Physiological Adaptation of Fenneropenaeus chinensis in Response to Saline–Alkaline Stress Revealed by a Combined Proteomics and Metabolomics Method
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Materials and Experimental Treatment
2.2. TMT-Labeled Quantitative Proteomics
2.3. LC-MS/MS Non-Target Metabolomics
2.4. Data Analysis
2.4.1. Proteomics Data Analysis
2.4.2. Metabolomics Data Analysis
3. Results
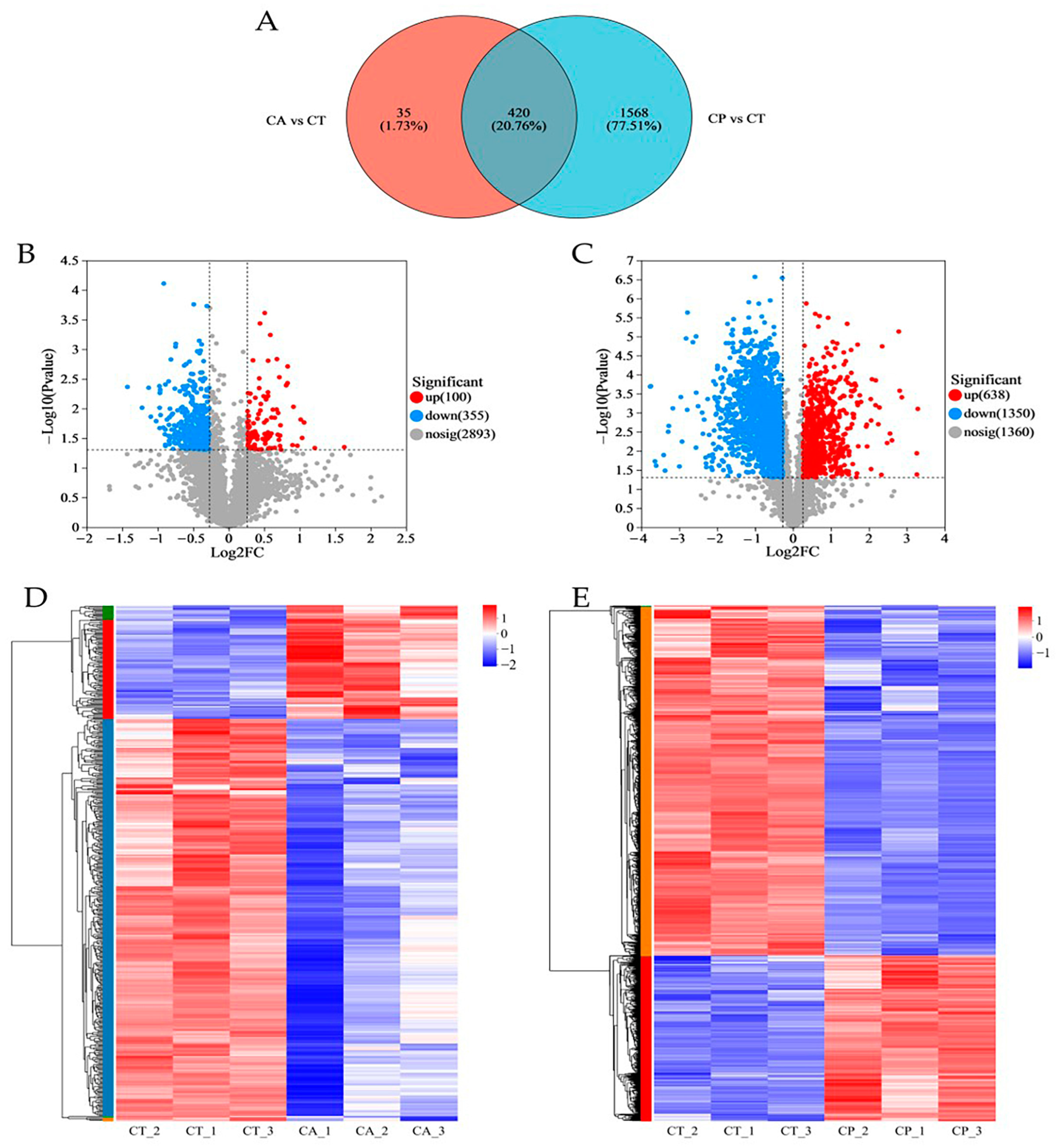
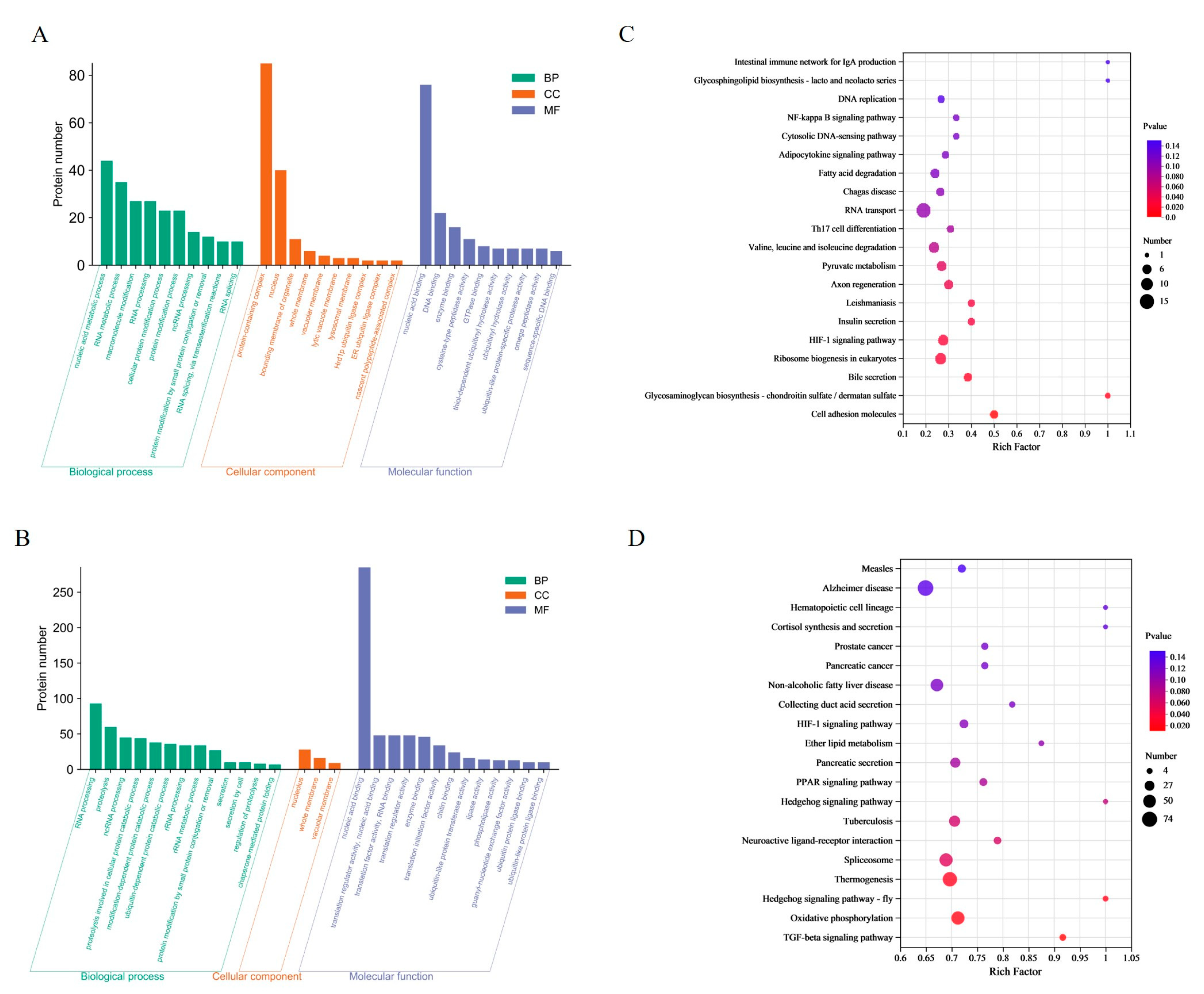
3.1. Proteomics Analysis
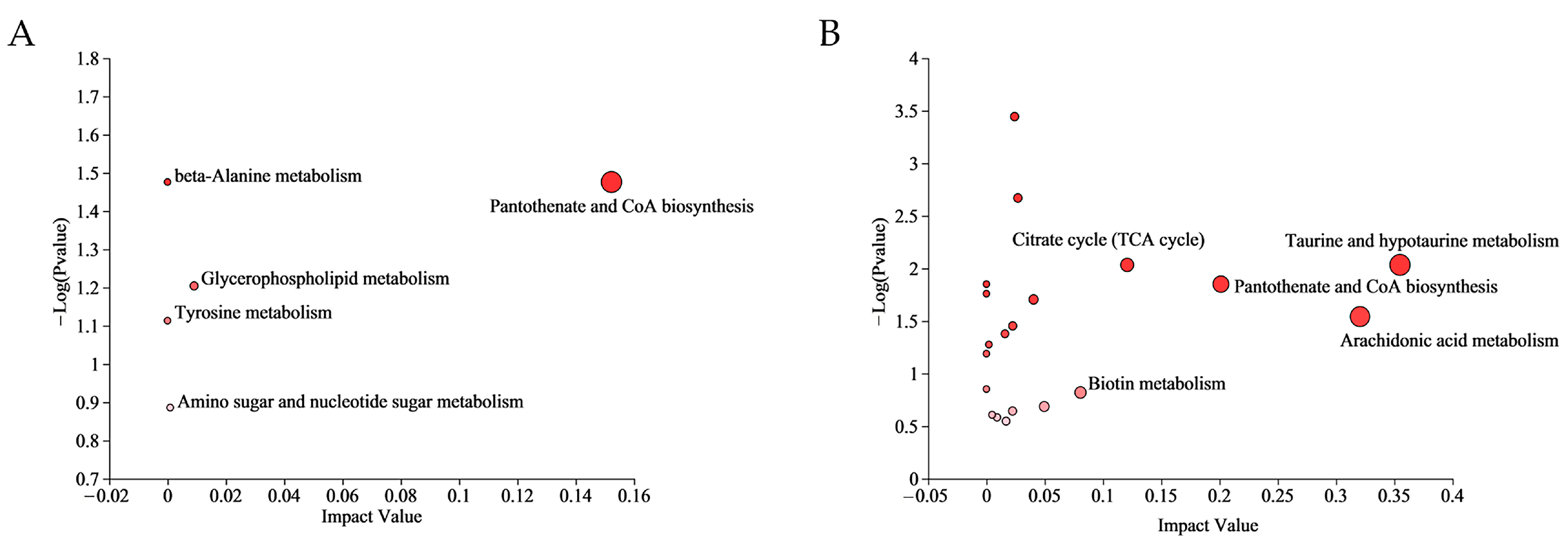
3.2. Metabolomics Analysis
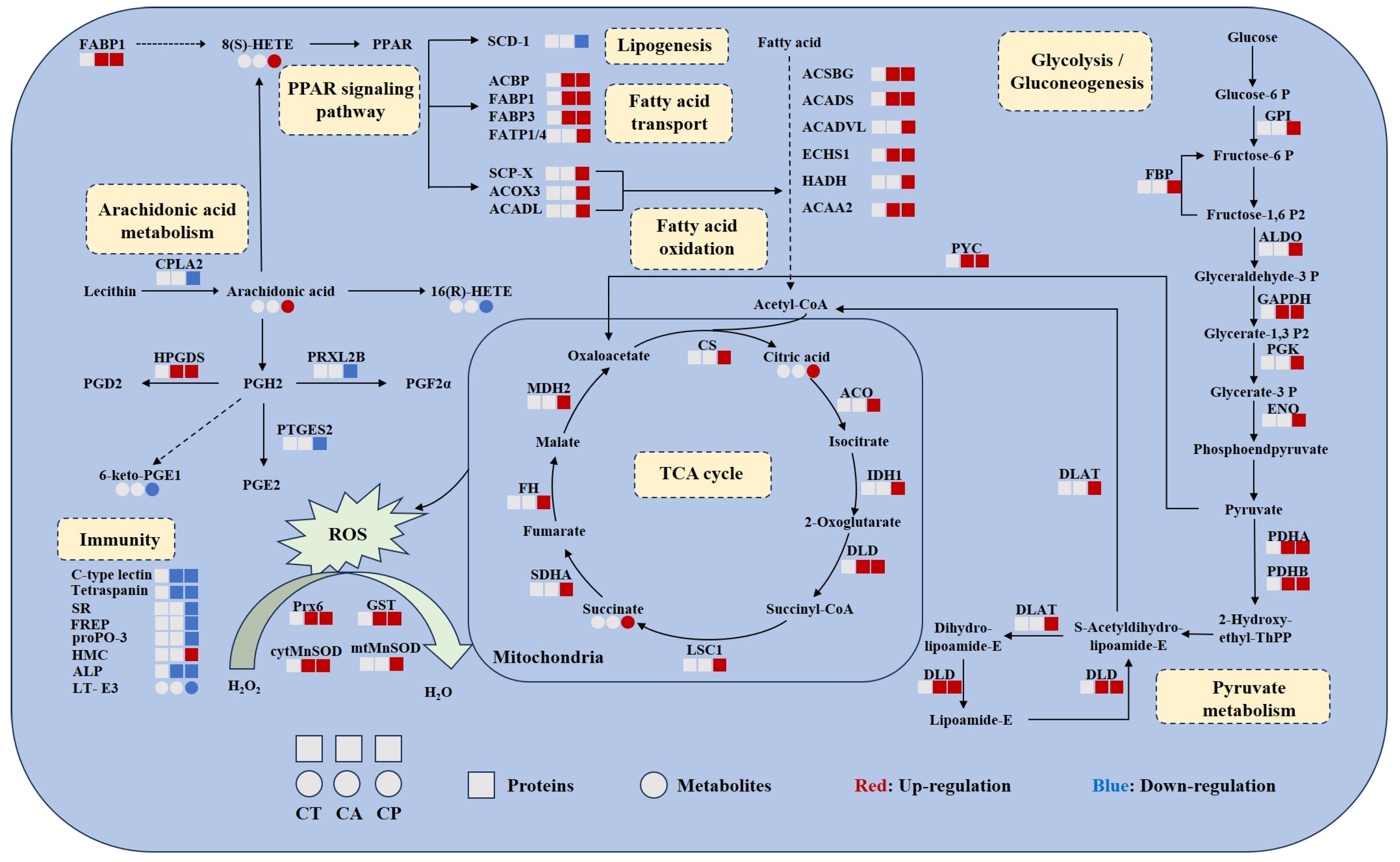
3.3. Multi-Omics Identification of Key Proteins and Metabolites
4. Discussion
4.1. Enhanced Carbohydrate and Energy Metabolism
4.2. Oxidative Stress Occurred under CA and CP Stress
4.3. CA and CP Stress Caused Lipid Metabolism Disorders
4.4. CA and CP Stress Triggered Immune Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Z.L.; Lai, Q.F.; Zhou, K.; Rizalita, R.E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gonzalez, R.J.; Patrick, M.L.; Grosell, M.; Zhang, C.; Feng, Q.; Du, J.; Walsh, P.J.; Wood, C.M. Unusual physiology of scale-less carp, Gymnocypris przewalskii, in Lake Qinghai: A high altitude alkaline saline lake. Comp. Biochem. Physiol. A 2003, 134, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Li, J.; Wang, J.; Li, Z.; Li, J. Characterization, functional analysis, and expression levels of three carbonic anhydrases in response to pH and saline-alkaline stresses in the ridgetail white prawn Exopalaemon carinicauda. Cell Stress Chaperones 2019, 24, 503–515. [Google Scholar] [CrossRef]
- Wang, M.; Kong, J.; Meng, X.; Luan, S.; Luo, K.; Sui, J.; Chen, B.; Cao, J.; Shi, X. Evaluation of genetic parameters for growth and cold tolerance traits in Fenneropenaeus chinensis juveniles. PLoS ONE 2017, 12, e0183801. [Google Scholar] [CrossRef]
