Mapping the Future: Revealing Habitat Preferences and Patterns of the Endangered Chilean Dolphin in Seno Skyring, Patagonia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
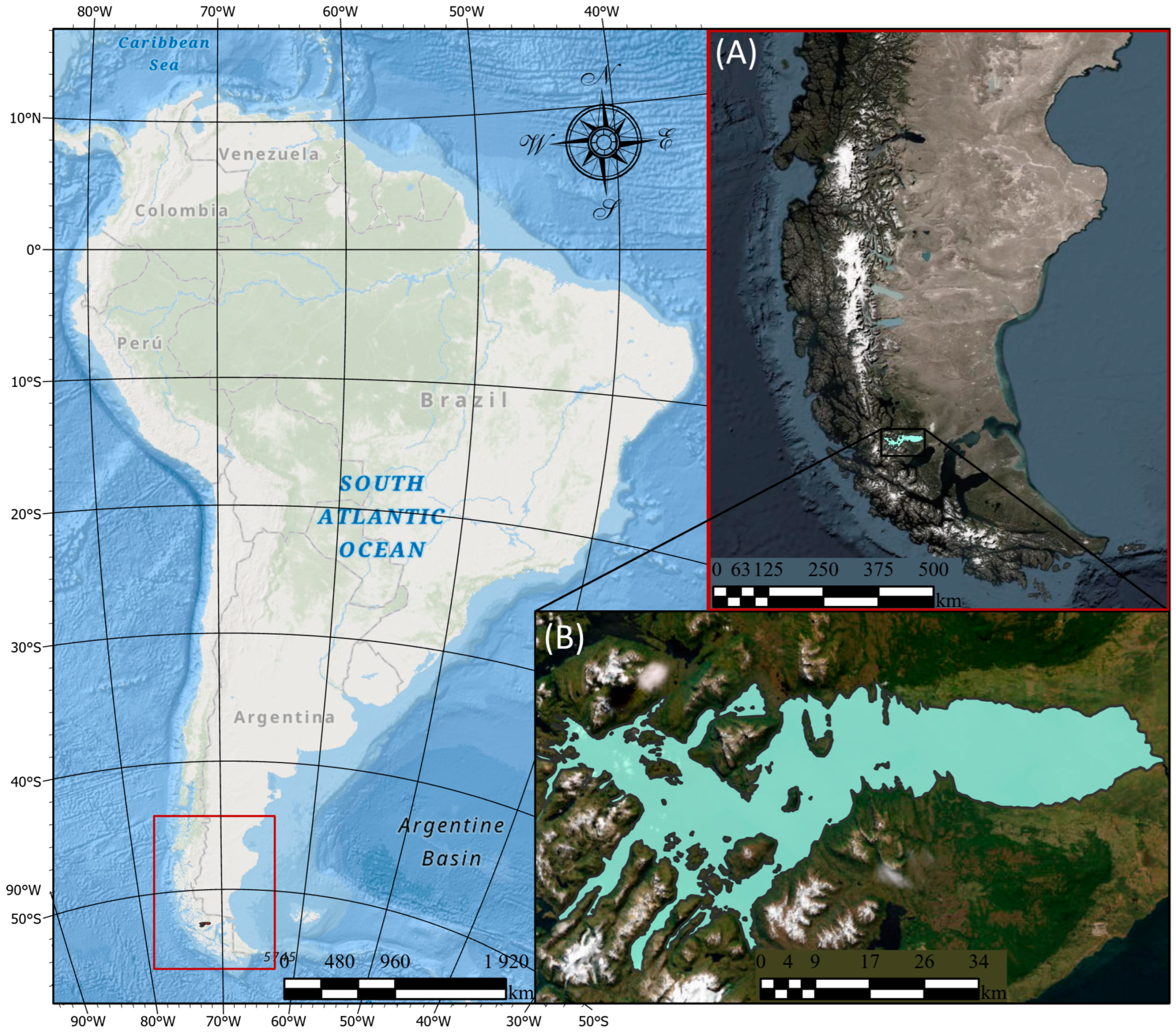
2.1. Study Area Description
2.2. Methodological Framework
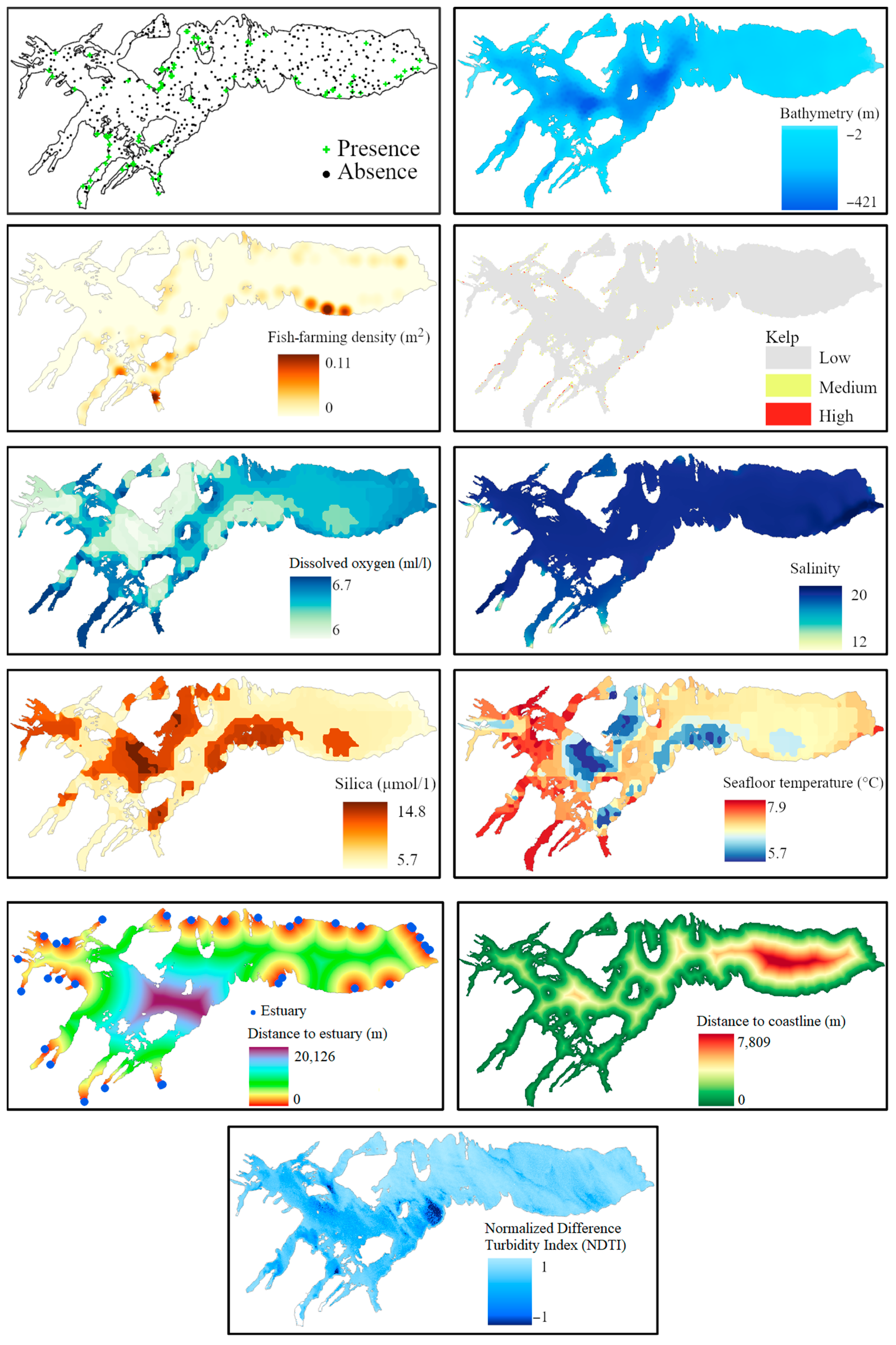
2.2.1. Data
2.2.2. Methods for Species Distribution Modeling
Random Forest (RF)
Generalized Linear Model (GLM)
Artificial Neural Network (ANN)
2.2.3. Model Evaluation Metrics
Area under the Curve (AUC) of a Receiver Operating Characteristic (ROC)
Root Mean Square Error (RMSE)
True Skill Statistic (TSS)
3. Results
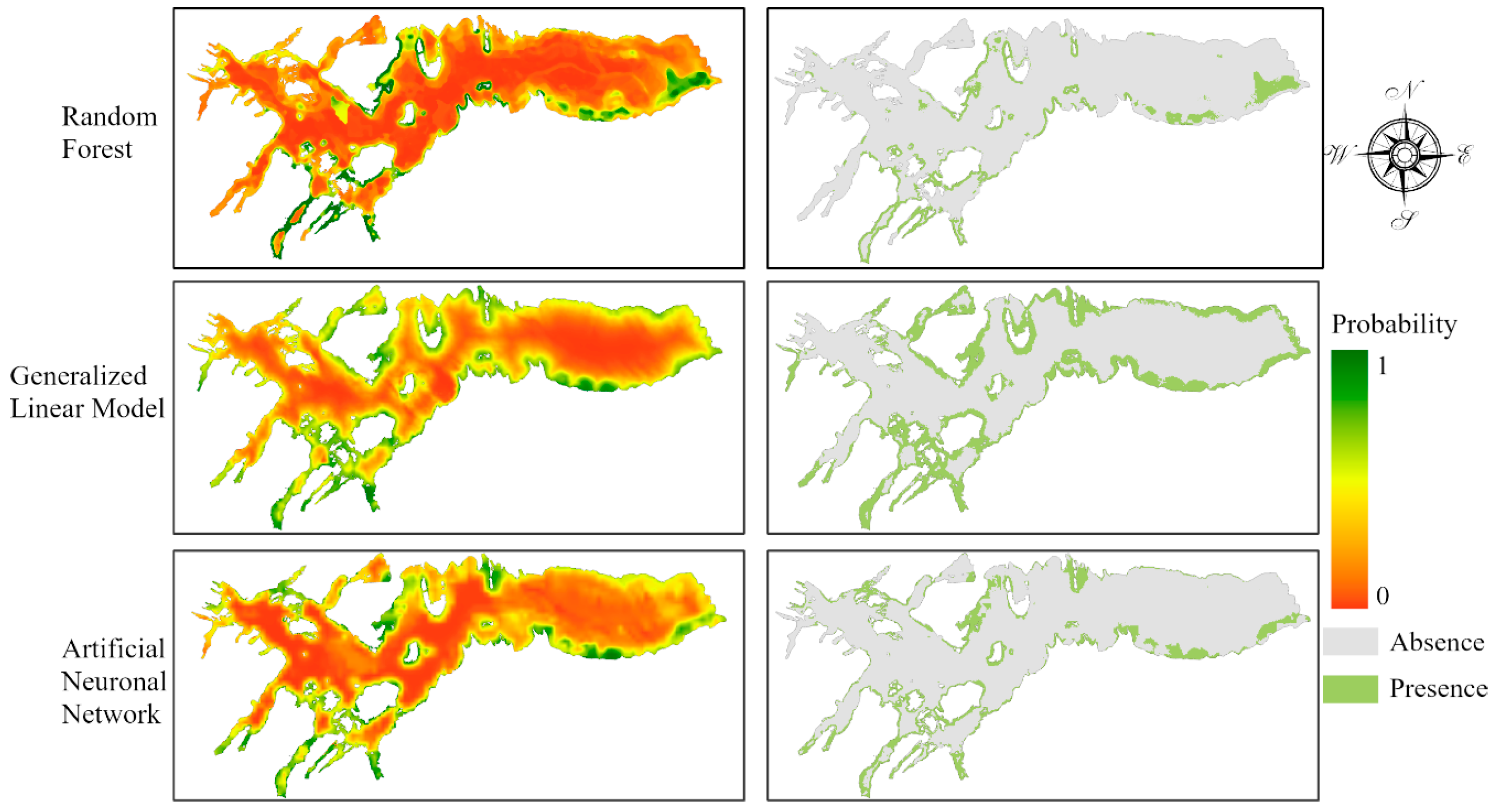
3.1. Model Validation
3.2. Chilean Dolphin Spatial Distribution Map
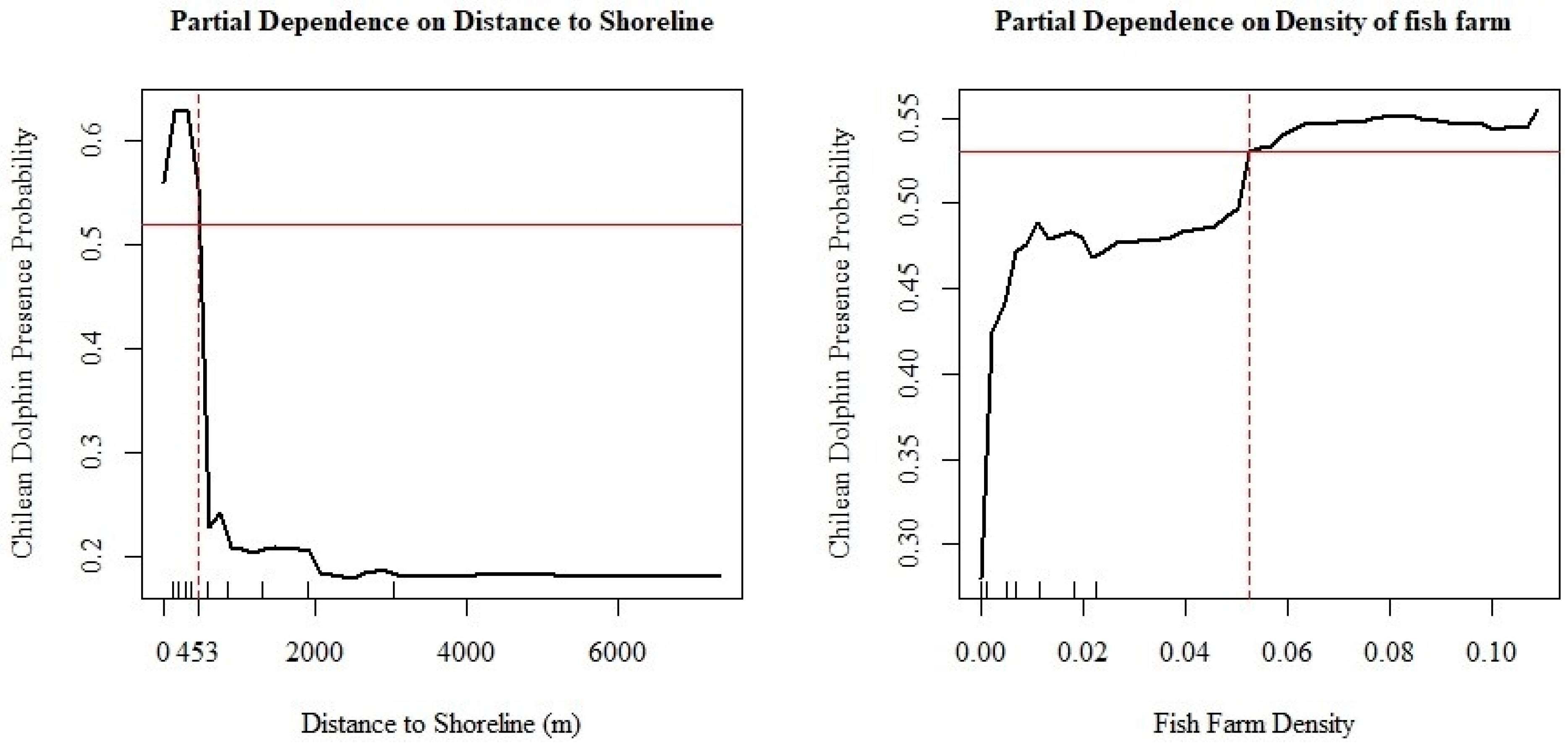
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics 2019; FAO: Rome, Italy, 2021; ISBN 978-92-5-135410-0. [Google Scholar]
- Félix, F.; Mangel, J.C.; Alfaro-Shigueto, J.; Cocas, L.A.; Guerra, J.; Pérez-Alvarez, M.J.; Sepúlveda, M. Challenges and Opportunities for the Conservation of Marine Mammals in the Southeast Pacific with the Entry into Force of the U.S. Marine Mammal Protection Act. Reg. Stud. Mar. Sci. 2021, 48, 102036. [Google Scholar] [CrossRef]
- Mena, M.; Figueroa Fernández, A. Biodiversidad de Chile: Patrimonio y Desafíos, 3rd ed.; Ministerio del Medio Ambiente, Gobierno de Chile: Santiago, Chile, 2018. [Google Scholar]
- Reeves, R.R.; Crespo, E.A.; Dans, S.; Jefferson, T.A.; Karczmarski, L.; Laidre, K.; O’Corry-Crowe, G.; Pedraza, S.; Rojas-Bracho, L.; Secchi, E.R.; et al. Cephalorhynchus eutropia 2008. In IUCN 2010. IUCN Red List ofThreatened Species. Version 2010.4. Available online: www.iucnredlist.org (accessed on 20 May 2024).
