The Therapeutic Potential of the Specific Intestinal Microbiome (SIM) Diet on Metabolic Diseases
Abstract
:Simple Summary
Abstract
1. Introduction
2. SIM and Metabolic Diseases
3. Crosstalk of Gut Microbiome and Its Metabolites
4. Current Evidence of Food for the Simulation of the SIM
5. Soluble Non-Starch Carbohydrates (NSPs)
6. Oligosaccharides
7. Resistant Starch
8. Inulin
9. Disaccharides and Monosaccharides
10. Polyols
11. Evidence for Prebiotics or Potential Prebiotics and Metabolic Diseases
11.1. Obesity
11.2. NAFLD
11.3. Diabetes
11.4. Metabolic Cardiovascular Diseases
12. Dietary Effect Is More Promising than Prebiotics Administration
13. Comparison between Current Dietary Therapies on Metabolic Diseases
14. Adverse Effects on Gastrointestinal Symptoms and Tolerance
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | area-under-the-curve |
| SIM | specific intestinal microbiome |
| SCFAs | short-chain fatty acids |
| NAFLD | non-alcoholic fatty liver disease |
| TLR | toll-like receptors |
| GPR | G protein-coupled receptor |
| HDAC | histone deacetylase |
| GLP-1 | glucagon-Like Peptide-1 |
| PYY | peptide YY |
| HOMA | Homeostatic Model Assessment |
| GOS | galacto-oligosaccharides |
| FOS | fructo-oligosaccharides |
| MAFLD | metabolic-associated fatty liver disease |
| FGF | fibroblast growth factor |
| FXR | farnesoid X receptor |
| TGR5 | thiol guanosine receptor-5 |
| TUDCA | tauroursodeoxycholic acid |
| GDCA | glycodeoxycholic acid |
| Hs-CRP | high sensitivity C-reactive protein |
| ROS | reactive oxygen species |
| Akt | protein kinase B |
| p38 | mitogen-activated protein kinases |
| ERK | extracellular signal-regulated kinase 1 |
| TNF-α | tumor necrosis factor Alpha |
| IL | interleukin |
| NOD2 | nucleotide-binding oligomerization domain-containing protein 2 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| JNK | Jun N-terminal kinase |
| CPT1 | carnitine palmitoyltransferase |
| PPAR α | Peroxisome proliferator-activated receptor alpha |
| NASH | non-alcoholic steatohepatitis |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPX | glutathione peroxidase |
References
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.; Chan, J.C.; Chow, E. A diet high in FODMAPs as a novel dietary strategy in diabetes? Clin. Nutr. 2022, 41, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Law, B.Y.K.; Xu, Y. Effect of inulin-type carbohydrates on insulin resistance in patients with type 2 diabetes and obesity: A systematic review and meta-analysis. J. Diabetes Res. 2019, 2019, 5101423. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.; Pijl, H.; Van den Hoek, A.M.; Havekes, L.; Romijn, J.; Corssmit, E. Gut–brain axis: Regulation of glucose metabolism. J. Neuroendocrinol. 2006, 18, 883–894. [Google Scholar] [CrossRef]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 424615. [Google Scholar]
- Gill, P.A.; Muir, J.G.; Gibson, P.R.; van Zelm, M.C. A randomized dietary intervention to increase colonic and peripheral blood SCFAs modulates the blood B-and T-cell compartments in healthy humans. Am. J. Clin. Nutr. 2022, 116, 1354–1367. [Google Scholar] [CrossRef]
- Umirah, F.; Neoh, C.F.; Ramasamy, K.; Lim, S.M. Differential gut microbiota composition between type 2 diabetes mellitus patients and healthy controls: A systematic review. Diabetes Res. Clin. Pract. 2021, 173, 108689. [Google Scholar] [CrossRef]