- Lin, T.; Lai, Q.; Yao, Z.; Lu, J.; Zhou, K.; Wang, H. Combined effects of carbonate alkalinity and pH on survival, growth and haemocyte parameters of the Venus clam Cyclina sinensis. Fish Shellfish Immunol. 2013, 35, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.P.; Wood, C.M. The adaptations of fish to extremely alkaline environments. Comp. Biochem. Physiol. B 1996, 113, 665–673. [Google Scholar] [CrossRef]
- Fang, W.; Wang, H.; Lai, Q. Toxicity of carbonate–alkalinity and pH to larval Penaeus chinensis. J. Fishery Sci. China 2000, 4, 78–81. [Google Scholar]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in hepatopancreas of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquacult. Rep. 2024, 35, 101981. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhou, J.; Zou, J.; Fan, L. New insights into the immune regulation and tissue repair of Litopenaeus vannamei during temperature fluctuation using TMT-based proteomics. Fish Shellfish Immunol. 2020, 106, 975–981. [Google Scholar] [CrossRef]
- Ning, M.; Wei, P.; Shen, H.; Wan, X.; Jin, M.; Li, X.; Shi, H.; Qiao, Y.; Jiang, G.; Gu, W.; et al. Proteomic and metabolomic responses in hepatopancreas of whiteleg shrimp Litopenaeus vannamei infected by microsporidian Enterocytozoon hepatopenaei. Fish Shellfish Immunol. 2019, 87, 534–545. [Google Scholar] [CrossRef]
- Duan, Y.; Xing, Y.; Zhu, X.; Li, H.; Wang, Y.; Nan, Y. Integration of transcriptomic and metabolomic reveals carbonate alkalinity stress responses in the hepatopancreas of Litopenaeus vannamei. Aquat. Toxicol. 2023, 260, 106569. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, L.; Ji, C.; Yu, D. Proteomic and metabolomic responses in D-shape larval mussels Mytilus galloprovincialis exposed to cadmium and arsenic. Fish Shellfish Immunol. 2016, 58, 514–520. [Google Scholar] [CrossRef]