- Heinrich, S.; Reeves, R. Cephalorhynchus eutropia—The IUCN Red List of Threatened Species; IUCN Red List: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Pérez-Álvarez, M.J.; Alvarez, E.; Aguayo-Lobo, A.; Olavarría, C. Occurrence and Distribution of Chilean Dolphin (Cephalorhynchus eutropia) in Coastal Waters of Central Chile. N. Z. J. Mar. Freshw. Res. 2007, 41, 405–409. [Google Scholar] [CrossRef]
- Buscaglia, M.; Sielfeld, W.; Aguayo-Lobo, A. Dolphins Distributions (Mammalia: Delphinidae) in an Upwellings Zone (Chile). An. Del Inst. Patagon. 2020, 48, 7–28. [Google Scholar] [CrossRef]
- Aguayo, L.A. Progress Report on Small Cetacean Research in Chile. J. Fish. Res. Board Can. 1975, 32, 1123–1143. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.J.; Olavarría, C.; Moraga, R.; Baker, C.S.; Hamner, R.M.; Poulin, E. Microsatellite Markers Reveal Strong Genetic Structure in the Endemic Chilean Dolphin. PLoS ONE 2015, 10, e0123956. [Google Scholar] [CrossRef]
- Lescrauwaet, A.N.-C.; Gibbons, J. Mortality of Small Cetaceans and the Crab Bait Fishery in the Magallanes Area of Chile Since 1980; Casilla: Punta Arenas, Chile, 1994; Volume 15. [Google Scholar]
- Natalie, R.; Goodall, P. Report on the Small Cetaceans Stranded on the Coasts of Tierra Del Fuego; Porpoise Research Library: Burnaby, BC, Canada, 1978. [Google Scholar]
- Reyes, J.C.; Oporto, J.A. Gillnet Fisheries and Cetaceans in the Southeast Pacific; Porpoise Research Library: Burnaby, BC, Canada, 1994; Volume 15. [Google Scholar]
- Buschmann, A.H.; López, D.A.; Medina, A. A Review of the Environmental Effects and Alternative Production Strategies of Marine Aquaculture in Chile. Aquac. Eng. 1996, 15, 397–421. [Google Scholar] [CrossRef]
- Viddi, F.A.; Lescrauwaet, A.-K. Insights on Habitat Selection and Behavioural Patterns of Peale’s Dolphins (Lagenorhynchus australis) in the Strait of Magellan, Southern Chile. Aquat. Mamm. 2005, 31, 176–183. [Google Scholar] [CrossRef]
- Ribeiro, S.; Viddi, F.A.; Cordeiro, J.L.; Freitas, T.R.O. Fine-Scale Habitat Selection of Chilean Dolphins (Cephalorhynchus eutropia): Interactions with Aquaculture Activities in Southern Chiloé Island, Chile. J. Mar. Biol. Assoc. United Kingd. 2007, 87, 119–128. [Google Scholar] [CrossRef]