- Hu, J.; Guo, P.; Mao, R.; Ren, Z.; Wen, J.; Yang, Q.; Yan, T.; Yu, J.; Zhang, T.; Liu, Y. Gut microbiota signature of obese adults across different classifications. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 3933–3947. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and Meta-analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Zhou, W.; Sailani, M.R.; Contrepois, K.; Zhou, Y.; Ahadi, S.; Leopold, S.R.; Zhang, M.J.; Rao, V.; Avina, M.; Mishra, T. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 2019, 569, 663–671. [Google Scholar] [CrossRef]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: A systematic review and meta-analysis. Diabetologia 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.; Liu, Y.; Zhang, Y. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: A meta-analysis. Dig. Dis. Sci. 2019, 64, 3402–3412. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Nair, A.; Sarma, S.J. The impact of carbon and nitrogen catabolite repression in microorganisms. Microbiol. Res. 2021, 251, 126831. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Qiu, X.; Wen, Q.; Liu, M.; Zhou, D.; Chen, Q. Probiotics, pre-biotics and synbiotics in the treatment of pre-diabetes: A systematic review of randomized controlled trials. Front. Public Health 2021, 9, 645035. [Google Scholar] [CrossRef]
- Debnath, N.; Kumar, R.; Kumar, A.; Mehta, P.K.; Yadav, A.K. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol. Genet. Eng. Rev. 2021, 37, 105–153. [Google Scholar] [CrossRef]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.-K.; Kong, H.; Kim, S.B.; Kim, K. A novel bacterium, butyricimonas virosa, preventing HFD-induced diabetes and metabolic disorders in mice via GLP-1 receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipcik, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Moloney, G.M.; Viola, M.F.; Hoban, A.E.; Dinan, T.G.; Cryan, J.F. Faecal microRNAs: Indicators of imbalance at the host-microbe interface? Benef. Microbes 2018, 9, 175–183. [Google Scholar] [CrossRef]
- Chen, J.-j.; Zeng, B.-h.; Li, W.-w.; Zhou, C.-j.; Fan, S.-h.; Cheng, K.; Zeng, L.; Zheng, P.; Fang, L.; Wei, H. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav. Brain Res. 2017, 322, 34–41. [Google Scholar] [CrossRef]
- Xue, X.; Feng, T.; Yao, S.; Wolf, K.J.; Liu, C.-G.; Liu, X.; Elson, C.O.; Cong, Y. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J. Immunol. 2011, 187, 5879–5886. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Kebede, M.; Alquier, T.; Latour, M.; Poitout, V. Lipid receptors and islet function: Therapeutic implications? Diabetes Obes. Metab. 2009, 11, 10–20. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut microbiome dysbiosis and immunometabolism: New frontiers for treatment of metabolic diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Guo, H.-L.; Deng, X.; Zhu, T.-T.; Xiong, J.-F.; Xu, Y.-H.; Xu, Y. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- Brar, P.C.; Kohn, B. Use of the microbiome in the management of children with type 2 diabetes mellitus. Curr. Opin. Pediatr. 2019, 31, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Tayyeb, J.Z.; Popeijus, H.E.; Mensink, R.P.; Konings, M.C.; Mokhtar, F.B.; Plat, J. Short-chain fatty acids (except hexanoic acid) lower NF-kB transactivation, which rescues inflammation-induced decreased apolipoprotein AI transcription in HepG2 cells. Int. J. Mol. Sci. 2020, 21, 5088. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Tsoulou, C.; Ward, E.; Gower, E.; Bhudia, N.; Chowdhury, F.; Dean, T.W.; Faucher, N.; Gangar, A.; Dowell, S.J. Pharmacological properties of acid N-thiazolylamide FFA 2 agonists. Pharmacol. Res. Perspect. 2015, 3, e00141. [Google Scholar] [CrossRef]
- Ørgaard, A.; Jepsen, S.L.; Holst, J.J. Short-chain fatty acids and regulation of pancreatic endocrine secretion in mice. Islets 2019, 11, 103–111. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Tan, Q.; Deng, X.; Tsai, P.-J.; Chen, P.-H.; Ye, M.; Guo, J.; Su, Z. Nondigestible oligosaccharides with anti-obesity effects. J. Agric. Food Chem. 2019, 68, 4–16. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Potthoff, M.J.; Haberland, M.; Qi, X.; Matsuzaki, S.; Humphries, K.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J. Clin. Investig. 2008, 118, 3588–3597. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Kars, M.; Yang, L.; Gregor, M.F.; Mohammed, B.S.; Pietka, T.A.; Finck, B.N.; Patterson, B.W.; Horton, J.D.; Mittendorfer, B.; Hotamisligil, G.S. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 2010, 59, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.L.; van Werven, J.; Aarts, E.; Berends, F.; Janssen, I.; Stoker, J.; Schaap, F.G. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig. Dis. 2011, 29, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut microbiota and type 2 diabetes mellitus: Association, mechanism, and translational applications. Mediat. Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef]
