The Cytotoxic Effects of Human Mesenchymal Stem Cells Induced by Uranium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Uranium Treatment
2.2. Cell Viability Assay
2.3. Flow Cytometry Analysis
2.4. Caspase3/7 Staining
2.5. Immunofluorescence Staining of γH2AX Foci for DNA Damage Assay
2.6. TEM Analysis
2.7. Wound Healing Assay
2.8. Transwell Migration Assay
2.9. GJIC Assay
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
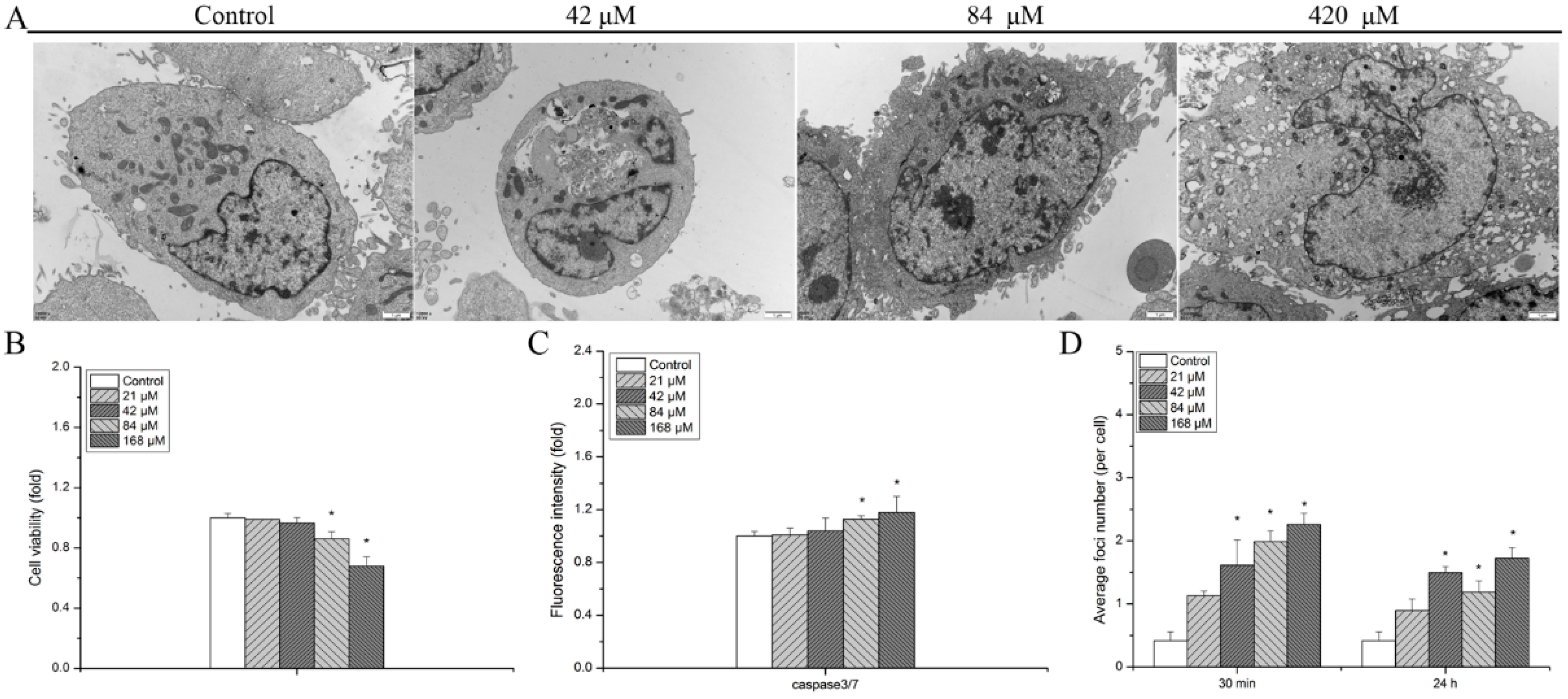
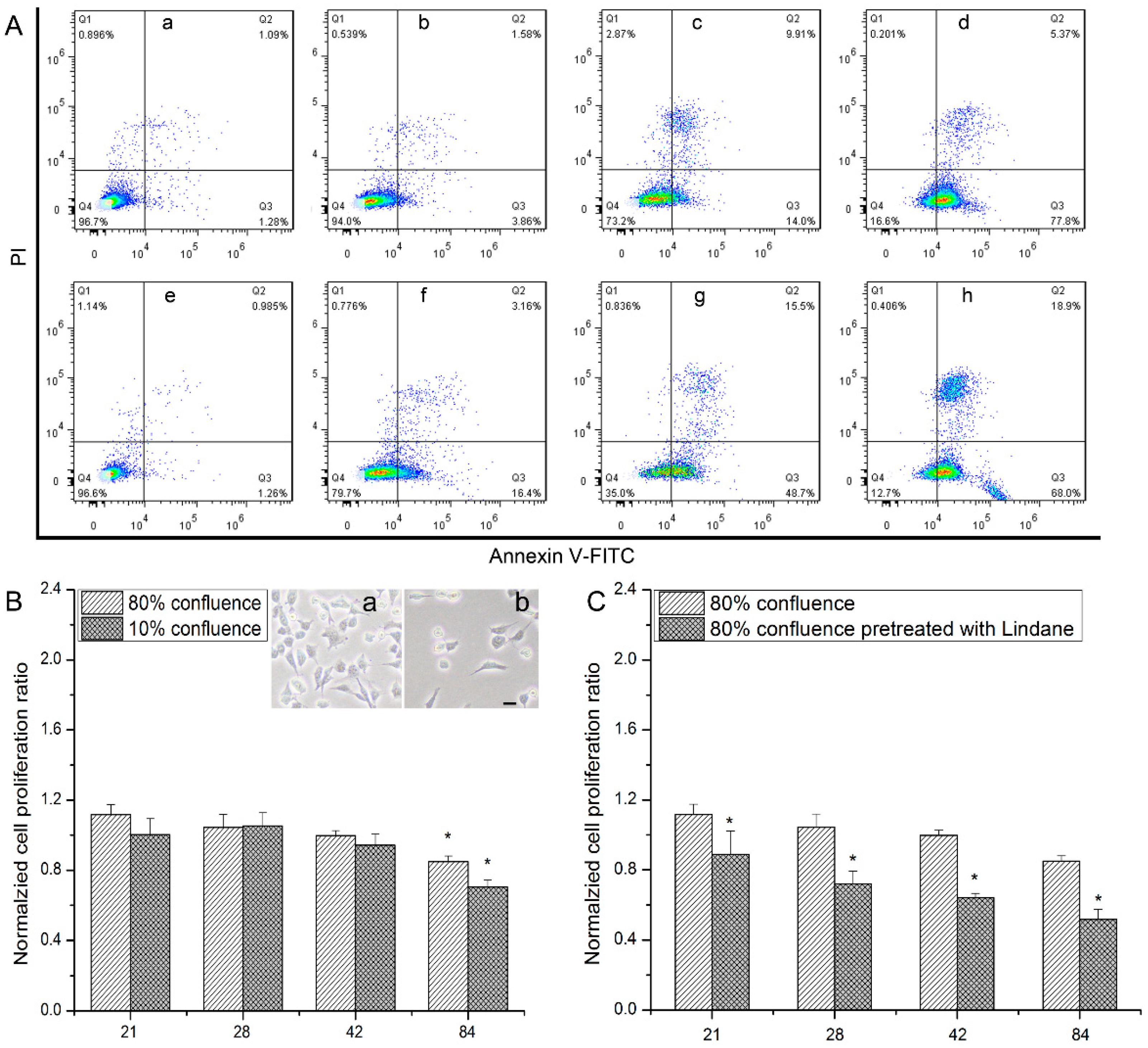
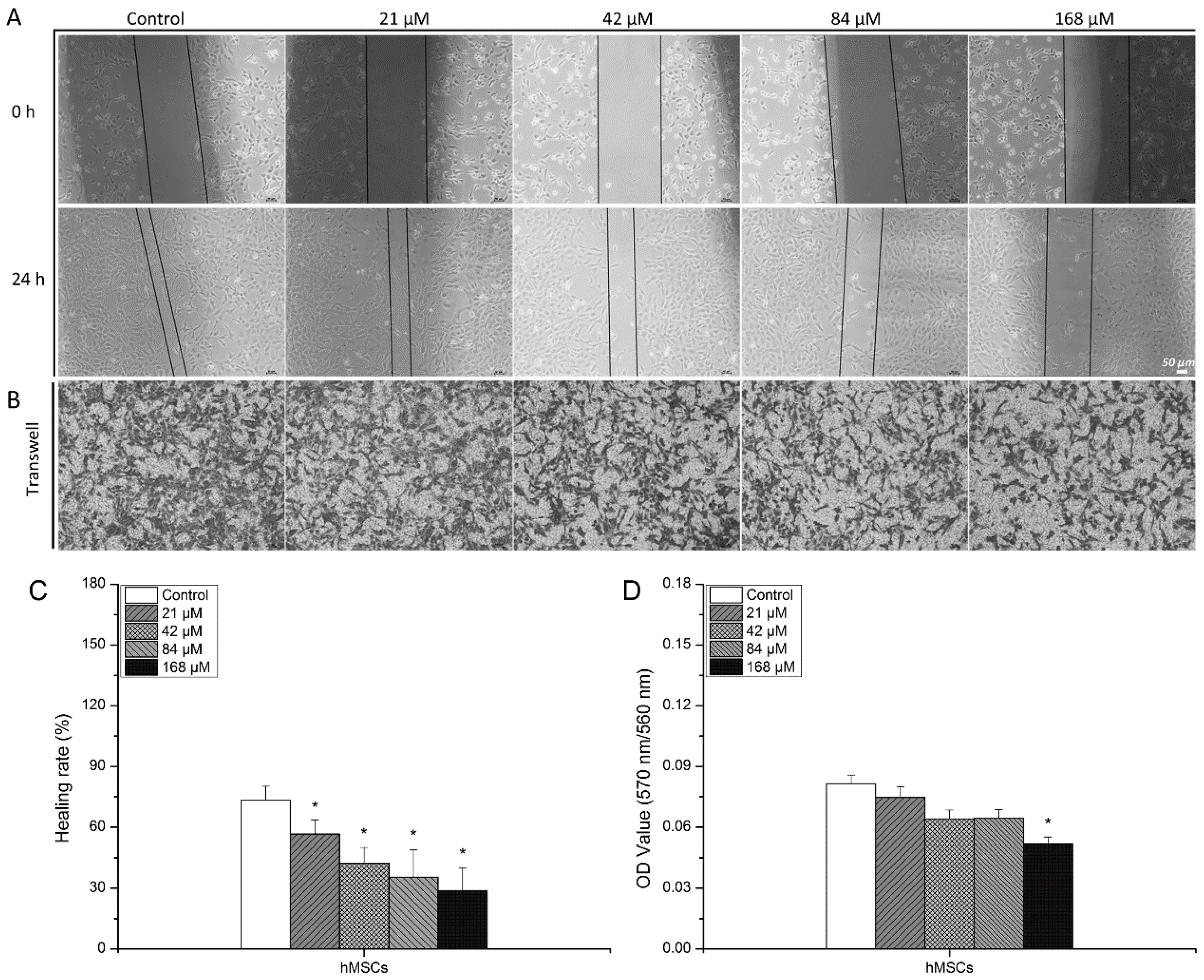