- Christie, C.A. Niveles de Organización Social Del Delfín Chileno Cephalorhynchus eutropia (Gray, 1846) y Delfín Austral Lagenorhynchus australis (Peale, 1848) En La Isla de Chiloé; Universidad Austral de Chile: Valdivia, Chile, 2005. [Google Scholar]
- Balkenhol Neumann, L.A. Distribución Geográfica Del Delfín Chileno Cephalorhynchus eutropia (Gray, 1846) y Delfín Austral, Lagenorhynchus australis (Peale, 1848), En Los Fiordos Comau y Reñihué. Xa Región; Universidad Austral de Chile: Valdivia, Chile, 2005. [Google Scholar]
- Heinrich, S.; Elwen, S.; Bräger, S. Patterns of Sympatry in Lagenorhynchus and Cephalorhynchus. In The Dusky Dolphin; Elesiver: Amsterdam, The Netherlands, 2010; pp. 313–332. [Google Scholar] [CrossRef]
- Heinrich, S.; Genov, T.; Fuentes Riquelme, M.; Hammond, P.S. Fine-scale Habitat Partitioning of Chilean and Peale’s Dolphins and Their Overlap with Aquaculture. Aquat. Conserv. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Ribeiro, S.; Viddi, F.A.; Freitas, T.R.O. Behavioural Responses of Chilean Dolphins (Cephalorhynchus eutropia) to Boats in Yaldad Bay, Southern Chile. Aquat. Mamm. 2005, 31, 234–242. [Google Scholar] [CrossRef]
- Viddi, F.A.; Harcourt, R.G.; Hucke-Gaete, R. Identifying Key Habitats for the Conservation of Chilean dolphins in the Fjords of Southern Chile. Aquat. Conserv. 2015, 26, 506–516. [Google Scholar] [CrossRef]
- Zamorano-Abramson, J.; Gibbons, J.; Capella, J. Diversidad y Distribución Estival de Cetáceos En Aguas Interiores Del Norte de Aisén, Chile. An. Del Inst. Patagon. 2010, 38, 151–157. [Google Scholar] [CrossRef]
- Lescrauwaet, A.-C.; Gibbons, J.; Guzman, L.; Schiavini, A. Abundance Estimation of Commerson’s Dolphin in the Eastern Area of the Strait of Magellan-Chile. Rev. Chil. Hist. Nat. 2000, 73, 473–478. [Google Scholar] [CrossRef]
