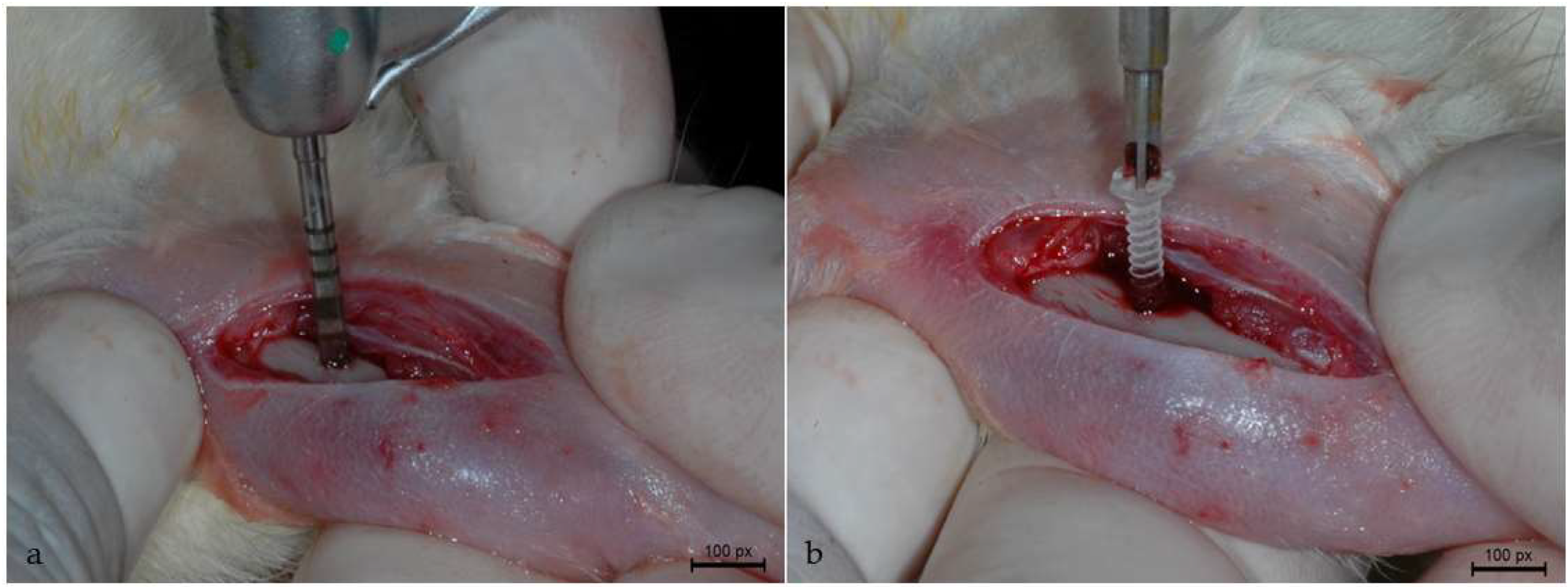
2.3. Surgical Procedure
2.4. Fluorochrome Application
2.5. Euthanasia and Sample Extraction
2.6. Micro-computed Tomography Analysis (Micro-CT)
2.7. Laboratory Processing
2.8. LASER Confocal Microscopy
2.9. Histomorphometric Analysis
2.10. Statistical Analysis
3. Results
3.1. Micro-CT Analysis
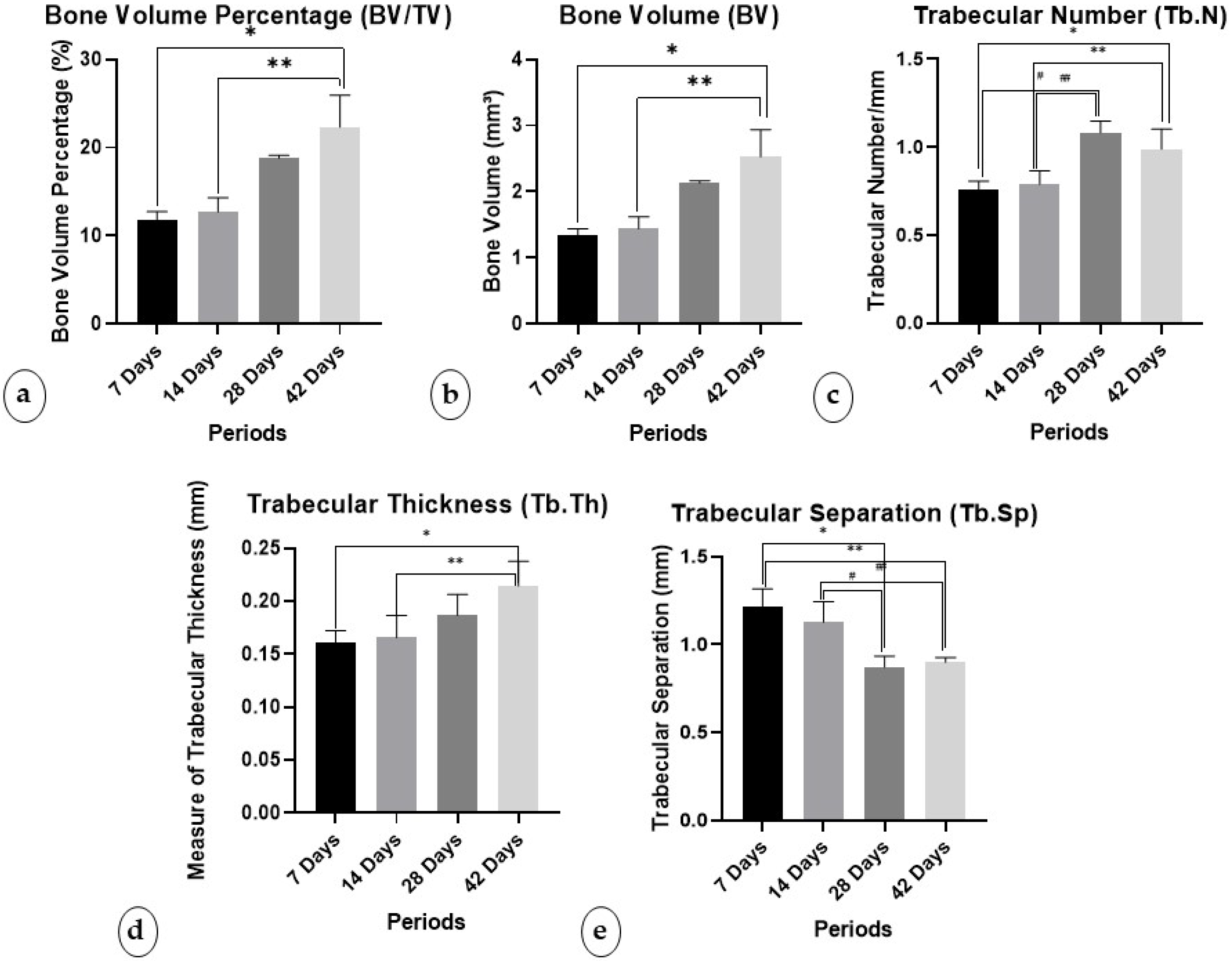
3.1.1. Percentage of Bone Volume/Trabecular Volume (BV/TV)
3.1.2. Bone Volume (BV)
3.1.3. Trabecular Number (Tb.N)
3.1.4. Trabecular Thickness (Tb.Th)
3.1.5. Trabecular Separation (Tb.Sp)
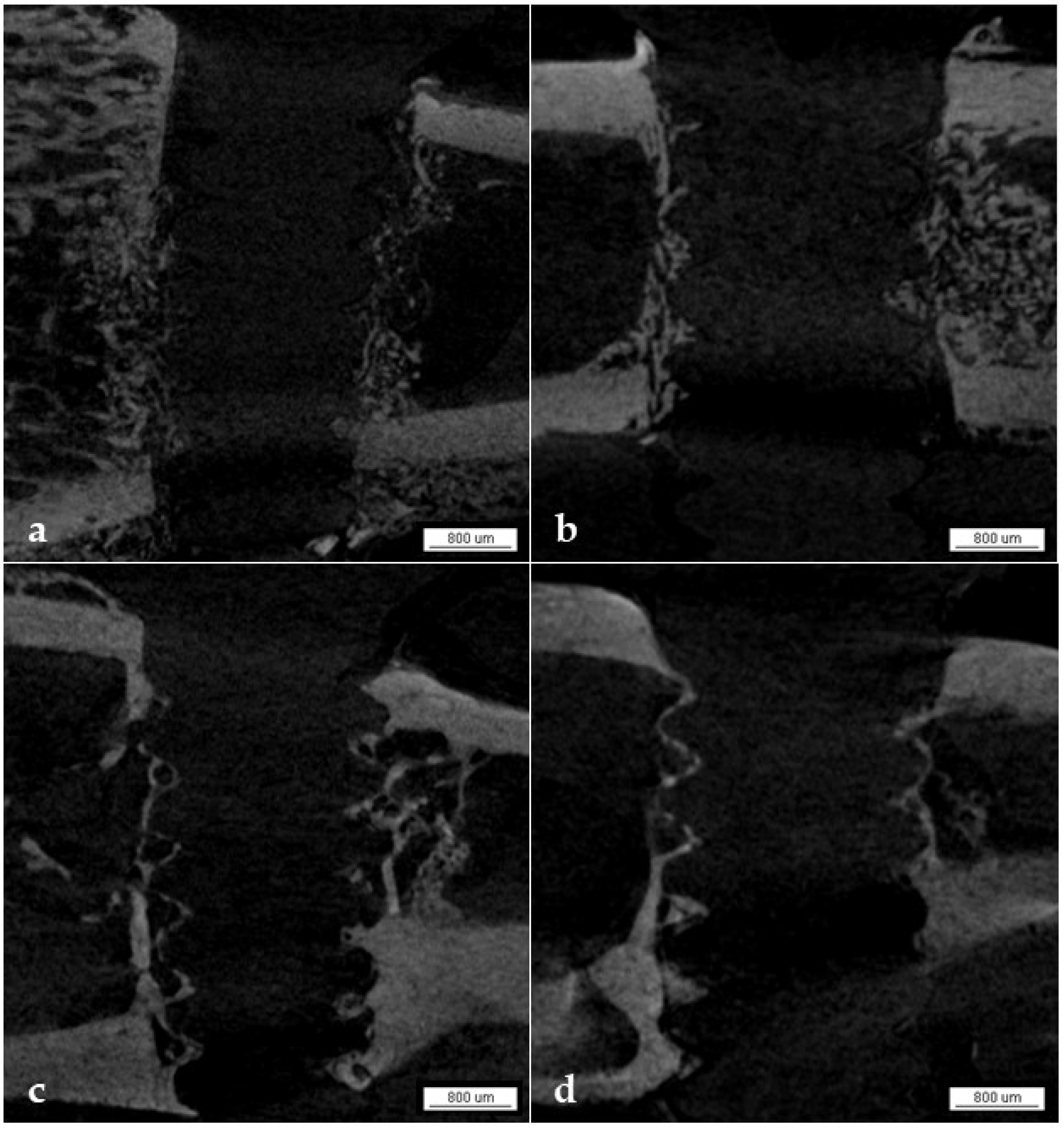
3.1.6. Qualitative Analysis
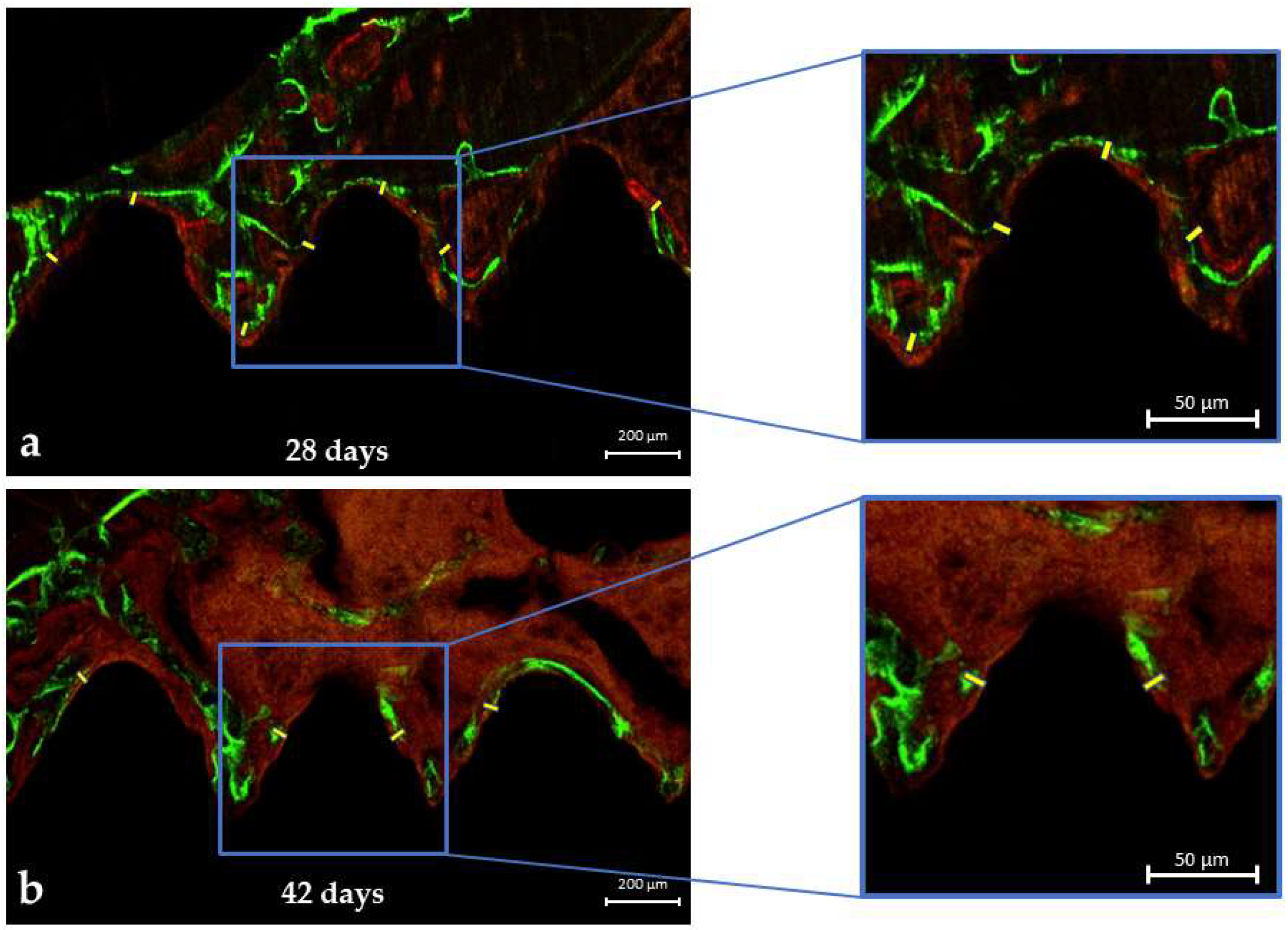
3.2. LASER Confocal Microscopy
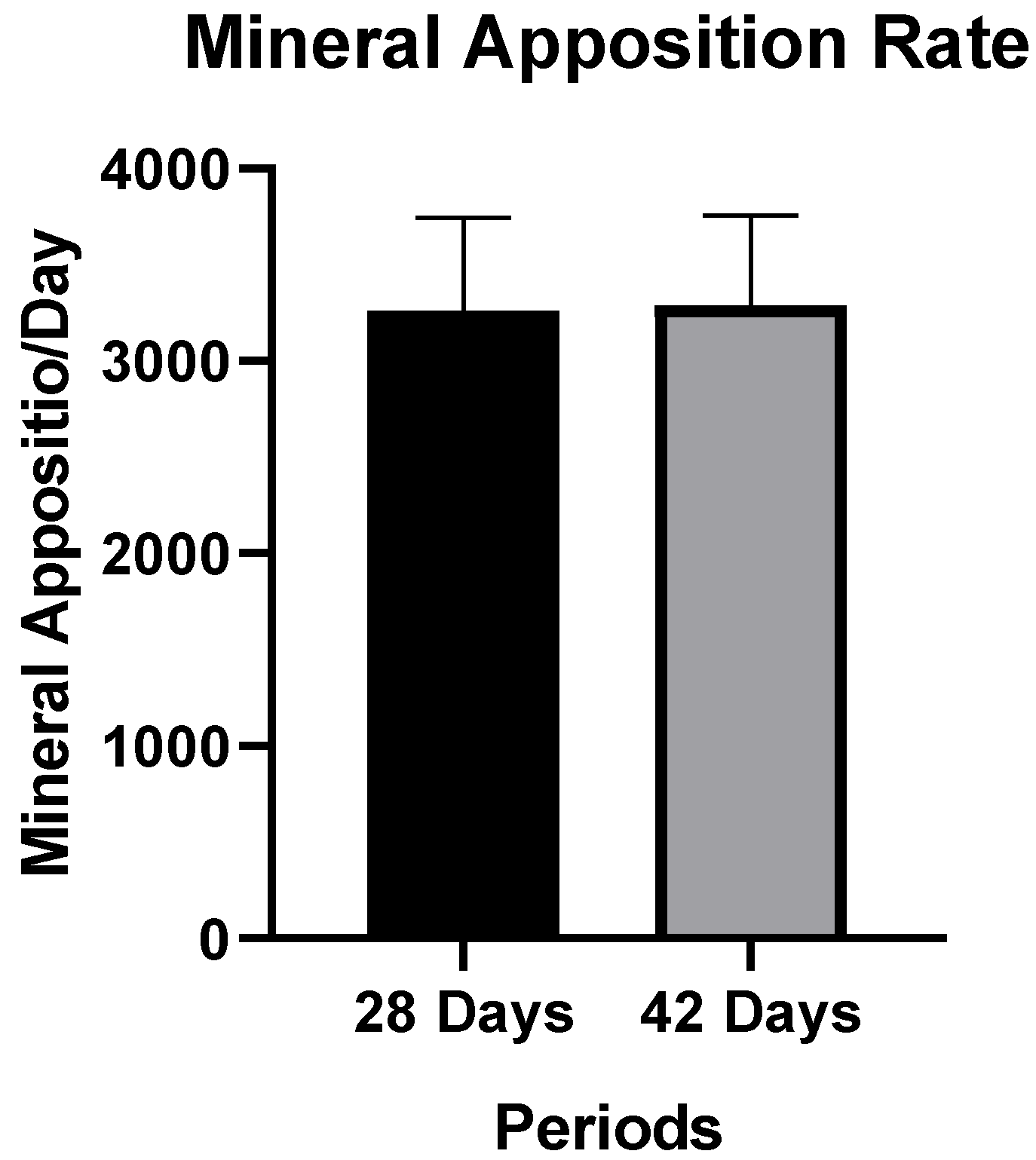
Mineral Apposition Rate (MAR)
3.3. Histomorphometric Analysis
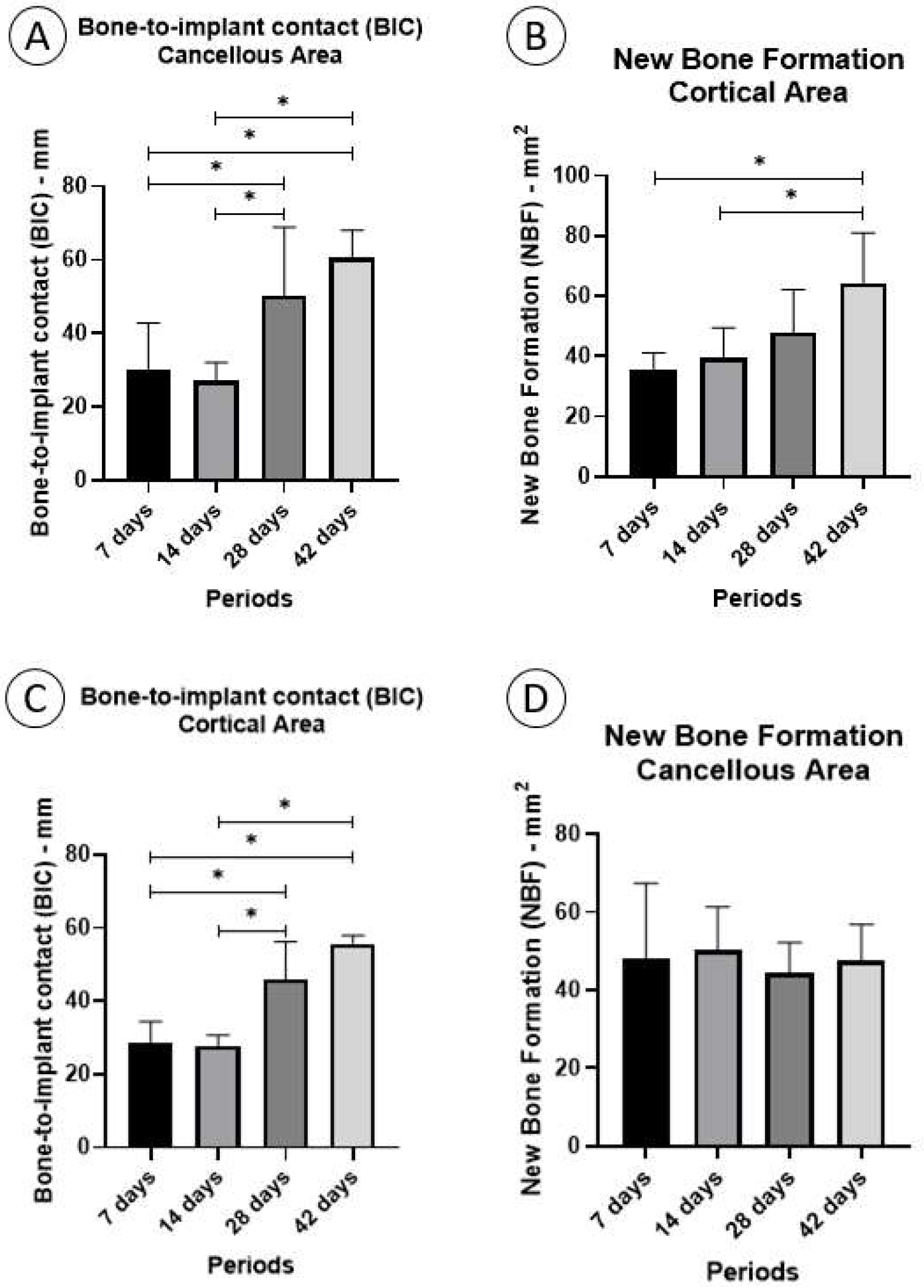
3.3.1. Fixation Device/Bone Contact (BIC%)
Cortical Area
3.3.2. Cancellous Area
3.3.3. New Bone Formation (NBF%)
Cortical Area
3.3.4. Cancellous Area
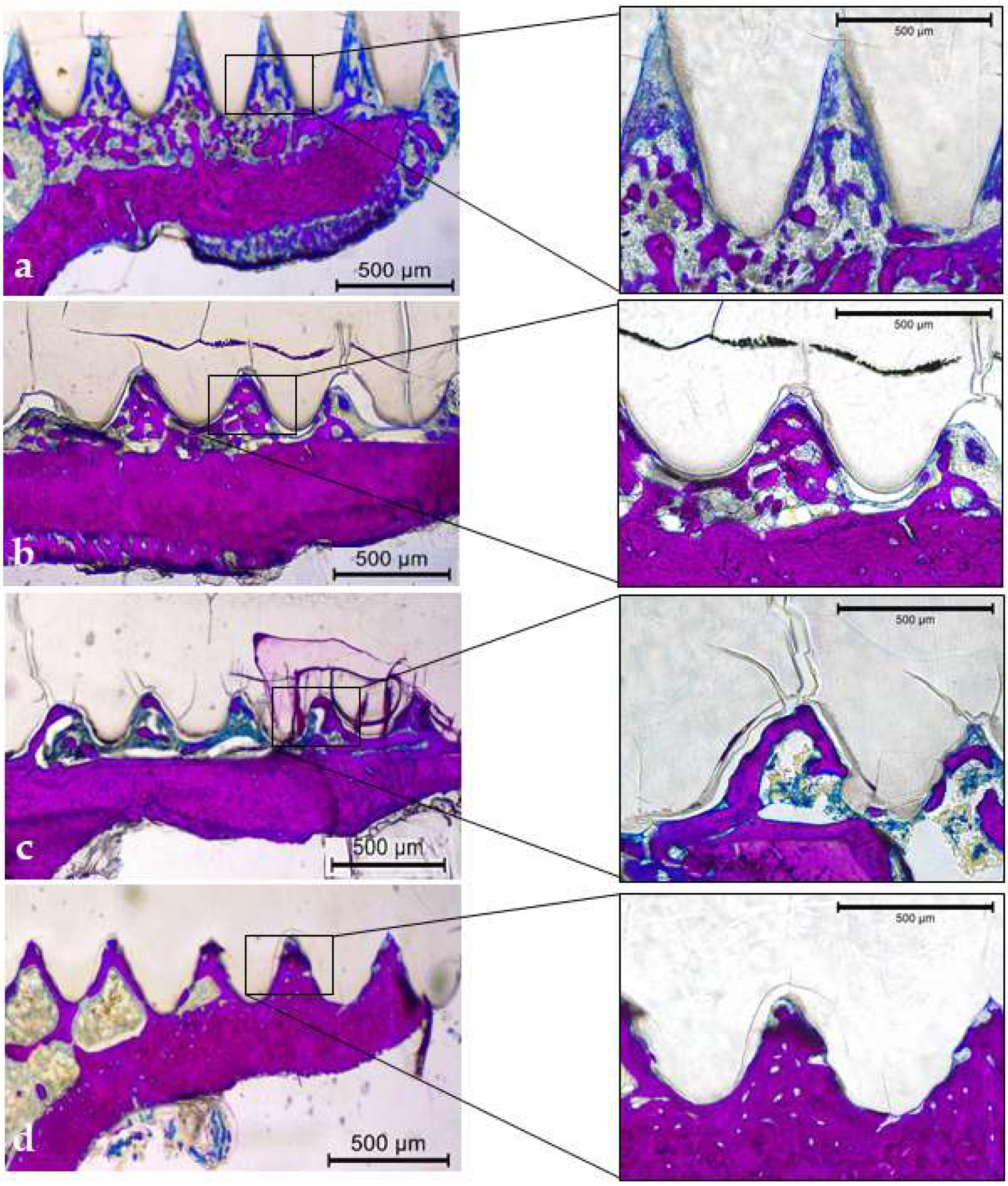
3.3.5. Qualitative Descriptive Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koutserimpas, C.; Alpantaki, K.; Chatzinikolaidou, M.; Chlouverakis, G.; Dohm, M.; Hadjipavlou, A.G. The effectiveness of biodegradable instrumentation in the treatment of spinal fractures. Injury 2018, 49, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Nastri, L.; Cecoro, G.; Guida, L. The Use of Poly-d,l-lactic Acid (PDLLA) Devices for Bone Augmentation Techniques: A Systematic Review. Molecules 2017, 22, 2214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, S.; Seo, S.B.; Kim, G.; Batsukh, S.; Son, K.H.; Byun, K. Poly-D,L-Lactic Acid Stimulates Angiogenesis and Collagen Synthesis in Aged Animal Skin. Int. J. Mol. Sci. 2023, 24, 7986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lazennec, J.Y.; Madi, A.; Rousseau, M.A.; Roger, B.; Saillant, G. Evaluation of the 96/4 PLDLLA polymer resorbable lumbar interbody cage in a long term animal model. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2006, 15, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Burstein, F.D. Resorbable distraction of the mandible: Technical evolution and clinical experience. J. Craniofac. Surg. 2008, 19, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Abd Alsaheb, R.A.; Aladdin, A.; Othman, N.Z.; Abd Malek, R.; Leng, O.M.; Aziz, R.; El Enshasy, H.A. Recent applications of polylactic acid in pharmaceutical and medical industries. J. Chem. Pharm. Res. 2015, 7, 51–63. [Google Scholar]
- Mazzonetto, R.; Paza, A.O.; Spagnoli, D.B. A retrospective evaluation of rigid fixation in orthognathic surgery using a biodegradable self-reinforced (70L:30DL) polylactide. Int. J. Oral Maxillofac. Surg. 2004, 33, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S. Heat adaptation of bioabsorbable craniofacial plates: A critical review of science and technology. J. Craniofac. Surg. 2009, 20, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S. Principles of development and use of absorbable internal fixation. Tissue Eng. 2000, 6, 425–433. [Google Scholar] [CrossRef]
- Eppley, B.L. Use of resorbable plates and screws in pediatric facial fractures. J. Oral Maxillofac. Surg. 2005, 63, 385–391. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngo, H.X.; Bai, Y.; Sha, J.; Ishizuka, S.; Toda, E.; Osako, R.; Kato, A.; Morioka, R.; Ramanathan, M.; Tatsumi, H.; et al. A narrative review of u-HA/PLLA, a bioactive resorbable reconstruction material: Applications in oral and maxillofacial surgery. Materials 2021, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Naseem, R.; Tzivelekis, C.; German, M.J.; Gentile, P.; Ferreira, A.M.; Dalgarno, K. Strategies for Enhancing Polyester-Based Materials for Bone Fixation Applications. Molecules 2021, 26, 