- Peng, M.; Li, Z.; Liu, X.; Niu, D.; Lan, T.; Ye, B.; Dong, Z.; Li, J. Tolerance, growth, and physiological responses of the juvenile razor clam (Sinonovacula constricta) to environmental Ca(2+) and Mg(2+) concentrations. Front. Physiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-P.; Lee, T.-H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A 2007, 148, 479–497. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Hwang, P.-P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. C 2008, 148, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Q.W.; Kong, X.; Liu, Y.F.; Li, J.H.; Du, G.C.; Lv, X.Q.; Ledesma-Amaro, R.; Chen, J.; Liu, L. Highly efficient neutralizer-free L-malic acid production using engineered Saccharomyces cerevisiae. Bioresource Technol. 2023, 370, 128580. [Google Scholar] [CrossRef]
- Tejero Rioseras, A.; Singh, K.D.; Nowak, N.; Gaugg, M.T.; Bruderer, T.; Zenobi, R.; Sinues, P.M. Real-time monitoring of tricarboxylic acid metabolites in exhaled breath. Anal. Chem. 2018, 90, 6453–6460. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Uribe, A.P.; Hernández-Cruz, E.Y.; Ramírez-Magaña, K.J.; Pedraza-Chaverri, J. Involvement of Tricarboxylic Acid Cycle Metabolites in Kidney Diseases. Biomolecules 2021, 11, 1259. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Li, J.; Li, Z. Comparative proteomic profiling in Chinese shrimp Fenneropenaeus chinensis under low pH stress. Fish Shellfish Immunol. 2022, 120, 526–535. [Google Scholar] [CrossRef]
- Xu, R.; Zheng, X. Hemocytes transcriptomes reveal metabolism changes and detoxification mechanisms in response to ammonia stress in Octopus minor. Ecotoxicology 2020, 29, 1441–1452. [Google Scholar] [CrossRef]
- Purandare, N.; Ghosalkar, E.; Grossman, L.I.; Aras, S. Mitochondrial oxidative phosphorylation in viral infections. Viruses 2023, 15, 2380. [Google Scholar] [CrossRef]