- He, S.; Kahles, F.; Rattik, S.; Nairz, M.; McAlpine, C.S.; Anzai, A.; Selgrade, D.; Fenn, A.M.; Chan, C.T.; Mindur, J.E. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 2019, 566, 115–119. [Google Scholar] [CrossRef]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging roles of bile acids in mucosal immunity and inflammation. Mucosal Immunol. 2019, 12, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.C.; de Las Heras, J.; Martínez-Chantar, M.L. Understanding gut-liver axis nitrogen metabolism in Fatty Liver Disease. Front. Endocrinol. 2022, 13, 1058101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; He, Y.; Chen, C.; Song, C.; Zhao, Y.; Bai, Y.; Wang, Y.; Pu, J.; Chen, J. Investigation of novel metabolites potentially involved in the pathogenesis of coronary heart disease using a UHPLC-QTOF/MS-based metabolomics approach. Sci. Rep. 2017, 7, 15357. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- He, L.-x.; Zhao, J.; Huang, Y.-s.; Li, Y. The difference between oats and beta-glucan extract intake in the management of HbA1c, fasting glucose and insulin sensitivity: A meta-analysis of randomized controlled trials. Food Funct. 2016, 7, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the human gut microbiota: From breakdown mechanisms to the impact on metabolic health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ke, M.-Y.; Li, W.-H.; Zhang, S.-Q.; Fang, X.-C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2014, 23, 210–218. [Google Scholar]
- Hopewell, R.; Yeater, R.; Ullrich, I. Soluble fiber: Effect on carbohydrate and lipid metabolism. Prog. Food Nutr. Sci. 1993, 17, 159–182. [Google Scholar] [PubMed]
- Hu, W.; Cassard, A.-M.; Ciocan, D. Pectin in metabolic liver disease. Nutrients 2022, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Shtriker, M.G.; Hahn, M.; Taieb, E.; Nyska, A.; Moallem, U.; Tirosh, O.; Madar, Z. Fenugreek galactomannan and citrus pectin improve several parameters associated with glucose metabolism and modulate gut microbiota in mice. Nutrition 2018, 46, 134–142.e3. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, M.; Shi, S.; Wang, H.; Li, N.; Su, J.; Liu, R.; Huang, Z.; Jin, H.; Ji, X. Hypoglycemic effect and mechanism of a pectic polysaccharide with hexenuronic acid from the fruits of Ficus pumila L. in C57BL/KsJ db/db mice. Carbohydr. Polym. 2017, 178, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, A.; Lapre, J.; De Vries, H.; Beynen, A. Dietary pectin with high viscosity lowers plasma and liver cholesterol concentration and plasma cholesteryl ester transfer protein activity in hamsters. J. Nutr. 1998, 128, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Krzysik, M.; Grajeta, H.; Prescha, A.; Weber, R. Effect of cellulose, pectin and chromium (III) on lipid and carbohydrate metabolism in rats. J. Trace Elem. Med. Biol. 2011, 25, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The potential of pectins to modulate the human gut microbiota evaluated by in vitro fermentation: A systematic review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef]
- Deng, Z.; Meng, C.; Huang, H.; Song, S.; Fu, L.; Fu, Z. The different effects of psyllium husk and orlistat on weight control, the amelioration of hypercholesterolemia and non-alcohol fatty liver disease in obese mice induced by a high-fat diet. Food Funct. 2022, 13, 8829–8849. [Google Scholar] [CrossRef]