- Gibbons, J.; Venegas, C.; Guzman, L.; Pizarro, G.; Bore, D.; Galvez, P.; Harlin, A.; Capella, J.; Brager, S. Programa de Monitoreo de Pequeños Cetáceos En La XII Región—Informe Final Fondo de Investigación Pesquera; FIPA: Alameda, CA, USA, 2002. [Google Scholar]
- Moreno, P.I.; François, J.P.; Villa-Martínez, R.P.; Moy, C.M. Millennial-Scale Variability in Southern Hemisphere Westerly Wind Activity over the Last 5000 Years in SW Patagonia. Quat. Sci. Rev. 2009, 28, 25–38. [Google Scholar] [CrossRef]
- Kilian, R.; Baeza, O.; Breuer, S.; Ríos, F.; Arz, H.; Lamy, F.; Wirtz, J.; Baque, D.; Korf, P.; Kremer, K.; et al. Late Glacial and Holocene Paleogeographical and Paleoecological Evolution of the Seno Skyring and Otway Fjord Systems in the Magellan Region. An. Del Inst. Patagon. 2013, 41, 5–26. [Google Scholar] [CrossRef]
- Lamy, F.; Kilian, R.; Arz, H.W.; Francois, J.-P.; Kaiser, J.; Prange, M.; Steinke, T. Holocene Changes in the Position and Intensity of the Southern Westerly Wind Belt. Nat. Geosci. 2010, 3, 695–699. [Google Scholar] [CrossRef]
- Rebolledo, L.; Lange, C.B.; Bertrand, S.; Muñoz, P.; Salamanca, M.; Lazo, P.; Iriarte, J.L.; Vargas, G.; Pantoja, S.; Dezileau, L. Late Holocene Precipitation Variability Recorded in the Sediments of Reloncaví Fjord (41°S, 72°W), Chile. Quat. Res. 2015, 84, 21–36. [Google Scholar] [CrossRef]
- Schneider, C.; Glaser, M.; Kilian, R.; Santana, A.; Butorovic, N.; Casassa, G. Weather Observations Across the Southern Andes at 53°S. Phys. Geogr. 2003, 24, 97–119. [Google Scholar] [CrossRef]
- Freitas, C.; Kovacs, K.M.; Lydersen, C.; Ims, R.A. A Novel Method for Quantifying Habitat Selection and Predicting Habitat Use. J. Appl. Ecol. 2008, 45, 1213–1220. [Google Scholar] [CrossRef]