- Tomanek, L. Proteomic responses to environmentally induced oxidative stress. J. Exp. Biol. 2015, 218, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Jia, S.; Gao, B.; Zhou, Q.; Xu, Y.; Liu, P.; Li, J. Application of proteomics and metabolomics to assess ammonia stress response and tolerance mechanisms of juvenile ornate rock lobster Panulirus ornatus. Sci. Total Environ. 2022, 837, 155751. [Google Scholar] [CrossRef]
- Zhong, J.; Fu, M.; Zeng, X.; Wang, Y. Molecular cloning and expression analysis of MnSOD from mud crab Scvlla paramamosain. J. Appl. Oceanogr. 2023, 42, 450–459. [Google Scholar]
- Li, Y.; Zhan, F.; Li, F.; Lu, Z.; Shi, F.; Xu, Z.; Yang, Y.; Zhao, L.; Qin, Z.; Lin, L. Immune function of cytosolic manganese superoxide dismutase from Macrobrachium rosenbergii in response to bacterial infection. Aquaculture 2021, 541, 736771. [Google Scholar] [CrossRef]
- Wei, X.F.; Liu, Y.J.; Li, S.W.; Ding, L.; Han, S.C.; Chen, Z.X.; Lu, H.; Wang, P.; Sun, Y.C. Stress response and tolerance mechanisms of NaHCO(3) exposure based on biochemical assays and multi-omics approach in the liver of crucian carp (Carassius auratus). Ecotoxicol. Environ. Saf. 2023, 253, 114633. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.J.; Knutson, S.T.; Soito, L.; Klomsiri, C.; Poole, L.B.; Fetrow, J.S. Analysis of the peroxiredoxin family: Using active-site structure and sequence information for global classification and residue analysis. Proteins 2011, 79, 947–964. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Gudkov, S.V. Radioprotective Role of Peroxiredoxin 6. Antioxidants 2019, 8, 15. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Su, X.; Li, T. A manganese superoxide dismutase in blood clam Tegillarca granosa: Molecular cloning, tissue distribution and expression analysis. Comp. Biochem. Physiol. B 2011, 159, 64–70. [Google Scholar] [CrossRef]