- Bacha, A.A.; Din, Z.U.; Khan, I. Effect of Psyllium husk fiber and lifestyle modification on human body insulin resistance. Nutr. Metab. Insights 2022, 15, 11786388221107797. [Google Scholar] [CrossRef] [PubMed]
- Ziai, S.A.; Larijani, B.; Akhoondzadeh, S.; Fakhrzadeh, H.; Dastpak, A.; Bandarian, F.; Rezai, A.; Badi, H.N.; Emami, T. Psyllium decreased serum glucose and glycosylated hemoglobin significantly in diabetic outpatients. J. Ethnopharmacol. 2005, 102, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Allgood, L.D.; Turner, J.; Oeltgen, P.R.; Daggy, B.P. Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hypercholesterolemia. Am. J. Clin. Nutr. 1999, 70, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.H.; He, J.; Leung, K.H.; Ma, R.C.; Lee, J.Y.; Varney, J.; Chan, J.C.; Muir, J.G.; Chow, E. Higher Short-Chain Fermentable Carbohydrates Are Associated with Lower Body Fat and Higher Insulin Sensitivity in People with Prediabetes. Nutrients 2023, 15, 5070. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Gallagher, E.; Horton, F.; Ellis, R.J.; Ijaz, U.Z.; Wu, H.; Jaiyeola, E.; Diribe, O.; Duparc, T.; Cani, P.D.; et al. Host–microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef]
- Tian, T.; Freeman, S.; Corey, M.; German, J.B.; Barile, D. Chemical characterization of potentially prebiotic oligosaccharides in brewed coffee and spent coffee grounds. J. Agric. Food Chem. 2017, 65, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Pastoriza, S.; Fernández-Arteaga, A.; Luzón, G.n.; Jiménez-Hernández, N.; D’Auria, G.; Francino, M.P.; Rufián-Henares, J.A.n. Spent coffee grounds extract, rich in mannooligosaccharides, promotes a healthier gut microbial community in a dose-dependent manner. J. Agric. Food Chem. 2019, 67, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Lightowler, H.; Thondre, S.; Holz, A.; Theis, S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory (oligofructose, inulin) reduces the postprandial blood glucose and insulin response to foods: Report of two double-blind, randomized, controlled trials. Eur. J. Nutr. 2018, 57, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, S.P.; Meyer, D.; Westerterp, K.R. Effects of oligofructose on appetite profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and energy intake. Br. J. Nutr. 2011, 106, 1757–1762. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, X.; Luo, Y.; Chen, B.; Ma, D.; Zhu, J. Effect of fructooligosaccharides supplementation on the gut microbiota in human: A systematic review and meta-analysis. Nutrients 2022, 14, 3298. [Google Scholar] [CrossRef]
- Wen, J.-j.; Li, M.-z.; Nie, S.-P. Dietary supplementation with resistant starch contributes to intestinal health. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cui, W.; Hu, X.; Ma, Z. Anti-hyperlipidemic and ameliorative effects of chickpea starch and resistant starch in mice with high fat diet induced obesity are associated with their multi-scale structural characteristics. Food Funct. 2022, 13, 5135–5152. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qiu, M.; Zhang, C.; Zhang, C.; Wang, N.; Zhao, F.; Liqiao, L.; Li, J.; Lyu-Bu, A.; Wang, T. Type 3 resistant starch from Canna edulis modulates obesity and obesity-related low-grade systemic inflammation in mice by regulating gut microbiota composition and metabolism. Food Funct. 2021, 12, 12098–12114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 4736. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Li, N.; Li, Q.; Ge, X.; Huang, Z.; Chen, F.; Liu, B.; Zeng, F. Pueraria lobata Resistant Starch Regulating Lipid Metabolism and Gut Microbiota in High-Fat Diet Mice. Starch-Stärke 2024, 2300123. [Google Scholar] [CrossRef]
