Morinda citrifolia Essential Oil: A Plant Resistance Biostimulant and a Sustainable Alternative for Controlling Phytopathogens and Insect Pests
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Morinda citrifolia Essential Oil
2.2. Gas Chromatography–Mass Spectrometry (CG-MS)
2.3. Purification of Anadenanthera Colubrina Gum
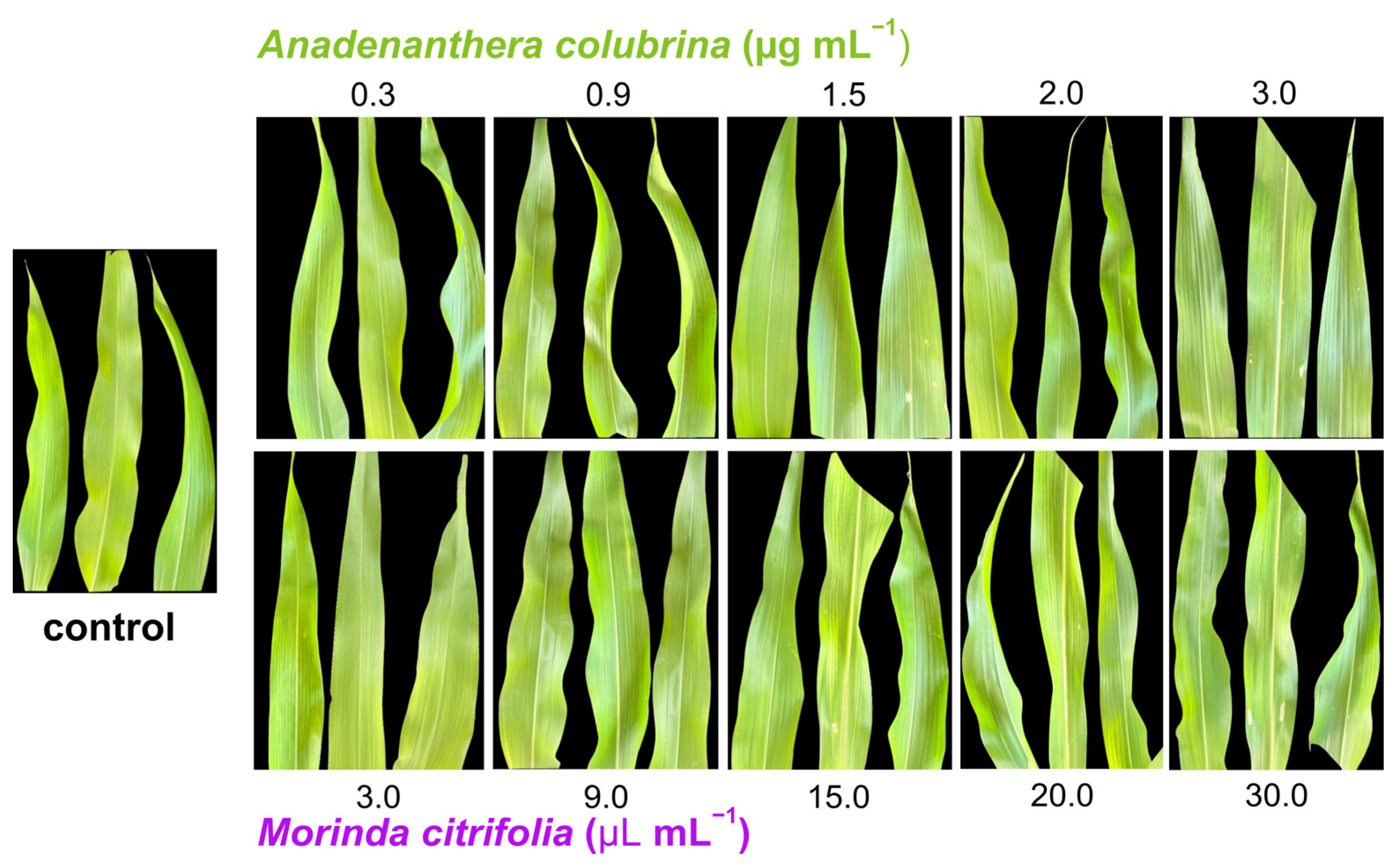
2.4. Phytotoxicity of M. citrifolia and Anadenanthera colubrina in Corn Leaf
2.5. Determination of Biochemical Effects on Corn Plants