992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Błaszczyk, B.; Kaspera, W.; Ficek, K.; Kajor, M.; Binkowski, M.; Stodolak-Zych, E.; Grajoszek, A.; Stojko, J.; Bursig, H.; Ładziński, P. Effects of Polylactide Copolymer Implants and Platelet-Rich Plasma on Bone Regeneration within a Large Calvarial Defect in Sheep. Biomed. Res. Int. 2018, 2018, 4120471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Xie, X.; Jiang, W.; Zhan, Y.; Zhang, Y.; Guo, Y.; Wang, Z.; Guo, N.; Guo, K.; Sun, J. Osteoinductive micro-nano guided bone regeneration membrane for in situ bone defect repair. Stem. Cell Res. Ther. 2024, 15, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de França, J.O.C.; da Silva Valadares, D.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers 2022, 14, 2317. [Google Scholar] [CrossRef] [PubMed]
- Bessho, K.; Fujimura, K.; Iizuka, T. Experimental long-term study of titanium ions eluted from pure titanium miniplates. J. Biomed. Mater. Res. 1995, 29, 901–904. [Google Scholar] [CrossRef]
- Böstman, O.M. Absorbable implants for the fixation of fractures. J. Bone Jt. Surg. Am. 1991, 73, 148–153. [Google Scholar] [CrossRef]
- NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. J. Physiol. 2010, 14, 2519–2521. [Google Scholar]
- Ramalho-Ferreira, G.; Faverani, L.P.; Prado, F.B.; Garcia, I.R., Jr.; Okamoto, R. Raloxifene enhances peri-implant bone healing in osteoporotic rats. Int. J. Oral Maxillofac. Surg. 2015, 44, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.D.; Hassumi, J.S.; Gomes-Ferreira, P.H.D.S.; Polo, T.O.B.; Ferreira, G.R.; Faverani, L.P.; Okamoto, R. Short term sodium alendronate administration improves the peri-implant bone quality in osteoporotic animals. J. Appl. Oral Sci. 2017, 25, 42–52. [Google Scholar] [CrossRef]
- Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Momesso, G.A.C.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Luvizuto, E.R.; Gruber, R.; et al. Raloxifene but not alendronate can compensate the impaired osseointegration in osteoporotic rats. Clin. Oral Investig. 2018, 22, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Palin, L.P.; Polo, T.O.B.; Batista, F.R.D.S.; Gomes-Ferreira, P.H.S.; Garcia Junior, I.R.; Rossi, A.C.; Freire, A.; Faverani, L.P.; Sumida, D.H.; Okamoto, R. Daily melatonin administration improves osseointegration in pinealectomized rats. J. Appl. Oral Sci. 2018, 26, e20170470. [Google Scholar] [CrossRef]
- Mulinari-Santos, G.; Souza Batista, F.R.; Kirchweger, F.; Tangl, S.; Gruber, R.; Okamoto, R. Losartan reverses impaired osseointegration in spontaneously hypertensive rats. Clin. Oral Implant. Res. 2018, 29, 1126–1134. [Google Scholar] [CrossRef]
- Luvizuto, E.R.; Dias, S.S.; Okamoto, T.; Dornelles, R.C.; Okamoto, R. Raloxifene therapy inhibits osteoclastogenesis during the alveolar healing process in rats. Arch. Oral Biol. 2011, 56, 984–990. [Google Scholar] [CrossRef]
- Ramalho-Ferreira, G.; Faverani, L.P.; Grossi-Oliveira, G.A.; Okamoto, T.; Okamoto, R. Alveolar bone dynamics in osteoporotic rats treated with raloxifene or alendronate: Confocal microscopy analysis. J. Biomed. Opt. 2015, 20, 038003. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Souza, F.Á.; Furtado, T.S.M.; Dayube, U.R.C.; Melo, W.M.; Nishioka, R.S.; Poli, P.P.; Maiorana, C.; de Carvalho, P.S.P. Comparative in vivo study of alloy titanium implants with two different surfaces: Biomechanical and SEM analysis. Clin. Oral Investig. 2019, 23, 4383–4397. [Google Scholar] [CrossRef] [PubMed]
- Sisti, K.E.; Piattelli, A.; Guastaldi, A.C.; Queiroz, T.P.; De Rossi, R. Nondecalcified histologic study of bone response to titanium implants topographically modified by laser with and without hydroxyapatite coating. Int. J. Periodontics Restor. Dent. 2013, 35, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.A.; Queiroz, T.P.; Sonoda, C.K.; Okamoto, R.; Margonar, R.; Guastaldi, A.C.; Nishioka, R.S.; Garcia Junior, I.R. Histometric analysis and topographic characterization of cp Ti implants with surfaces modified by laser with and without silica deposition. J. Biomed. Mater. Res. B 2014, 102, 1677–1688. [Google Scholar] [CrossRef]
- Queiroz, T.P.; de Molon, R.S.; Souza, F.Á.; Margonar, R.; Thomazini, A.H.A.; Guastaldi, A.C.; Hochuli-Vieira, E. In vivo evaluation of cp Ti implants with modified surfaces by laser beam with and without hydroxyapatite chemical deposition and without and with thermal treatment: Topographic characterization and histomorphometric analysis in rabbits. Clin. Oral Investig. 2017, 21, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Doll, C.; Thieme, N.; Schönmuth, S.; Voss, J.O.; Nahles, S.; Beck-Broichsitter, B.; Heiland, M.; Raguse, J.D. Enhanced radiographic visualization of resorbable foils for orbital floor reconstruction: A proof of principle. J. Craniomaxillofac. Surg. 2018, 46, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hollier, L.H., Jr.; Sharabi, S.E.; Koshy, J.C.; Stal, S. Facial trauma: General principles of management. J. Craniofac. Surg. 2010, 21, 1051–1053. [Google Scholar] [CrossRef]
- Brożyna, B.; Szymańska, H.; Ptaszyński, K.; Woszczyński, M.; Lechowska-Piskorowska, J.; Gajewska, M.; Rostkowska, J.; Chełmiński, K.; Bulski, W.; Krajewski, R. Tissue response after implantation of pure titanium and bioresorbable screws in scapula with postoperative irradiation: An experimental study on rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 443–452. [Google Scholar] [CrossRef]
- Martínez-Villalobos Castillo, S. Osteosíntesis maxilofacial con titanio. Rev. Esp. Cirug. Oral y Maxilofac. 2004, 26, 351–368. [Google Scholar] [CrossRef]