- Stewart, M.L.; Wilcox, M.L.; Bell, M.; Buggia, M.A.; Maki, K.C. Type-4 resistant starch in substitution for available carbohydrate reduces postprandial glycemic response and hunger in acute, randomized, double-blind, controlled study. Nutrients 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. RS5 produced more butyric acid through regulating the microbial community of human gut microbiota. J. Agric. Food Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.; Tovar, A.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, H.; Lv, Y.; Tao, H.; Jiang, Y.; Ni, Z.; Peng, L.; Chen, X. Inulin intervention attenuates hepatic steatosis in rats via modulating gut microbiota and maintaining intestinal barrier function. Food Res. Int. 2023, 163, 112309. [Google Scholar] [CrossRef]
- Tripkovic, L.; Muirhead, N.; Hart, K.; Frost, G.; Lodge, J. The effects of a diet rich in inulin or wheat fibre on markers of cardiovascular disease in overweight male subjects. J. Hum. Nutr. Diet. 2015, 28, 476–485. [Google Scholar] [CrossRef]
- De Luis, D.; de La Fuente, B.; Izaola, O.; Conde, R.; Gutierrez, S.; Morillo, M. Randomized clinical trial with a inulin enriched cookie on risk cardiovascular factor in obese patients. Nutr. Hosp. 2010, 25, 53–59. [Google Scholar] [PubMed]
- Alptekin, İ.M.; Çakıroğlu, F.P.; Kiremitci, S.; Reçber, T.; Nemutlu, E. Inulin may prevent steatosis by suppressing cannabinoid receptor-1 and patatin-like phospholipase-3 expression in liver. Nutrition 2022, 103, 111742. [Google Scholar] [CrossRef]
- Yao, C.K.; Chu, N.H.S.; Tan, V.P.Y. Breath hydrogen testing in East and Southeast Asia. J. Clin. Gastroenterol. 2018, 52, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bhavadharini, B.; Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Res. Care 2020, 8, e000826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Shi, B.M. Milk in the prevention and management of type 2 diabetes: The potential role of milk proteins. Diabetes/Metab. Res. Rev. 2019, 35, e3187. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Marks, V.; Salminen, S. Blood glucose and plasma insulin responses to fat-free milk and low-lactose fat-free milk in young type 1 diabetics. Z. Ernährungswissenschaft 1987, 26, 226–229. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Mahalak, K.; Hu, W.; Bittinger, K.; Moustafa, A.; Jones, S.M.; Narrowe, A.; Tomasula, P. An in vitro analysis of how lactose modifies the gut microbiota structure and function of adults in a donor-independent manner. Front. Nutr. 2023, 9, 1040744. [Google Scholar] [CrossRef]
- Chu, N.; Ling, J.; Jie, H.; Leung, K.; Poon, E. The potential role of lactulose pharmacotherapy in the treatment and prevention of diabetes. Front. Endocrinol. 2022, 13, 956203. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Choe, J.-y.; Patel, C.R. Intestinal absorption of fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A. Fructose Consumption and the Metabolic Syndrome: Association or Causality? Praxis 2016, 105, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.H.; Svendsen, P.; Albarrán-Juárez, J.; Moestrup, S.K.; Bentzon, J.F. High-fructose feeding does not induce steatosis or non-alcoholic fatty liver disease in pigs. Sci. Rep. 2021, 11, 2807. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, V.H.; Sievenpiper, J.L.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Santaren, I.D.; Blanco Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A. Total fructose intake and risk of hypertension: A systematic review and meta-analysis of prospective cohorts. J. Am. Coll. Nutr. 2014, 33, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Mayes, P.A. Intermediary metabolism of fructose. Am. J. Clin. Nutr. 1993, 58, 754S–765S. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Cherrington, A.D.; Mann, S.L.; Davis, S.N. Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults. J. Clin. Endocrinol. Metab. 2000, 85, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Havel, P.J. Fructose consumption: Recent results and their potential implications. Ann. N. Y. Acad. Sci. 2010, 1190, 15–24. [Google Scholar] [CrossRef]