- Bosso, L.; Panzuto, R.; Balestrieri, R.; Smeraldo, S.; Chiusano, M.L.; Raffini, F.; Canestrelli, D.; Musco, L.; Gili, C. Integrating Citizen Science and Spatial Ecology to Inform Management and Conservation of the Italian Seahorses. Ecol. Inform. 2024, 79, 102402. [Google Scholar] [CrossRef]
- Overly, K.E.; Lecours, V. Mapping Queen Snapper (Etelis oculatus) Suitable Habitat in Puerto Rico Using Ensemble Species Distribution Modeling. PLoS ONE 2024, 19, e0298755. [Google Scholar] [CrossRef]
- Strickland, M.D.; McDonald, L.L. Introduction to the Special Section on Resource Selection. J. Wildl. Manag. 2006, 70, 321–323. [Google Scholar] [CrossRef]
- Leduc, R. Biogeography. In Encyclopedia of Marine Mammals; Elesiver: Amsterdam, The Netherlands, 2009; pp. 112–115. [Google Scholar]
- Forcada, J. Distribution. In Encyclopedia of Marine Mammals; Elesiver: Amsterdam, The Netherlands, 2009; pp. 316–321. [Google Scholar] [CrossRef]
- Cribb, N.; Miller, C.; Seuront, L. Towards a Standardized Approach of Cetacean Habitat: Past Achievements and Future Directions. Open J. Mar. Sci. 2015, 5, 335–357. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Bosso, L.; Fasola, M.; Santicchia, F.; Aloise, G.; Lioy, S.; Tricarico, E.; Ruggieri, L.; Bovero, S.; Mori, E.; et al. Different Facets of the Same Niche: Integrating Citizen Science and Scientific Survey Data to Predict Biological Invasion Risk under Multiple Global Change Drivers. Glob. Chang. Biol. 2023, 29, 5509–5523. [Google Scholar] [CrossRef]
- Curd, A.; Chevalier, M.; Vasquez, M.; Boyé, A.; Firth, L.B.; Marzloff, M.P.; Bricheno, L.M.; Burrows, M.T.; Bush, L.E.; Cordier, C.; et al. Applying Landscape Metrics to Species Distribution Model Predictions to Characterize Internal Range Structure and Associated Changes. Glob. Chang. Biol. 2023, 29, 631–647. [Google Scholar] [CrossRef]
- Redfern, J.; Ferguson, M.; Becker, E.; Hyrenbach, K.; Good, C.; Barlow, J.; Kaschner, K.; Baumgartner, M.; Forney, K.; Ballance, L.; et al. Techniques for Cetacean–Habitat Modeling. Mar. Ecol. Prog. Ser. 2006, 310, 271–295. [Google Scholar] [CrossRef]