- Gareb, B.; Van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; De Visscher, J.G.A.M.; Hoppenreijs, T.J.; Bergsma, J.E.; Van Minnen, B.; Stegenga, B.; Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152. [Google Scholar] [CrossRef] [PubMed]
- Prokop, A.; Höfl, A.; Hellmich, M.; Jubel, A.; Andermahr, J.; Emil Rehm, K.; Hahn, U. Degradation of poly-L/DL-lactide versus TCP composite pins: A three-year animal study. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Smit, T.H.; Krijnen, M.R.; van Dijk, M.; Wuisman, P.I. Application of polylactides in spinal cages: Studies in a goat model. J. Mater. Sci. Mater. Med. 2006, 17, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, V.; Suuronen, R.; Teittinen, M.; Nurmenniemi, P. Comparison of the stability of bioabsorbable and titanium osteosynthesis materials for rigid internal fixation in orthognathic surgery. A prospective randomized controlled study in 101 patients with 192 osteotomies. Int. J. Oral Maxillofac. Surg. 2010, 39, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Raghoebar, G.M.; Liem, R.S.; Bos, R.R.; van der Wal, J.E.; Vissink, A. Resorbable screws for fixation of autologous bone grafts. Clin. Oral Implant. Res. 2006, 17, 288–293. [Google Scholar] [CrossRef]
- Olate, S.; Unibazo, A.; Uribe, F.; Alister, J.P.; Martinez, F. Bioresorbable bioactive ostoesynthesys (Hidroxiapatite/Poly L-lactide) in Le Fort Segmented Osteotomy. Int. J. Odontostomat. 2018, 12, 137–141. [Google Scholar] [CrossRef]
- Ferretti, C. A prospective trial of poly-L-lactic/polyglycolic acid co-polymer plates and screws for internal fixation of mandibular fractures. Int. J. Oral Maxillofac. Surg. 2008, 37, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Reilly, M. Degradation characteristics of PLLA-PGA bone fixation devices. J. Craniofac. Surg. 1997, 8, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Yuan, Y.; Jiang, L.Q.; Xia, Y.; Wang, Y.; Xu, S.G.; Zhou, P.Y. Removing a