2.6. Molecular Docking
2.6.1. Ligands
2.6.2. Target Modeling
2.6.3. Molecular Docking Calculations
2.6.4. Molecular Dynamic Simulations
2.7. Curvularia Leaf Spot Preventative Control
2.8. Inhibition of Mycelial Growth of Curvularia lunatalll
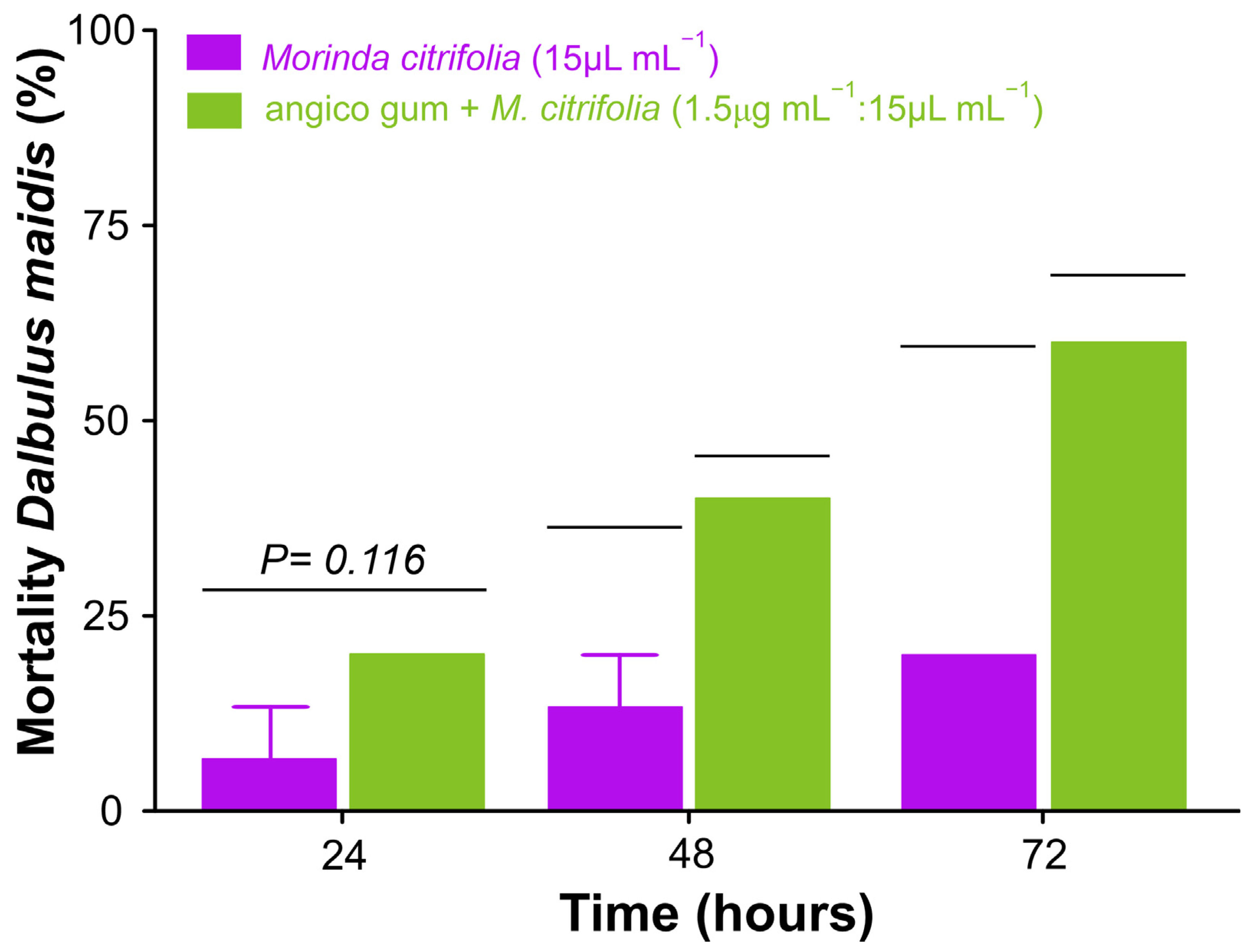
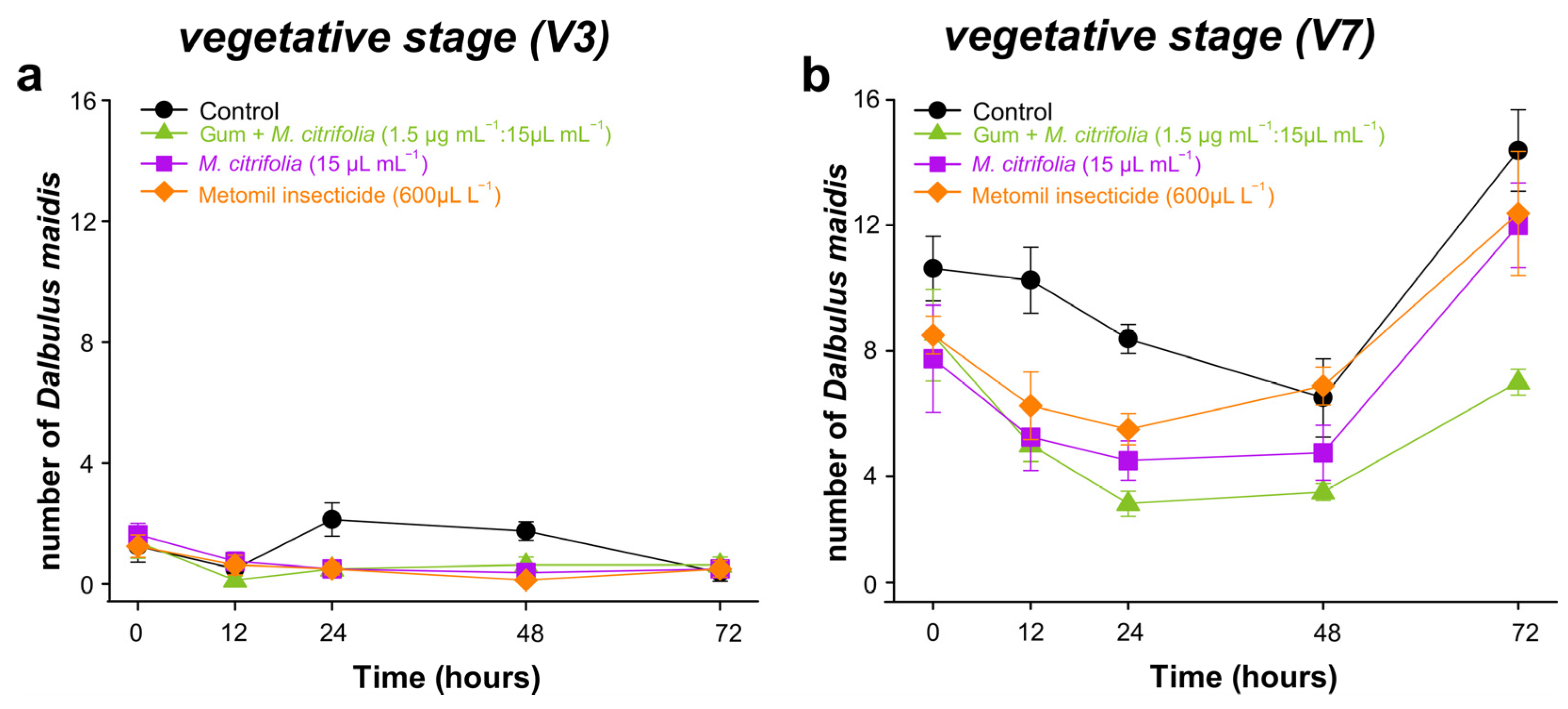
2.9. Essential Oil and Angico Gum Effect on Dalbulus maidis
2.9.1. Toxicity
2.9.2. Field Repellency
2.10. Statistical Analysis
3. Results
3.1. Gas Chromatographic Analysis (GC-MS) of Morinda citrifolia Essential Oil