- Li, C.; Zhou, H.M. The role of manganese superoxide dismutase in inflammation defense. Enzym. Res. 2011, 2011, 387176. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Granillo-Luna, O.N.; Peregrino-Uriarte, A.B.; Gómez-Jiménez, S.; Yepiz-Plascencia, G. Mitochondrial manganese superoxide dismutase from the shrimp Litopenaeus vannamei: Molecular characterization and effect of high temperature, hypoxia and reoxygenation on expression and enzyme activity. J. Therm. Biol. 2020, 88, 102519. [Google Scholar] [CrossRef] [PubMed]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta 2015, 1852, 826–838. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef]
- Doi, A.M.; Pham, R.T.; Hughes, E.M.; Barber, D.S.; Gallagher, E.P. Molecular cloning and characterization of a glutathione S-transferase from largemouth bass (Micropterus salmoides) liver that is involved in the detoxification of 4-hydroxynonenal. Biochem. Pharmacol. 2004, 67, 2129–2139. [Google Scholar] [CrossRef]
- Rolim, A.E.; Henrique-Araújo, R.; Ferraz, E.G.; de Araújo Alves Dultra, F.K.; Fernandez, L.G. Lipidomics in the study of lipid metabolism: Current perspectives in the omic sciences. Gene 2015, 554, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Schoeman, J.C.; Di, X.; Lamont, L.; Harms, A.C.; Hankemeier, T. A comprehensive UHPLC–MS/MS method for metabolomics profiling of signaling lipids: Markers of oxidative stress, immunity and inflammation. Anal. Chim Acta 2024, 1297, 342348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lu, K.; Lai, Y.; Wang, L.; Wang, F.; Li, N.; Peng, Y.; Gong, H. Effects of dietary 25-hydroxyvitamin D3 on growth, calcium–phosphorus metabolism, lipid metabolism and immunity of Litopenaeus vannamei at low salinity. Aquacult. Rep. 2024, 35, 101965. [Google Scholar] [CrossRef]
- Lee, M.C.; Park, J.C.; Kim, D.H.; Kang, S.; Shin, K.H.; Park, H.G.; Han, J.; Lee, J.S. Interrelationship of salinity shift with oxidative stress and lipid metabolism in the monogonont rotifer Brachionus koreanus. Comp. Biochem Physiol. A 2017, 214, 79–84. [Google Scholar] [CrossRef]