- Croll, D.A.; Tershy, B.R.; Hewitt, R.P.; Demer, D.A.; Fiedler, P.C.; Smith, S.E.; Armstrong, W.; Popp, J.M.; Kiekhefer, T.; Lopez, V.R.; et al. An Integrated Approch to the Foraging Ecology of Marine Birds and Mammals. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 1353–1371. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.J.; Olavarría, C.; Moraga, R.; Baker, C.S.; Hamner, R.M.; Poulin, E. Historical Dimensions of Population Structure in a Continuously Distributed Marine Species: The Case of the Endemic Chilean dolphin. Sci. Rep. 2016, 6, 35507. [Google Scholar] [CrossRef]
- Cañadas, A.; Sagarminaga, R.; De Stephanis, R.; Urquiola, E.; Hammond, P.S. Habitat Preference Modelling as a Conservation Tool: Proposals for Marine Protected Areas for Cetaceans in Southern Spanish Waters. Aquat. Conserv. 2005, 15, 495–521. [Google Scholar] [CrossRef]
- Acha, E.M.; Mianzan, H.W.; Guerrero, R.A.; Favero, M.; Bava, J. Marine Fronts at the Continental Shelves of Austral South America. J. Mar. Syst. 2004, 44, 83–105. [Google Scholar] [CrossRef]
- Pinilla, E.; Soto, C.; San Martin, J.; Valdebenito, P.; Soto, G.; Reche, P. Determinación de Las Escalas de Intercambio de Agua En y Canales de La Patagonia Chilena, 2021–2022; IFOP: Paris, France, 2022. [Google Scholar]
- Reche, P.; Artal, O.; Pinilla, E.; Ruiz, C.; Venegas, O.; Arriagada, A.; Falvey, M. CHONOS: Oceanographic Information Website for Chilean Patagonia. Ocean. Coast. Manag. 2021, 208, 105634. [Google Scholar] [CrossRef]
- Sibson, R. A Brief Description of Natural Neighbor Interpolation. In Interpreting Multivariate Data; Barnett, V., Ed.; John Wiley & Sons: New York, NY, USA, 1981; pp. 21–36. [Google Scholar]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Chapman and Hall: London, UK, 1986. [Google Scholar]
- ESRI ArcGIS Pro, version 3.3; Environmental Systems Research Institute: Redlands, CA, USA, 2024.