3.2. Biochemical Effects
3.3. Phytotoxicity of M. citrifolia and Anadenanthera colubrina in Corn Leaf
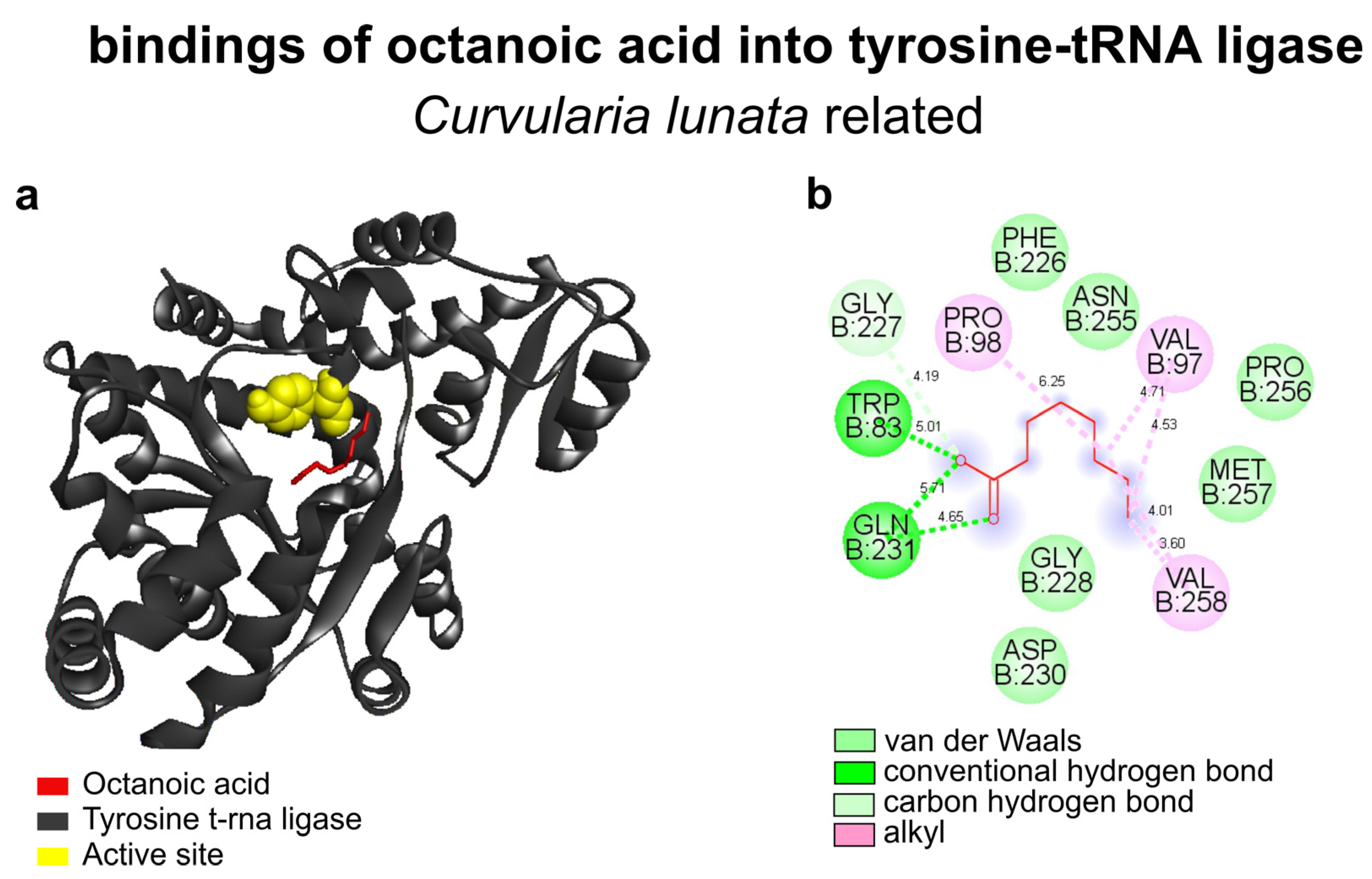
3.4. Molecular Docking
3.4.1. Interactions of Morinda citrifolia Components and Fungal Tyrosine-tRNA Ligase
3.4.2. Molecular Dynamic Simulation
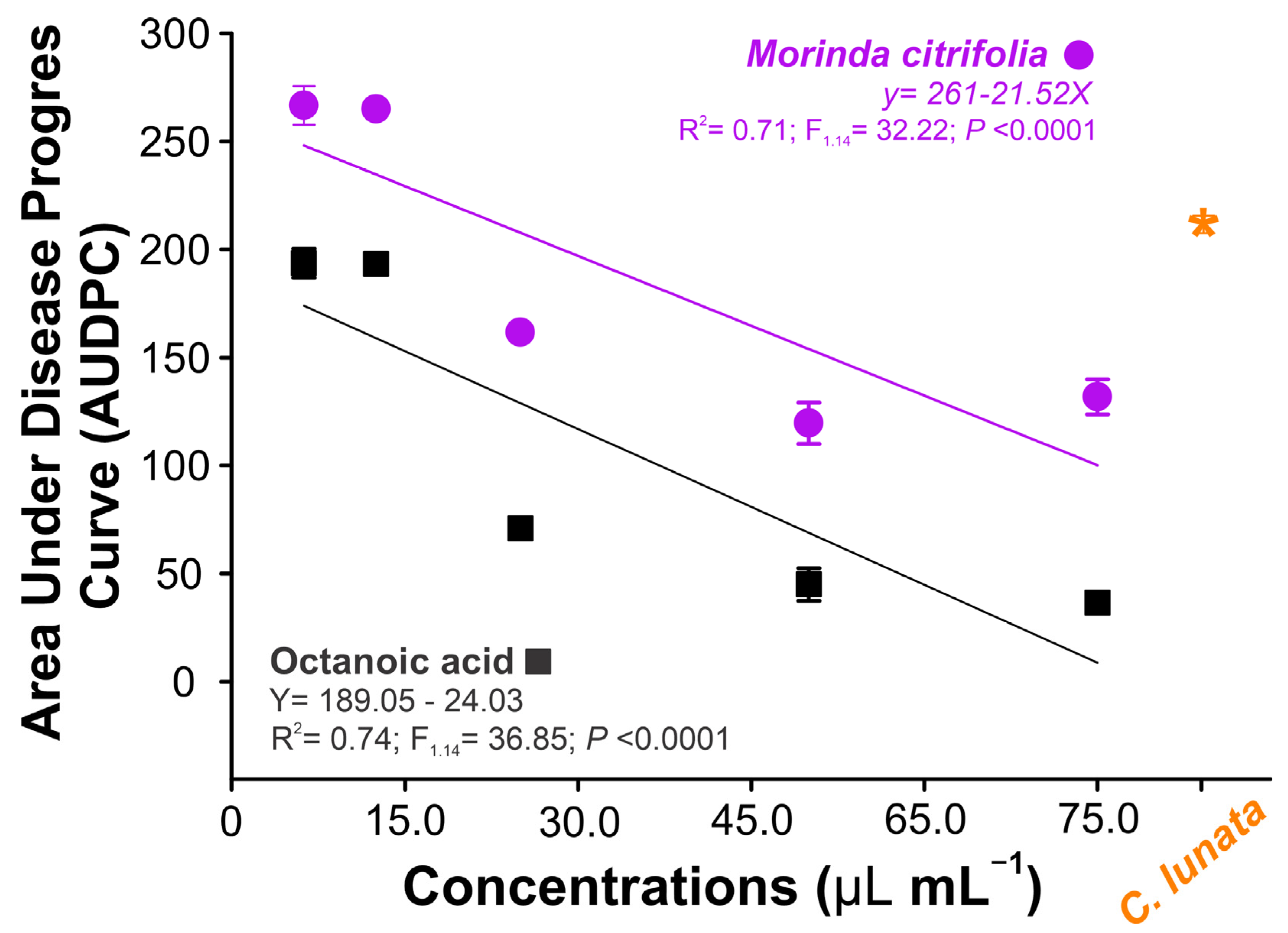
3.5. Curvularia Leaf Spot Control
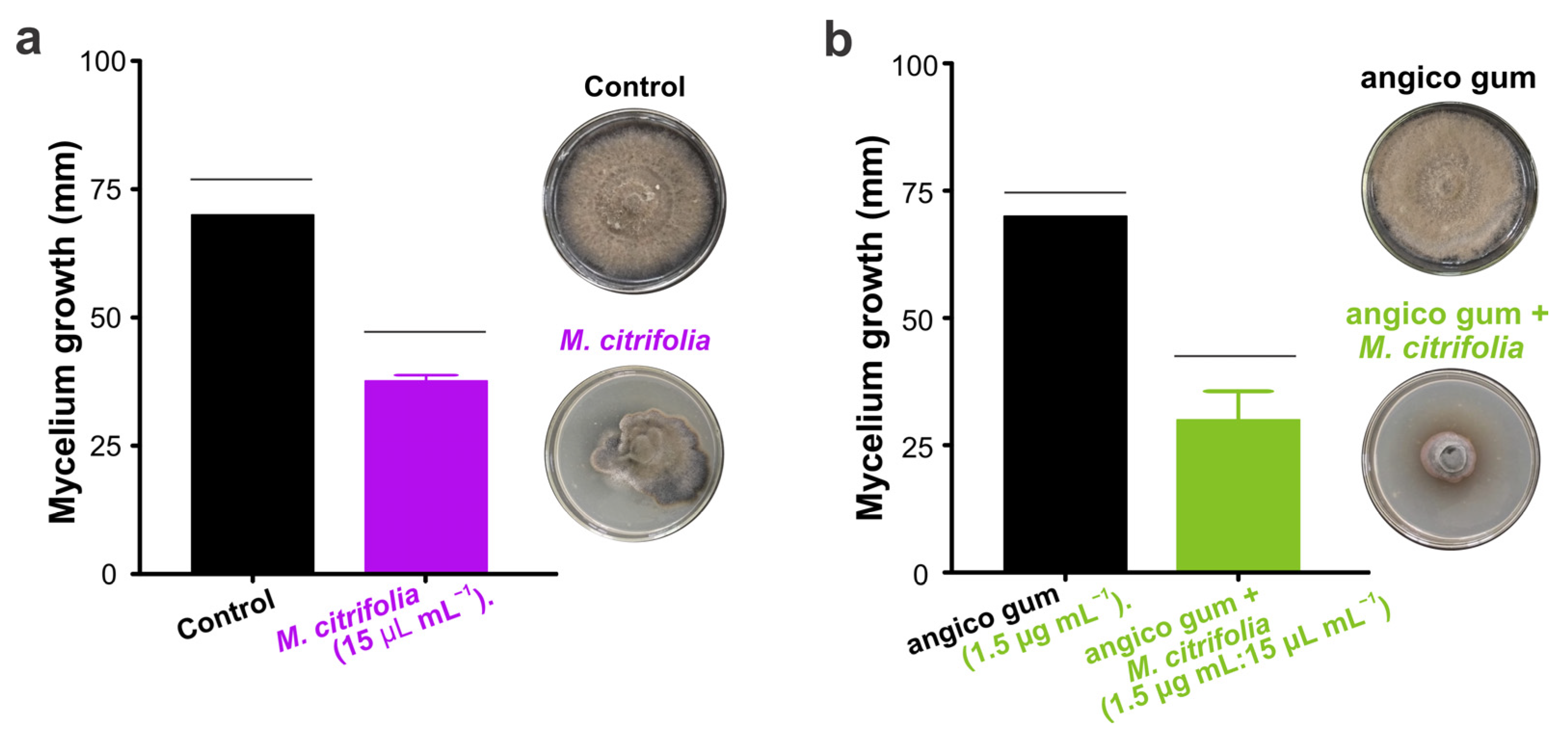
3.6. Effect of Morinda citrifolia Essential Oil and Gum Anadenanthera colubrina on the Development of Curvularia lunata
3.7. Preventive Measure
3.7.1. Toxicity in Field to Vector Dalbulus maidis
3.7.2. Repellence in Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quan, Q.; Yi, F.; Liu, H. Fertilizer response to climate change: Evidence from corn production in China. Sci. Total Environ. 2024, 928, 172226. [Google Scholar] [CrossRef] [PubMed]
- CONAB. Companhia Nacional de Abastecimento. Available online: https://www.conab.gov.br/ (accessed on 24 February 2024).