- Ran, Z.; Li, S.; Zhang, R.; Xu, J.; Liao, K.; Yu, X.; Zhong, Y.; Ye, M.; Yu, S.; Ran, Y.; et al. Proximate, amino acid and lipid compositions in Sinonovacula constricta (Lamarck) reared at different salinities. J. Sci. Food Agric. 2017, 97, 4476–4483. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, M.; Li, S.; Wei, X.; Ding, L.; Han, S.; Wang, P.; Lv, B.; Chen, Z.; Sun, Y. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef]
- Qin, Z.; Ge, Q.; Wang, J.; Li, M.; Zhang, X.; Li, J.; Li, J. Metabolomic responses based on transcriptome of the hepatopancreas in Exopalaemon carinicauda under carbonate alkalinity stress. Ecotoxicol. Environ. Saf. 2023, 268, 115723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, S.; Liu, Y.; Ding, L.; Wei, X.; Wang, P.; Sun, Y. Metabolomics of rainbow trout liver under heat stress. J. Fish. Sci. China 2022, 29, 1168–1178. [Google Scholar]
- Zhu, Q.; Wu, Y.; Mai, J.; Guo, G.; Meng, J.; Fang, X.; Chen, X.; Liu, C.; Zhong, S. Comprehensive metabolic profiling of inflammation indicated key roles of glycerophospholipid and arginine metabolism in coronary artery disease. Front. Immunol. 2022, 13, 829425. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Placín, C.; Castillejo-Rufo, A.; Estarás, M.; González, A. Membrane lipid derivatives: Roles of arachidonic acid and its metabolites in pancreatic physiology and pathophysiology. Molecules 2023, 28, 4316. [Google Scholar] [CrossRef]
- Zheng, X.; Chi, C.; Xu, C.; Liu, J.; Zhang, C.; Zhang, L.; Huang, Y.; He, C.; He, C.; Jia, X.; et al. Effects of dietary supplementation with icariin on growth performance, antioxidant capacity and non-specific immunity of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immun. 2019, 90, 264–273. [Google Scholar] [CrossRef]