- Update, A.S. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Laughlin, M.R. Normal roles for dietary fructose in carbohydrate metabolism. Nutrients 2014, 6, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pozo, S.E.; Schold, J.; Nakagawa, T.; Sánchez-Lozada, L.G.; Johnson, R.J.; Lillo, J.L. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: Role of uric acid in the hypertensive response. Int. J. Obes. 2010, 34, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: Acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.S. Suitability of sugar alcohols as antidiabetic supplements: A review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- García-Sanmartín, J.; Bobadilla, M.; Mirpuri, E.; Grifoll, V.; Pérez-Clavijo, M.; Martínez, A. Agaricus mushroom-enriched diets modulate the microbiota-gut-brain axis and reduce brain oxidative stress in mice. Antioxidants 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Drewnowski, A.; Lê, K.-A. New metrics of dietary carbohydrate quality. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Gibb, R.D.; Sloan, K.J.; McRorie, J.W., Jr. Psyllium is a natural nonfermented gel-forming fiber that is effective for weight loss: A comprehensive review and meta-analysis. J. Am. Assoc. Nurse Pract. 2023, 35, 468–476. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the metabolic syndrome profile by soluble fibre–guar gum–in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef]
- Visuthranukul, C.; Chamni, S.; Kwanbunbumpen, T.; Saengpanit, P.; Chongpison, Y.; Tepaamorndech, S.; Panichsillaphakit, E.; Uaariyapanichkul, J.; Nonpat, N.; Chomtho, S. Effects of inulin supplementation on body composition and metabolic outcomes in children with obesity. Sci. Rep. 2022, 12, 13014. [Google Scholar] [CrossRef]
- Rodriguez, J.; Hiel, S.; Neyrinck, A.M.; Le Roy, T.; Pötgens, S.A.; Leyrolle, Q.; Pachikian, B.D.; Gianfrancesco, M.A.; Cani, P.D.; Paquot, N. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut 2020, 69, 1975–1987. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Liu, J.; Huang, Z.; Yang, X.; Qin, P.; Chen, C.; Luo, X.; Li, Y.; Wu, Y. Consumption of Dairy Products and the Risk of Overweight or Obesity, Hypertension, and Type 2 Diabetes Mellitus: A Dose–Response Meta-Analysis and Systematic Review of Cohort Studies. Adv. Nutr. 2022, 13, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Tsunashima, H.; Omagari, K.; Hara, M.; Yasuda, I.; Miyakawa, H.; Kikuchi, K. Fructo-oligosaccharides and intestinal barrier function in a methionine–choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS ONE 2017, 12, e0175406. [Google Scholar] [CrossRef]
- Takai, A.; Kikuchi, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Matsumoto, K.; Tsunashima, H.; Onda, T.; Kuniyoshi, N.; Nariyama, T. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Qian, L.; Siliceo, S.L.; Long, X.; Nychas, E.; Liu, Y.; Ismaiah, M.J.; Leung, H.; Zhang, L.; Gao, Q. Resistant starch decreases intrahepatic triglycerides in patients with NAFLD via gut microbiome alterations. Cell Metab. 2023, 35, 1530–1547.e1538. [Google Scholar] [CrossRef]
- Salehi-Sahlabadi, A.; Teymoori, F.; Ahmadirad, H.; Mokhtari, E.; Azadi, M.; Seraj, S.S.; Hekmatdoost, A. Nutrient patterns and non-alcoholic fatty liver disease in Iranian Adul: A case-control study. Front. Nutr. 2022, 9, 977403. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: Meta-analyses and meta-regression models of intervention studies. Am. J. Clin. Nutr. 2008, 88, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.D.; McRorie, J.W., Jr.; Russell, D.A.; Hasselblad, V.; D’Alessio, D.A. Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am. J. Clin. Nutr. 2015, 102, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; An, H.; Fang, D.; Wang, W.; Han, Y.; Lian, C. Plantamajoside protects H9c2 cells against hypoxia/reoxygenation-induced injury through regulating the akt/Nrf2/HO-1 and NF-κB signaling pathways. J. Recept. Signal Transduct. 2022, 42, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; Qu, H.; Hughes, S.O.; He, M.; Wagner, S.E.; Foushee, H.R.; Shewchuk, R.M. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am. J. Clin. Nutr. 2011, 94, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yu, H.; Liu, L.; Lu, T.; Li, J.; Ji, Y.; Le, Z.; Bao, L.; Ma, W.; Xiao, R. Milk powder Co-supplemented with inulin and resistant dextrin improves glycemic control and insulin resistance in elderly type 2 diabetes mellitus: A 12-week randomized, double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2018, 62, 1800865. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shang, C.; Xin, L.; Xiang, M.; Wang, Y.; Shen, Z.; Jiao, L.; Ding, F.; Cui, X. Beneficial effects of psyllium on the prevention and treatment of cardiometabolic diseases. Food Funct. 2022, 13, 7473–7486. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Steiniger, J.; Reich, S.; Weickert, M.; Harsch, I.; Machowetz, A.; Mohlig, M.; Spranger, J.; Rudovich, N.; Meuser, F. Arabinoxylan fibre consumption improved glucose metabolism, but did not affect serum adipokines in subjects with impaired glucose tolerance. Horm. Metab. Res. 2006, 38, 761–766. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef]
- Letexier, D.; Diraison, F.; Beylot, M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am. J. Clin. Nutr. 2003, 77, 559–564. [Google Scholar] [CrossRef]
- Aldubayan, M.A.; Mao, X.; Laursen, M.F.; Pigsborg, K.; Christensen, L.H.; Roager, H.M.; Nielsen, D.S.; Hjorth, M.F.; Magkos, F. Supplementation with inulin-type fructans affects gut microbiota and attenuates some of the cardiometabolic benefits of a plant-based diet in individuals with overweight or obesity. Front. Nutr. 2023, 10, 1108088. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Preston, T.; Tedford, C.; Brignardello, J.; Garcia-Perez, I.; Holmes, E.; Wallis, G.A.; Morrison, D.J.; Frost, G.S. Effects of inulin propionate ester incorporated into palatable food products on appetite and resting energy expenditure: A randomised crossover study. Nutrients 2019, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Clemente, G.; Luongo, D.; Lasorella, G.; Fiume, I.; Brouns, F.; Bornet, F.; Patti, L.; Cipriano, P.; Rivellese, A.A. Effects of short-chain fructo-oligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clin. Nutr. 2004, 23, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, W.; Wezendonk, B.; Srikumar, T.; Van den Heuvel, E. Effect of nondigestible oligosaccharides on large-bowel functions, blood lipid concentrations and glucose absorption in young healthy male subjects. Eur. J. Clin. Nutr. 1999, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Forcheron, F.; Beylot, M. Long-term administration of inulin-type fructans has no significant lipid-lowering effect in normolipidemic humans. Metabolism 2007, 56, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Giliberti, R.; Cavaliere, S.; Mauriello, I.E.; Ercolini, D.; Pasolli, E. Host phenotype classification from human microbiome data is mainly driven by the presence of microbial taxa. PLoS Comput. Biol. 2022, 18, e1010066. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Hansen, L.; Bahl, M.I.; Frandsen, H.L.; Carvalho, V.; Gøbel, R.J.; Dalgaard, M.D.; Plichta, D.R.; Sparholt, M.H.; Vestergaard, H. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 2016, 1, 16093. [Google Scholar] [CrossRef]
- Willis, H.J.; Thomas, W.; Willis, D.J.; Slavin, J.L. Feasibility of measuring gastric emptying time, with a wireless motility device, after subjects consume fiber-matched liquid and solid breakfasts. Appetite 2011, 57, 38–44. [Google Scholar] [CrossRef]