- Hamzat, O.T.H.; Ganiyu, S.A.; Obembe, O.M.; Ajayi, A.M.; Owolade, O.F. Response of maize (Zea mays L.) cultivars to leaf blight and Curvularia leaf spot under application of Titanium dioxide in forest—Savanna transition agro ecological zone of Nigeria. Arch. Phytopathol. Plant Prot. 2022, 55, 913–925. [Google Scholar] [CrossRef]
- Sabato, E.O.; Landau, E.C.; Barros, B.A.; Oliveira, C.M. Differential transmission of phytoplasma and spiroplasma to maize caused by variation in the environmental temperature in Brazil. Eur. J. Plant Pathol. 2020, 157, 163–171. [Google Scholar] [CrossRef]
- Faria, R.D.; Fanela, T.L.M.; Sartori, M.M.P.; Lopes, J.R.S.; Lourenção, A.L.; Baldin, E.L.L. Evaluation of resistance of Bt and non-Bt maize genotypes to Dalbulus maidis (Hemiptera: Cicadellidae) and associated mollicutes. Phytoparasitica 2022, 50, 997–1009. [Google Scholar] [CrossRef]
- Oliveira, C.M.d.; Frizzas, M.R. Eight decades of Dalbulus maidis (DeLong & Wolcott) (Hemiptera, Cicadellidae) in Brazil: What we know and what we need to know. Neotrop. Entomol. 2022, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Manzar, N.; Kashyap, A.S.; Maurya, A.; Rajawat, M.V.S.; Sharma, P.K.; Srivastava, A.K.; Roy, M.; Saxena, A.K.; Singh, H.V. Multi-Gene phylogenetic approach for identification and diversity analysis of Bipolaris maydis and Curvularia lunata isolates causing foliar blight of Zea mays. J. Fungus 2022, 8, 802. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Venkatesan, R.; Deepa, S.; Sana, S.S.; Arumugam, S.; Karami, A.M.; Vetcher, A.A.; Kim, S.-C. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci. Rep. 2023, 13, 18838. [Google Scholar] [CrossRef] [PubMed]
- Dalcin, M.S.; Dias, B.L.; Viteri Jumbo, L.O.; Oliveira, A.C.S.S.; Araújo, S.H.C.; Moura, W.S.; Mourão, D.S.C.; Ferreira, T.P.S.; Campos, F.S.; Cangussu, A.S.R.; et al. Potential action mechanism and inhibition efficacy of Morinda citrifolia essential oil and octanoic acid against Stagonosporopsis cucurbitacearum infestations. Molecules 2022, 27, 5173. [Google Scholar] [CrossRef]
- Osorio, P.; Dias, F.; Mourão, D.; Araujo, S.; Toledo, P.; Silva, A.C.; Viera, W.; Câmara, M.; Moura, W.; Aguiar, R.; et al. Essential oil of Noni, Morinda citrifolia L., fruits controls the rice stem-rot disease without detrimentally affect beneficial fungi and ladybeetles. Ind. Crops Prod. 2021, 170, 113728. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Oyebamiji, A.K.; Olugbeko, S.C. Mosquito repellent and antibacterial efficiency of facile and low-cost silver nanoparticles synthesized using the leaf extract of Morinda citrifolia. Plasmonics 2021, 16, 1645–1656. [Google Scholar] [CrossRef]
- Perina, F.J.; de Andrade, C.C.L.; Moreira, S.I.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun zeylanicum oil and trans-cinnamaldehyde against Alternaria brown spot in tangerine: Direct effects and induced resistance. Phytoparasitica 2019, 47, 575–589. [Google Scholar] [CrossRef]
- Britto, I.O.; Araújo, S.H.C.; Toledo, P.F.S.; Lima, G.D.A.; Salustiano, I.V.; Alves, J.R.; Mantilla-Afanador, J.G.; Kohlhoff, M.; Oliveira, E.E.; Leite, J.P.V. Potential of Ficus carica extracts against Euschistus heros: Toxicity of major active compounds and selectivity against beneficial insects. Pest Manag. Sci. 2021, 77, 4638–4647. [Google Scholar] [CrossRef] [PubMed]
- Nosé, N.P.; Dalcin, M.S.; Dias, B.L.; Toloy, R.S.; Mourão, D.S.C.; Giongo, M.; Cangussu, A.; da Cruz Araujo, S.H.; dos Santos, G.R. Noni essential oil associated with adjuvants in the production of phytoalexins and in the control of soybean anthracnosis. J. Med. Plant Res. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Dias, B.L.; Dalcin, M.S.; Costa, P.F.d.; Santos, P.R.R.d.; Sousa, R.R.d.; Dias, F.R.; Mourão, D.d.S.C.; Santos, G.R.d. Morinda citrifolia compounds such elicitor substances of biochemical responses in melon plants: Morinda citrifolia compounds such elicitor substances of biochemical responses in melon plants. Comun. Sci. 2023, 14, e3919. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Patricia, M.; Shihabul, A.; Norazlina, M.R.; Ramlah George, M.R.; Noorakmar, A.W.; Lee, J.S.; Jumardi, R.; Jinap, S.; Zaidul, I.S.M. A review on functional and nutritional properties of noni fruit seed (Morinda citrifolia L.) and its oil. Food Biosci. 2021, 41, 101000. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pratibha; Petkoska, A.T.; Singla, M. Natural Gums for Fruits and Vegetables Preservation: A Review. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–37. [Google Scholar]
- Santos, J.S.; Marinho, R.R.; Ekundi-Valentim, E.; Rodrigues, L.; Yamamoto, M.H.; Teixeira, S.A.; Muscara, M.N.; Costa, S.K.; Thomazzi, S.M. Beneficial effects of Anadenanthera colubrina (Vell.) Brenan extract on the inflammatory and nociceptive responses in rodent models. J. Ethnopharmacol. 2013, 148, 218–222. [Google Scholar] [CrossRef]