- Baughman, C.; Jones, B.; Bartz, K.; Young, D.; Zimmerman, C. Reconstructing Turbidity in a Glacially Influenced Lake Using the Landsat TM and ETM+ Surface Reflectance Climate Data Record Archive, Lake Clark, Alaska. Remote Sens. 2015, 7, 13692–13710. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Duran-Llacer, I.; González-Rodríguez, L.; Cardenas, R.; Urrutia, R. Retrieving Water Turbidity in Araucanian Lakes (South-Central Chile) Based on Multispectral Landsat Imagery. Remote Sens. 2021, 13, 3133. [Google Scholar] [CrossRef]
- Bid, S.; Siddique, G. Identification of Seasonal Variation of Water Turbidity Using NDTI Method in Panchet Hill Dam, India. Model. Earth Syst. Environ. 2019, 5, 1179–1200. [Google Scholar] [CrossRef]
- ESRI ArcGIS Pro, version 2.9.0; Environmental Systems Research Institute: Redlands, CA, USA, 2020.
- Carlucci, R.; Cipriano, G.; Paoli, C.; Ricci, P.; Fanizza, C.; Capezzuto, F.; Vassallo, P. Random Forest Population Modelling of Striped and Common-Bottlenose Dolphins in the Gulf of Taranto (Northern Ionian Sea, Central-Eastern Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 204, 177–192. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Olshen, R.; Stone, C. Classification and Regression Trees. Wadsworth Int. 1984, 37, 237–251. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Regression Trees. In Classification and Regression Trees; CRC: New York, NY, USA, 2017; pp. 216–265. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling Species Presence-only Data with Random Forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized Linear and Generalized Additive Models in Studies of Species Distributions: Setting the Scene. Ecol. Modell. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models. Stat. Med. 1992, 11, 981–982. [Google Scholar] [CrossRef]
- Venables, W.N.; Dichmont, C.M. GLMs, GAMs and GLMMs: An Overview of Theory for Applications in Fisheries Research. Fish. Res. 2004, 70, 319–337. [Google Scholar] [CrossRef]
- Stock, B.C.; Ward, E.J.; Eguchi, T.; Jannot, J.E.; Thorson, J.T.; Feist, B.E.; Semmens, B.X. Comparing Predictions of Fisheries Bycatch Using Multiple Spatiotemporal Species Distribution Model Frameworks. Can. J. Fish. Aquat. Sci. 2020, 77, 146–163. [Google Scholar] [CrossRef]
- McCulloch, W.S.; Pitts, W. A Logical Calculus of the Ideas Immanent in Nervous Activity. Bull. Math. Biophys. 1943, 5, 115–133. [Google Scholar] [CrossRef]
- Hopfield, J.J. Neural Networks and Physical Systems with Emergent Collective Computational Abilities. Proc. Natl. Acad. Sci. USA 1982, 79, 2554–2558. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Generalized Linear Models. In Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; pp. 183–210. [Google Scholar] [CrossRef]
- Ripley, B.D. Pattern Recognition and Neural Networks; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-521-46086-6. [Google Scholar]
- McPherson, J.M.; Jetz, W. Effects of Species’ Ecology on the Accuracy of Distribution Models. Ecography 2007, 30, 135–151. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Denny, M. Introduction to RStudio: Customizing RStudio. [CrossRef]
- Breiman, L. RandomForest: Breiman and Cutler’s Random Forests for Classification and Regression. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Viddi, F.A.; Bedriñana-Romano, L.; Hucke-Gaete, R. Potenciales Riesgos e Impactos En Cetáceos de La Acuicultura Industrial de Salmón, En La Patagonia Chilena; Patagonia: Valdivia, Chile, 2023. [Google Scholar]
- Pérez-Alvarez, M.J. Evaluación de La Interacción Entre El Delfín Chileno (Cephalorhynchus eutropia) y Actividades de Pesca Costera y Acuicultura a Lo Largo de Su Distribución: Fase 1; FIPA: Alameda, CA, USA, 2018. [Google Scholar]
- Marini, C.; Fossa, F.; Paoli, C.; Bellingeri, M.; Gnone, G.; Vassallo, P. Predicting Bottlenose Dolphin Distribution along Liguria Coast (Northwestern Mediterranean Sea) through Different Modeling Techniques and Indirect Predictors. J. Environ. Manag. 2015, 150, 9–20. [Google Scholar] [CrossRef]
- Purdon, J.; Shabangu, F.W.; Yemane, D.; Pienaar, M.; Somers, M.J.; Findlay, K. Species Distribution Modelling of Bryde’s Whales, Humpback Whales, Southern Right Whales, and Sperm Whales in the Southern African Region to Inform Their Conservation in Expanding Economies. PeerJ 2020, 8, e9997. [Google Scholar] [CrossRef]