- Li, F.; Xiang, J. Recent advances in researches on the innate immunity of shrimp in China. Dev. Comp. Immunol. 2013, 39, 11–26. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Li, J.; He, Y. Comparative proteomic and transcriptomic analysis reveals high pH-induced expression signatures of Chinese shrimp Fenneropenaeus chinensis. Funct. Integr. Genom. 2021, 21, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Boonchuen, P.; Jaree, P.; Somboonviwat, K.; Somboonwiwat, K. Regulation of shrimp prophenoloxidase activating system by lva-miR-4850 during bacterial infection. Sci. Rep. 2021, 11, 3821. [Google Scholar] [CrossRef]
- Tao, Y.; Qiang, J.; Wang, H.; Xu, P.; Ma, X.; Zhao, W. Acute toxicity of high pH stress and its effect on enzymes activity and histological structure of gill and hepatopancreas in Procambarus clarkii. J. Fish. China 2016, 40, 1694–1704. [Google Scholar]
- Zhang, L.; Cui, D.; Ma, X.; Han, B.; Han, L. Comparative analysis of rice reveals insights into the mechanism of colored rice via widely targeted metabolomics. Food Chem. 2023, 399, 133926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Aweya, J.J.; Feng, Q.; Zheng, Z.; Yao, D.; Zhao, Y.; Chen, X.; Zhang, Y. Ammonia stress affects the structure and function of hemocyanin in Penaeus vannamei. Ecotoxicol. Environ. Saf. 2022, 241, 113827. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Wang, Q.; Sun, H.; Liu, Y.; Li, J.; He, Y. Physiological Adaptation of Fenneropenaeus chinensis in Response to Saline–Alkaline Stress Revealed by a Combined Proteomics and Metabolomics Method. Biology 2024, 13, 488. https://doi.org/10.3390/biology13070488
Gao T, Wang Q, Sun H, Liu Y, Li J, He Y. Physiological Adaptation of Fenneropenaeus chinensis in Response to Saline–Alkaline Stress Revealed by a Combined Proteomics and Metabolomics Method. Biology. 2024; 13(7):488. https://doi.org/10.3390/biology13070488
Chicago/Turabian StyleGao, Tian, Qiong Wang, Huarui Sun, Yang Liu, Jitao Li, and Yuying He. 2024. "Physiological Adaptation of Fenneropenaeus chinensis in Response to Saline–Alkaline Stress Revealed by a Combined Proteomics and Metabolomics Method" Biology 13, no. 7: 488. https://doi.org/10.3390/biology13070488