- Delgobo, C.L.; Gorin, P.A.J.; Jones, C.; Iacomini, M. Gum heteropolysaccharide and free reducing mono- and oligosaccharides of Anadenanthera colubrina. Phytochemistry 1998, 47, 1207–1214. [Google Scholar] [CrossRef]
- Oliveira, R.W.G.; de Oliveira, J.M.; da Paz, F.B.; Muniz, E.C.; de Moura, E.M.; Costa, J.C.S.; do Nascimento, M.O.; Carvalho, A.L.M.; Pinheiro, I.M.; Mendes, A.N.; et al. Films composed of white angico gum and chitosan containing chlorhexidine as an antimicrobial agent. Int. J. Biol. Macromol. 2023, 235, 123905. [Google Scholar] [CrossRef] [PubMed]
- Luiz, C.; da Rocha Neto, A.C.; Franco, P.O.; Di Piero, R.M. Emulsions of essential oils and aloe polysaccharides: Antimicrobial activity and resistance inducer potential against Xanthomonas fragariae. Trop. Plant Pathol. 2017, 42, 370–381. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of Essential Oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Veloso, R.A.; de Souza Ferreira, T.P.; Dias, B.L.; Mourâ, D.d.S.C.; de Araujo Filho, R.N.; Glaria, R.S.L.; Barros, A.M.; de Souza Ferreira, T.P.; Chapla, V.M.; Cangussu, A.S.R. Chemical composition and bioactivity of essential oil from Morinda citrifolia L. fruit. J. Med. Plant Res. 2020, 14, 208–214. [Google Scholar] [CrossRef]
- Guimarães, L.G.d.L.; Cardoso, M.d.G.; Zacaroni, L.M.; Lima, R.K.d.; Pimentel, F.A.; Morais, A.R.d. Influência da luz e da temperatura sobre a oxidação do óleo essencial de capim-limão (Cymbopogon citratus (DC) Stapf). Quim. Nova 2008, 31, 1476–1480. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2007. [Google Scholar]
- Silva, A.G.d.; Rodrigues, J.F.; Paula, R.C.M.d. Composição e propriedades reológicas da goma do angico (Anadenanthera macrocarpa Benth). Polímeros 1998, 8, 34–40. [Google Scholar] [CrossRef]
- Brum, R.; Castro, H.G.; Gama, F.R.; Cardon, C.H.; Santos, G.R. Phytotoxicity of essential oils in watermelon, bean and rice plants. J. Biotechnol. Biodivers. 2014, 5, 101–109. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Amako, K.; Chen, G.-X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of active oxygen in chloroplasts. In Molecular Biology of Free Radical Scavenging Systems; Kyle, D.O.C., Arntzen, C., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1992; pp. 127–287. [Google Scholar]
- Wirth, S.J.; Wolf, G.A. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J. Microbiol. Methods 1990, 12, 197–205. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Sasisekharan, V. Conformation of Polypeptides and Proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Anson, M.L., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 23, pp. 283–437. [Google Scholar]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous Automated Model EvaluatiOn (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins Struct. Funct. Bioinf. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2010, 27, 343–350. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model 1999, 17, 57–61. [Google Scholar]
- de Souza Moura, W.; de Souza, S.R.; Campos, F.S.; Sander Rodrigues Cangussu, A.; Macedo Sobrinho Santos, E.; Silva Andrade, B.; Borges Gomes, C.H.; Fernandes Viana, K.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crops Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- BIOVIA Dassault Systemes. Discovery Studio Modeling Environment; BIOVIA Dassault Systemes: San Diego, CA, USA, 2017. [Google Scholar]
- Hospital, A.; Andrio, P.; Fenollosa, C.; Cicin-Sain, D.; Orozco, M.; Gelpí, J.L. MDWeb and MDMoby: An integrated web-based platform for molecular dynamics simulations. Bioinformatics 2012, 28, 1278–1279. [Google Scholar] [CrossRef]
- Chaudhary, M.; Kumar, N.; Baldi, A.; Chandra, R.; Arockia Babu, M.; Madan, J. Chloro and bromo-pyrazole curcumin Knoevenagel condensates augmented anticancer activity against human cervical cancer cells: Design, synthesis, in silico docking and in vitro cytotoxicity analysis. J. Biomol. Struct. Dyn. 2020, 38, 200–218. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.S.; da Cruz, J.N.; Gomes Silva, S.; da Costa, W.A.; de Sousa, S.H.B.; Bezerra, F.W.F.; Teixeira, E.; da Silva, N.J.N.; de Aguiar Andrade, E.H.; de Jesus Chaves Neto, A.M.; et al. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J. Supercrit. Fluids 2019, 145, 74–84. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; da Cruz, J.N.; Almeida da Costa, W.; Silva, S.G.; Brito, M.d.P.; de Menezes, S.A.F.; de Jesus Chaves Neto, A.M.; de Aguiar Andrade, E.H.; de Carvalho Junior, R.N. Chemical composition, antimicrobial properties of Siparuna guianensis essential oil and a molecular docking and dynamics molecular study of its major chemical constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.R.; Brum, R.B.C.S.; De Castro, H.G.; Gonçalves, C.G.; Fidelis, R.R. Effect of essential oils of medicinal plants on leaf blotch in Tanzania grass. Rev. Ciênc. Agron. 2013, 44, 587–593. [Google Scholar] [CrossRef]