- Chu, N.H.S.; Yao, C.K.; Tan, V.P.Y. Food Therapy in Sinosphere Asia. J. Clin. Gastroenterol. 2018, 52, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Schloissnig, S.; Arumugam, M.; Sunagawa, S.; Mitreva, M.; Tap, J.; Zhu, A.; Waller, A.; Mende, D.R.; Kultima, J.R.; Martin, J. Genomic variation landscape of the human gut microbiome. Nature 2013, 493, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Portincasa, P.; Jamioł-Milc, D.; Maciejewska-Markiewicz, D.; Skonieczna-Żydecka, K. The relationship between prebiotic supplementation and anthropometric and biochemical parameters in patients with NAFLD—A systematic review and meta-analysis of randomized controlled trials. Nutrients 2020, 12, 3460. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-metabolic benefits of plant-based diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Papamichou, D.; Panagiotakos, D.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 531–543. [Google Scholar] [CrossRef]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The low-carbohydrate diet: Short-term metabolic efficacy versus longer-term limitations. Nutrients 2021, 13, 1187. [Google Scholar] [CrossRef] [PubMed]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Storz, M.A.; Müller, A.; Niederreiter, L.; Zimmermann-Klemd, A.M.; Suarez-Alvarez, M.; Kowarschik, S.; Strittmatter, M.; Schlachter, E.; Pasluosta, C.; Huber, R. A cross-sectional study of nutritional status in healthy, young, physically-active German omnivores, vegetarians and vegans reveals adequate vitamin B12 status in supplemented vegans. Ann. Med. 2023, 55, 2269969. [Google Scholar] [CrossRef]
- Yao, C.K.; Fung, J.; Chu, N.H.S.; Tan, V.P.Y. Dietary Interventions in Liver Cirrhosis. J. Clin. Gastroenterol. 2018, 52, 663–673. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Yao, C.K.; Ardalan, Z.S.; Thwaites, P.A.; Kalantar-Zadeh, K.; Gibson, P.R.; Muir, J.G. Supplementing dietary fibers with a low FODMAP diet in irritable bowel syndrome: A randomized controlled crossover trial. Clin. Gastroenterol. Hepatol. 2022, 20, 2112–2120.e7. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Mysonhimer, A.R.; Holscher, H.D. Gastrointestinal effects and tolerance of nondigestible carbohydrate consumption. Adv. Nutr. 2022, 13, 2237–2276. [Google Scholar] [CrossRef]
- Clevers, E.; Törnblom, H.; Simrén, M.; Tack, J.; Van Oudenhove, L. Relations between food intake, psychological distress, and gastrointestinal symptoms: A diary study. United Eur. Gastroenterol. J. 2019, 7, 965–973. [Google Scholar] [CrossRef]

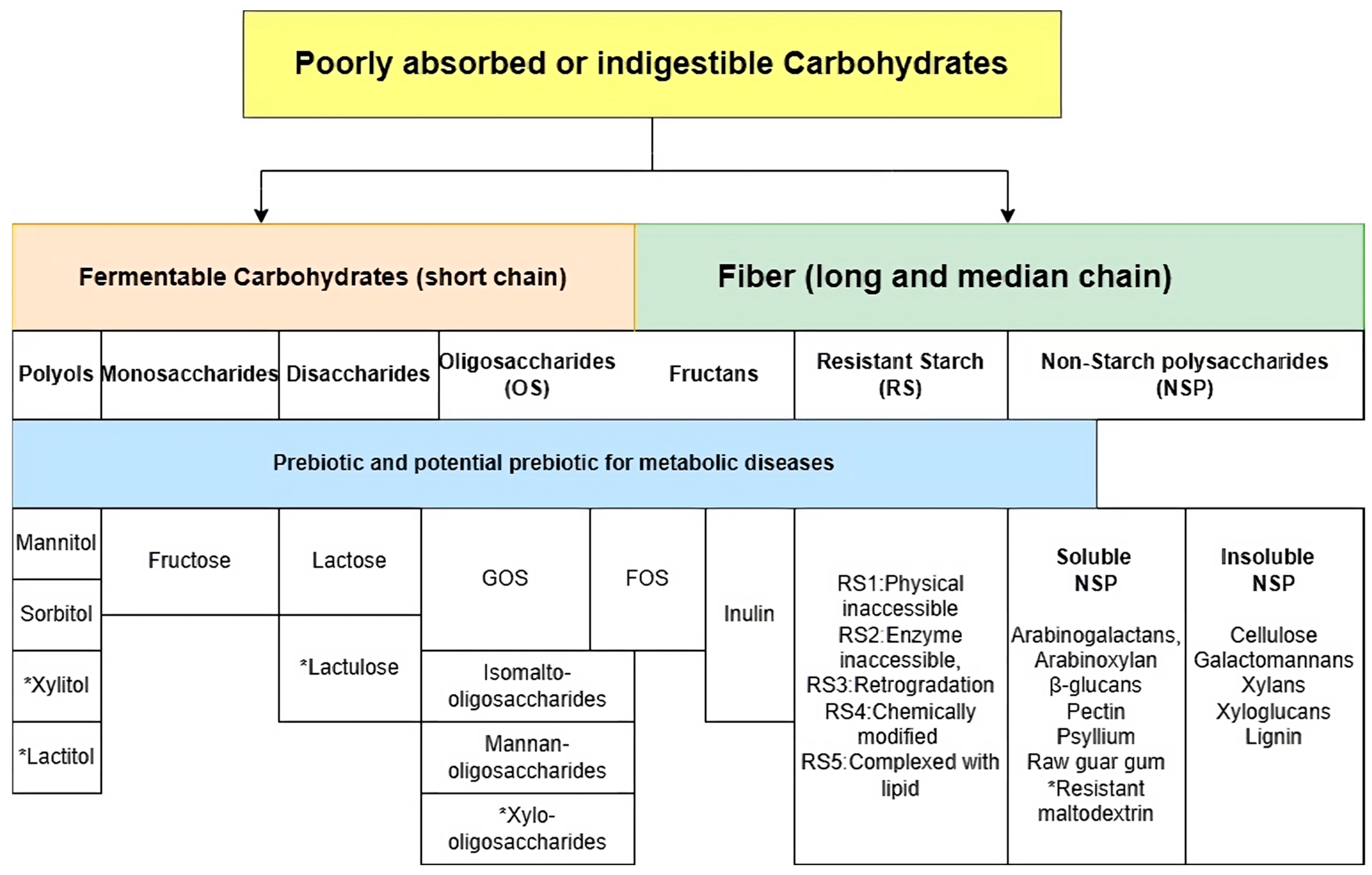
| Soluble NSP | Oligosaccharides | Resistant Starch and Inulin | Di- and Mono-Saccharides | Polyols |
|---|---|---|---|---|
| Oat bran | Wheat | Lentils | Dairy | Mushrooms |
| Barley | Pulses | Oats | Some Fruits (Apples, pears, etc.) | Cherry |
| Seeds | Figs | Barley | Dates | |
| Apples | Garlic | Banana | ||
| Oranges | Onion | |||
| Carrots | Nuts | |||
| Genus [70] | Genus [77,80] | Genus [84,85,87] | Family [99] | Family [117] |
| Bacteroides | Akkermansia | Akkermansia | Bacteroidaceae | Verrucomicrobia |
| Roseburia | Barnesiella | Ruminococcus | Lactobacillaceae | Phylum [117] |
| Coprobacillus | Odoribacter | Victivallis | Enterococcaceae | ↓ Cyanobacteria |
| Species [69] | Coprococcus | Comamonas | Streptococcaceae | Genus [117] |
| Lachnospira eligens | Butyricicoccus | Lactobacillus | Genus [113,114] | Bacteroides |
| Faecalibacterium prausnitzii | Bifidobacterium | Bifidobacterium | Anaerostipes | Phascolarctobacterium |
| Turicibacter | Coprococcus | ↓ Escherichia-Shigella | ||
| Phascolarctobacterium | Ruminococcus | |||
| Blautia | Erysipelatoclostridium | |||
| ↓ Desulfovibrio |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, N.H.S.; Chow, E.; Chan, J.C.N. The Therapeutic Potential of the Specific Intestinal Microbiome (SIM) Diet on Metabolic Diseases. Biology 2024, 13, 498. https://doi.org/10.3390/biology13070498
Chu NHS, Chow E, Chan JCN. The Therapeutic Potential of the Specific Intestinal Microbiome (SIM) Diet on Metabolic Diseases. Biology. 2024; 13(7):498. https://doi.org/10.3390/biology13070498
Chicago/Turabian StyleChu, Natural H. S., Elaine Chow, and Juliana C. N. Chan. 2024. "The Therapeutic Potential of the Specific Intestinal Microbiome (SIM) Diet on Metabolic Diseases" Biology 13, no. 7: 498. https://doi.org/10.3390/biology13070498