metal foreign object successfully from a patient’s retroperitoneal space using laparoscopy and a novel navigation system. Ann. R Coll. Surg. Engl. 2018, 100, e114–e117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, Y.; Sha, J.; Kanno, T.; Miyamoto, K.; Hideshima, K.; Matsuzaki, Y. Comparison of the bone regenerative capacity of three-dimensional uncalcined and unsintered Hydroxyapatite/Poly-d/l-Lactide and beta-tricalcium phosphate used as bone graft substitutes. J. Invest. Surg. 2021, 34, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Scorsato, P.S.; Rahal, S.C.; Cestari, T.M.; Mamprim, M.J.; Doiche, D.P.; Teixeira, D.D.B.; Siqueira, R.C.; Felix, M. Evaluation of the degradation of two bioabsorbable interference screws: An in-vivo study in sheep. Acta Cir. Bras. 2022, 37, e370405. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Pan, Y.H.; Tzeng, J.J.; Wu, T.L.; Fong, T.H.; Feng, S.W.; Huang, H.M. Development and Testing of X-Ray Imaging-Enhanced Poly-L-Lactide Bone Screws. PLoS ONE 2015, 10, e0140354. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.R.M.; Rozema, F.B.; Boering, G.; Nijenhius, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W.B. Degradation of and tissue reaction to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in rats. Biomaterials 1991, 12, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; Rozema, F.R.; Bos, R.R.; Boering, G.; de Bruijn, W.C.; Pennings, A.J. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polyactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Marumo, K.; Sato, Y.; Suzuki, H.; Kurosaka, D. MRI study of bioabsorbable poly-L-lactic acid devices used for fixation of fracture and osteotomies. J. Orthop. Sci. 2006, 11, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Peiji, W.; Qirong, D.; Jianzhong, Q.; Huayi, W.; Kailong, Z.; Nan, Y. Intramedullary fixation in digital replantation using bioabsorbable poly-DL-lactic acid rods. J. Hand. Surg. Am. 2012, 37, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.J. An investigation of the normal variations in alveolar bone trabeculation. Oral Surg. Oral Med. Oral Pathol. 1962, 15, 1453–1463. [Google Scholar] [CrossRef]