3.1. Detrimental Effects Observed in hMSCs after Exposure to Uranyl Nitrate
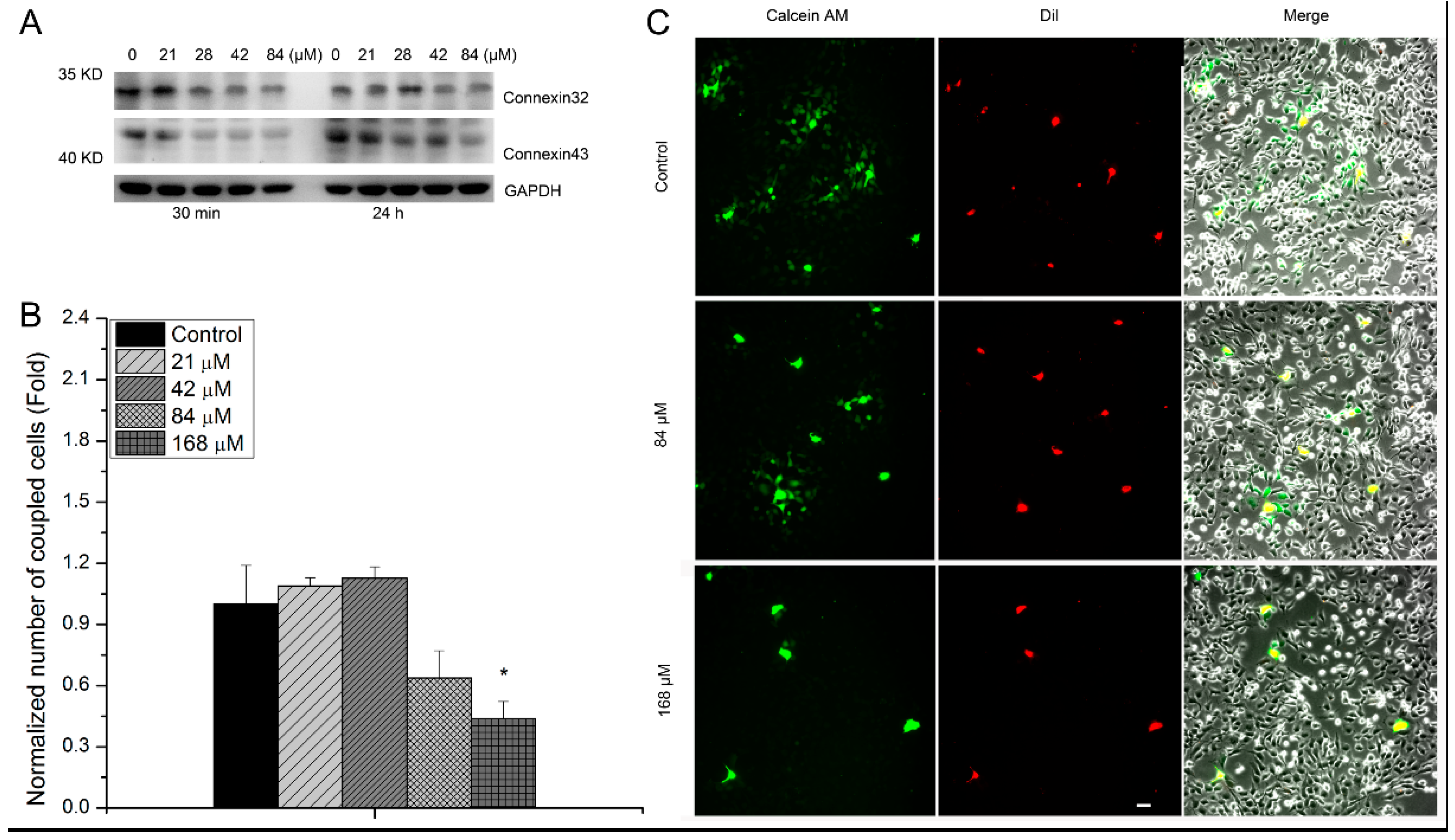
3.2. The Relevance of GJIC to Cytotoxic Effects of hMSCs Induced by Uranyl Nitrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2016 Report: Report to the General Assembly, with Scientific Annexes, United Nations; United Nations Scientific Committee on the Effects of Atomic Radiation: Vienna, Austrua, 2017; Available online: https://www.unscear.org/unscear/uploads/documents/publications/UNSCEAR_2016_Annex-C.pdf (accessed on 10 July 2024).