- Maia, F.G.M.; Armesto, C.; Zancan, W.L.A.; Maia, J.B.; Abreu, M.S.d.A. Efeito da temperatura no crescimento micelial, produção e germinação de conídios de Colletotrichum spp. isolados de mangueira com sintomas de antracnose. Biosci. J. 2011, 27, 205–210. [Google Scholar]
- Shaner, G.; Finney, R.E. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Ismail, S.; Man, C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012, 92, 593–597. [Google Scholar] [CrossRef]
- Martins, P.; Sbaite, P.; Benites, C.; Maciel, M. Thermal characterization of orange, lemongrass, and basil essential oils. In Proceedings of the International Conference on Chemical and Process Engineering, Florence, Italy, 8–11 May 2011; pp. 463–468. [Google Scholar]
- Chambre, D.R.; Moisa, C.; Lupitu, A.; Copolovici, L.; Pop, G.; Copolovici, D.-M. Chemical composition, antioxidant capacity, and thermal behavior of Satureja hortensis essential oil. Sci. Rep. 2020, 10, 21322. [Google Scholar] [CrossRef]
- Thombare, N.; Mahto, A.; Singh, D.; Chowdhury, A.R.; Ansari, M.F. Comparative FTIR characterization of various natural gums: A criterion for their identification. J. Polym. Environ. 2023, 31, 3372–3380. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Optimizing sampling and extraction methods for plant-parasitic and entomopathogenic nematodes. Plants 2021, 10, 629. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Silva, É.O.d.; Alves, E.; Ferreira, T.C.; Albuquerque, C.A.C.d. Óleos essenciais no controle da pinta bacteriana e na ativação de respostas bioquímicas em tomateiro. Summa Phytopathol. 2017, 43, 212–217. [Google Scholar] [CrossRef]
- Hanaka, A.; Lechowski, L.; Mroczek-Zdyrska, M.; Strubińska, J. Oxidative enzymes activity during abiotic and biotic stresses in Zea mays leaves and roots exposed to Cu, methyl jasmonate and Trigonotylus caelestialium. Physiol. Mol. Biol. Plants 2018, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 165102. [Google Scholar] [CrossRef]
- Singh, G.; Arya, S.K. Antifungal and insecticidal potential of chitinases: A credible choice for the eco-friendly farming. Biocatal. Agric. Biotechnol. 2019, 20, 101289. [Google Scholar] [CrossRef]
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Martínez-Álvarez, J.C.; López-Meyer, M.; Lizárraga-Sánchez, G.J.; Félix-Gastélum, R.; Castro-Martínez, C.; Maldonado-Mendoza, I.E. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. Springerplus 2016, 5, 330. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.S.; Ganguly, S.; Kumar, P.; Poddar, R.; Kumar, A. Homology model, molecular dynamics simulation and novel pyrazole analogs design of Candida albicans CYP450 lanosterol 14 α-demethylase, a target enzyme for antifungal therapy. J. Biomol. Struct. Dyn. 2017, 35, 1446–1463. [Google Scholar] [CrossRef]
- Sun, J.; Lv, P.-C.; Zhu, H.-L. Tyrosyl-tRNA synthetase inhibitors: A patent review. Expert. Opin. Ther. Pat. 2017, 27, 557–564. [Google Scholar] [CrossRef]
- Victoria Arellano, A.D.; Guatimosim, E.; da Silva, G.M.; Frank, A.K.; Dallagnol, L.J. Fungi causing leaf spot diseases in Lolium multiflorum in Brazil. Mycol. Prog. 2021, 20, 1175–1190. [Google Scholar] [CrossRef]
- Mourão, D.D.S.C.; Ferreira de Souza Pereira, T.; Souza, D.J.d.; Chagas Júnior, A.F.; Dalcin, M.S.; Veloso, R.A.; Leão, E.U.; Santos, G.R.d. Essential oil of Cymbopogon citratus on the control of the Curvularia Leaf spot disease on maize. Medicines 2017, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Han, S.; Wu, Z.; Pan, C.; Wang, M.; Tang, Y.; Zhang, Q.-H.; Tan, G.; Han, B. A push–pull strategy for controlling the tea green leafhopper (Empoasca flavescens F.) using semiochemicals from Tagetes erecta and Flemingia macrophylla. Pest Manag. Sci. 2022, 78, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Pozebon, H.; Stürmer, G.R.; Arnemann, J.A. Corn Stunt Pathosystem and Its Leafhopper Vector in Brazil. J. Econ. Entomol. 2022, 115, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z. Non-host plant essential oil volatiles with potential for a ‘push-pull’ strategy to control the tea green leafhopper, Empoasca vitis. Entomol. Exp. Appl. 2015, 156, 77–87. [Google Scholar] [CrossRef]
- Abdelkader, H.; Abdelkader, B.; Yahia, B. Toxicity and repellency of Eucalyptus globulus L. essential oil against Aphis fabae scopoli, 1763 (Homoptera: Aphididae). J. Entomol. Res. 2020, 44, 147–152. [Google Scholar] [CrossRef]