- Capella, J.; Gibbons, J. Mamíferos Marinos Del Canal Fitzroy, Isla Riesco, Región de Magallanes—Informe Final Para Sociedad Mina Invierno SA; Mina Invierno: Punta Arenas, Chile, 2013. [Google Scholar]
- Capella, J.; Gibbons, J. Monitoreo de Biodiversidad de Mamíferos y Aves Marinas En El Estero Poca Esperanza y Oeste de Almirante Montt, Provincia de Última Esperanza; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Capella, J.; Gibbons, J. Monitoreo de La Biodiversidad de Mamíferos y Aves Marinas y Costeras En Seno Taraba, Región de Magallanes—Informe Para Multiexport; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Capella, J.; Gibbons, J. Monitoreo de La Biodiversidad de Mamíferos y Aves Marinas y Costeras Del Seno Skyring, Region de Magallanes—Informe Final Para Australis Mar; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Capella, J.; Gibbons, J. Monitoreo de Aves y Mamíferos Marinos En Estero Córdova, Norte de Isla Desolación, Provincia de Magallanes—Informe Técnico Para Australis Mar y Bluriver SpA; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Capella, J.; Gibbons, J. Monitoreo de Aves y Mamíferos Marinos En Golfo Xaultegua, Comuna de Río Verde, Provincia de Magallanes—Informe Técnico Para Australis Mar y Bluriver SpA; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Capella, J.; Gibbons, J. Monitoreo de Aves y Mamíferos Marinos En Seno Glacier, Peninsula Muñoz-Gamero, Provincia de Magallanes, Verano 2020, Informe Técnico Para Australis Mar; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Capella, J.; Gibbons, J. Biodiversidad de Mamíferos y Aves Marinas En Seno Staines, Provincia de Última Esperanza, XII Región, Monitoreo de Otoño, Junio 2020—Informe Final Para Aquachile SA; Mina Invierno: Punta Arenas, Chile, 2020. [Google Scholar]
- Pichler, F.B.; Olavarría, B.C. Resolving Chilean Dolphin (Cephalorhynchus eutropia, Gray 1846) Synonymy by Sequencing DNA Extracted from Teeth of Museum Specimens. Rev. Biol. Mar. Oceanogr. 2001, 36, 117–121. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Cabello, F.; Young, K.; Carvajal, J.; Varela, D.A.; Henríquez, L. Salmon Aquaculture and Coastal Ecosystem Health in Chile: Analysis of Regulations, Environmental Impacts and Bioremediation Systems. Ocean. Coast. Manag. 2009, 52, 243–249. [Google Scholar] [CrossRef]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental Issues in Chilean Salmon Farming: A Review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Acevedo, J.; Aguayo-Lobo, A.; Haro, D.; Garrido, G.; Olave, C. Occurrence of the Commersonʼs Dolphin (Cephalorhynchus commersonii) in Fitz Roy Channel, Pacific Coast of Chilean Patagonia. Aquat. Mamm. 2019, 45, 266–273. [Google Scholar] [CrossRef]



| Models | AUC | |||
|---|---|---|---|---|
| Training | Validation | RMSE | TSS | |
| RF | 1 | 0.97 | 0.22 | 0.91 |
| GLM | 0.82 | 0.81 | 1.70 | 0.52 |
| ANN | 0.94 | 0.88 | 0.37 | 0.68 |
| Variable | Importance |
|---|---|
| Distance to shoreline | 111.4 |
| Fish farm density | 50 |
| Salinity | 48 |
| Bathymetry | 35 |
| Turbidity | 31 |
| River mouths | 28 |
| Dissolved oxygen | 26 |
| Seafloor temperature | 21 |
| Silica | 20 |
| Kelp | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, L.; Cuellar, Y.; Gibbons, J.; Pinilla Matamala, E.; Demers, S.; Capella, J. Mapping the Future: Revealing Habitat Preferences and Patterns of the Endangered Chilean Dolphin in Seno Skyring, Patagonia. Biology 2024, 13, 514. https://doi.org/10.3390/biology13070514
Perez L, Cuellar Y, Gibbons J, Pinilla Matamala E, Demers S, Capella J. Mapping the Future: Revealing Habitat Preferences and Patterns of the Endangered Chilean Dolphin in Seno Skyring, Patagonia. Biology. 2024; 13(7):514. https://doi.org/10.3390/biology13070514
Chicago/Turabian StylePerez, Liliana, Yenny Cuellar, Jorge Gibbons, Elias Pinilla Matamala, Simon Demers, and Juan Capella. 2024. "Mapping the Future: Revealing Habitat Preferences and Patterns of the Endangered Chilean Dolphin in Seno Skyring, Patagonia" Biology 13, no. 7: 514. https://doi.org/10.3390/biology13070514
APA StylePerez, L., Cuellar, Y., Gibbons, J., Pinilla Matamala, E., Demers, S., & Capella, J. (2024). Mapping the Future: Revealing Habitat Preferences and Patterns of the Endangered Chilean Dolphin in Seno Skyring, Patagonia. Biology, 13(7), 514. https://doi.org/10.3390/biology13070514