- Emonde, C.K.; Eggers, M.E.; Wichmann, M.; Hurschler, C.; Ettinger, M.; Denkena, B. Radiopacity Enhancements in Polymeric Implant Biomaterials: A Comprehensive Literature Review. ACS Biomater. Sci. Eng. 2024, 10, 1323–1334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamani-Valeriano, H.L.; Silva, N.P.; Nímia, H.H.; Pereira-Silva, M.; Oliveira, M.E.d.F.S.; Rodrigues, L.G.d.S.; Tavares, P.M.H.; Hadad, H.; de Jesus, L.K.; Santos, A.F.P.; et al. Bone Incorporation of a Poly (L-Lactide-Co-D, L-Lactide) Internal Fixation Device in a Rat’s Tibia: Microtomographic, Confocal LASER, and Histomorphometric Analysis. Biology 2024, 13, 471. https://doi.org/10.3390/biology13070471
Mamani-Valeriano HL, Silva NP, Nímia HH, Pereira-Silva M, Oliveira MEdFS, Rodrigues LGdS, Tavares PMH, Hadad H, de Jesus LK, Santos AFP, et al. Bone Incorporation of a Poly (L-Lactide-Co-D, L-Lactide) Internal Fixation Device in a Rat’s Tibia: Microtomographic, Confocal LASER, and Histomorphometric Analysis. Biology. 2024; 13(7):471. https://doi.org/10.3390/biology13070471
Chicago/Turabian StyleMamani-Valeriano, Harrisson Lucho, Nelson Padilha Silva, Heloisa Helena Nímia, Maísa Pereira-Silva, Maria Eduarda de Freitas Santana Oliveira, Letícia Gabriella de Souza Rodrigues, Paulo Matheus Honda Tavares, Henrique Hadad, Laís Kawamata de Jesus, Ana Flávia Piquera Santos, and et al. 2024. "Bone Incorporation of a Poly (L-Lactide-Co-D, L-Lactide) Internal Fixation Device in a Rat’s Tibia: Microtomographic, Confocal LASER, and Histomorphometric Analysis" Biology 13, no. 7: 471. https://doi.org/10.3390/biology13070471