- Da Costa, R.V.; de Almeida, R.E.M.; Cota, L.V.; da Silva, D.D.; lima, L.S.; de Sousa, C.W.A.; de Souza, M.R. Corn stunt disease complex increases charcoal rot (Macrophomina phaseolina) under field conditions. Trop. Plant Pathol. 2023, 48, 283–292. [Google Scholar] [CrossRef]
- Sombra, K.E.; de Aguiar, C.V.; de Oliveira, S.J.; Barbosa, M.G.; Zocolo, G.J.; Pastori, P.L. Potential pesticide of three essential oils against Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2020, 80, 617–628. [Google Scholar] [CrossRef]
- Patiño-Bayona, W.R.; Nagles Galeano, L.J.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Herrera Daza, E.; Suárez, L.E.C.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Effects of essential oils from 24 plant species on Sitophilus zeamais motsch (Coleoptera, Curculionidae). Insects 2021, 12, 532. [Google Scholar] [CrossRef]
- Hammoud, Z.; Ben Abada, M.; Greige-Gerges, H.; Elaissari, A.; Mediouni Ben Jemâa, J. Insecticidal effects of natural products in free and encapsulated forms: An overview. J. Nat. Pestic. Res. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Tschoeke, P.H.; Oliveira, E.E.; Dalcin, M.S.; Silveira-Tschoeke, M.C.A.C.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Ferreira, T.P.; Bastos, I.M.A.S.; Rezende, S.M.; Viteri Jumbo, L.O.; Didonet, J.; Andrade, B.S.; Melo, T.S.; Smagghe, G.; Oliveira, E.E.; et al. Essential oil from Negramina (Siparuna guianensis) plants controls aphids without impairing survival and predatory abilities of non-target ladybeetles. Environ. Pollut. 2019, 255, 113153. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, L.; Xia, H.; Yu, C.; Dou, K.; Li, Y.; Chen, J. Omics for understanding synergistic action of validamycin A and Trichoderma asperellum GDFS1009 against maize sheath blight pathogen. Sci. Rep. 2017, 7, 40140. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Sun, J.; Wang, X.; Zou, L.; Fu, B.; Chen, J. Reprogrammed endophytic microbial community in maize stalk induced by Trichoderma asperellum biocontrol agent against Fusarium diseases and mycotoxin accumulation. Fungal Biol. 2019, 123, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Shabbir, M.Z.; Zhang, T.; Bai, S.; Chen, J.; Wang, Z. Myco-synergism boosts herbivory-induced maize defense by triggering antioxidants and phytohormone signaling. Front. Plant Sci. 2022, 13, 790504. [Google Scholar] [CrossRef] [PubMed]

| Chemical Class | Compound Name (IUPAC) | % | R.T. (min) | I.T. |
|---|---|---|---|---|
| Fatty acid | Octanoic acid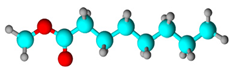 | 58.43 | 14.239 | 12.78 |
| Fatty acid | Hexanoic acid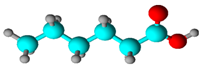 | 9.46 | 8.435 | 7.81 |
| Fatty acid esters | Pent-4-enyl hexanoate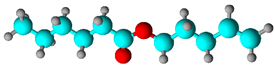 | 8.17 | 14.449 | 14.35 |
| Fatty acid methyl ester | Methyl octanoate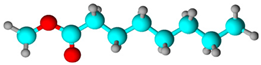 | 7.26 | 10.606 | 10.52 |
| Treatment | Velocity of Growth Mycelial (mm/day ± SE) Curvularia lunata | VCM (mm/day) | ||||
|---|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | ||
| Control | 18.2 ± 0.3 | 8.7 ± 0.5 | 8.2 ± 0.2 | 0.0 ± 0 | 0.0 ± 0 | 11.67 |
| Morinda citrifolia | 3.0 ± 0.3 | 4.2 ± 0.3 | 6.4 ± 0.5 | 3.2 ± 1.0 | 2.1 ± 2.1 | 3.78 |
| angico gum | 14.0 ± 0.9 | 2.0 ± 0.7 | 16.4 ± 0.4 | 2.6 ± 1.3 | 0.0 ± 0 | 8.75 |
| Angico gum + M. citrifolia | 1.9 ± 1.1 | 1.3 ± 0.7 | 4.8 ± 2.5 | 3.6 ± 0.6 | 3.5 ± 0 | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, B.L.; Sarmento, R.A.; Venzon, M.; Jumbo, L.O.V.; dos Santos, L.S.S.; de Souza Moura, W.; Mourão, D.d.S.C.; Fernandes, P.R.d.S.; Neitzke, T.R.; Oliveira, J.V.d.A.; et al. Morinda citrifolia Essential Oil: A Plant Resistance Biostimulant and a Sustainable Alternative for Controlling Phytopathogens and Insect Pests. Biology 2024, 13, 479. https://doi.org/10.3390/biology13070479
Dias BL, Sarmento RA, Venzon M, Jumbo LOV, dos Santos LSS, de Souza Moura W, Mourão DdSC, Fernandes PRdS, Neitzke TR, Oliveira JVdA, et al. Morinda citrifolia Essential Oil: A Plant Resistance Biostimulant and a Sustainable Alternative for Controlling Phytopathogens and Insect Pests. Biology. 2024; 13(7):479. https://doi.org/10.3390/biology13070479
Chicago/Turabian StyleDias, Bruna Leticia, Renato Almeida Sarmento, Madelaine Venzon, Luis Oswaldo Viteri Jumbo, Lucas Samuel Soares dos Santos, Wellington de Souza Moura, Dalmarcia de Souza Carlos Mourão, Paulo Ricardo de Sena Fernandes, Taila Renata Neitzke, João Victor de Almeida Oliveira, and et al. 2024. "Morinda citrifolia Essential Oil: A Plant Resistance Biostimulant and a Sustainable Alternative for Controlling Phytopathogens and Insect Pests" Biology 13, no. 7: 479. https://doi.org/10.3390/biology13070479