- Arzuaga, X.; Gehlhaus, M.; Strong, J. Modes of action associated with uranium induced adverse effects in bone function and development. Toxicol. Lett. 2015, 236, 123–130. [Google Scholar] [CrossRef] [PubMed]
- LaCerte, C.; Xie, H.; Aboueissa, A.-M.; Wise, J.P. Particulate depleted uranium is cytotoxic and clastogenic to human lung epithelial cells. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 697, 33–37. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Kitahara, K.; Suzuki, K.; Blyth, B.J.; Suya, N.; Konishi, T.; Terada, Y.; Shimada, Y. Cellular localization of uranium in the renal proximal tubules during acute renal uranium toxicity. J. Appl. Toxicol. 2015, 35, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Kathren, R.L.; Tolmachev, S.Y. Natural Uranium Tissue Content of Three Caucasian Males. Health Phys. 2015, 109, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Brooks, K.; Stewart, M.; Anderson, B.; Shi, L.; McClain, D.; Page, N. Genomic instability in human osteoblast cells after exposure to depleted uranium: Delayed lethality and micronuclei formation. J. Environ. Radioact. 2003, 64, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Tasat, D.R.; Orona, N.S.; Mandalunis, P.M.; Cabrini, R.L.; Ubios, A.M. Ultrastructural and metabolic changes in osteoblasts exposed to uranyl nitrate. Arch. Toxicol. 2007, 81, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Milgram, S.; Carrière, M.; Thiebault, C.; Malaval, L.; Gouget, B. Cytotoxic and phenotypic effects of uranium and lead on osteoblastic cells are highly dependent on metal speciation. Toxicology 2008, 250, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ubios, A.; Guglielmotti, M.; Steimetz, T.; Cabrini, R. Uranium inhibits bone formation in physiologic alveolar bone modeling and remodeling. Environ. Res. 1991, 54, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ. Int. 2020, 145, 106107–106122. [Google Scholar] [CrossRef]
- Bozal, C.B.; Martinez, A.B.; Cabrini, R.L.; Ubios, A.M. Effect of ethane-1-hydroxy-1,1-bisphosphonate (EHBP) on endochondral ossification lesions induced by a lethal oral dose of uranyl nitrate. Arch. Toxicol. 2005, 79, 475–481. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- White, A.C.; Lowry, W.E. Refining the role for adult stem cells as cancer cells of origin. Trends Cell Biol. 2015, 25, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting cancer stemness in the clinic: From hype to hope. Cell Stem Cell 2018, 24, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Huang, B.; Wang, N. Forces in stem cells and cancer stem cells. Cells Dev. 2022, 170, 203776. [Google Scholar] [CrossRef] [PubMed]
- He, J.-L.; You, Y.-X.; Pei, X.; Jiang, W.; Zeng, Q.-M.; Chen, B.; Wang, Y.-H.; Chen, E.-Q.; Tang, H.; Gao, X.-F.; et al. Tracking of Stem Cells in Chronic Liver Diseases: Current Trends and Developments. Stem Cell Rev. Rep. 2024, 20, 447–454. [Google Scholar] [CrossRef]
- Bright, S.; Kadhim, M. The future impacts of non-targeted effects. Int. J. Radiat. Biol. 2018, 94, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Hosseini, M.-J.; Ghazi-Khansari, M.; Pourahmad, J. Depleted uranium induces disruption of energy homeostasis and oxidative stress in isolated rat brain mitochondria. Metallomics 2013, 5, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.; Postnov, A.; Sukhorukov, V.; Popov, M.; Uzokov, J.; Orekhov, A. Significance of Mitochondrial Dysfunction in the Pathogenesis of Parkinson’s Disease. Front. Biosci. 2024, 29, 36. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Ma, T.; Guan, H.; Yang, Z.-H.; Liu, X.-D.; Wang, Y.; Jiang, Y.-G.; Zhou, P.-K. Inhibitory effect of uranyl nitrate on DNA double-strand break repair by depression of a set of proteins in the homologous recombination pathway. Toxicol. Res. 2017, 6, 711–718. [Google Scholar] [CrossRef]
- Yellowhair, M.; Romanotto, M.R.; Stearns, D.M.; Lantz, R.C. Uranyl acetate induced DNA single strand breaks and AP sites in Chinese hamster ovary cells. Toxicol. Appl. Pharmacol. 2018, 349, 29–38. [Google Scholar] [CrossRef]
- Xu, M.; Frelon, S.; Simon, O.; Lobinski, R.; Mounicou, S. Non-denaturating isoelectric focusing gel electrophoresis for uranium–protein complexes quantitative analysis with LA-ICP MS. Anal. Bioanal. Chem. 2014, 406, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Eb-Levadoux, Y.; Frelon, S.; Simon, O.; Arnaudguilhem, C.; Lobinski, R.; Mounicou, S. In vivo identification of potential uranium protein targets in zebrafish ovaries after chronic waterborne exposure. Metallomics 2017, 9, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Vidaud, C.; Robert, M.; Paredes, E.; Ortega, R.; Avazeri, E.; Jing, L.; Guigonis, J.-M.; Bresson, C.; Malard, V. Deciphering the uranium target proteins in human dopaminergic SH-SY5Y cells. Arch. Toxicol. 2019, 93, 2141–2154. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, W.; Chu, J.; Chen, C.; Li, X.; Tang, W.; Xia, B.; Xiong, Z. Uranium inhibits mammalian mitochondrial cytochrome c oxidase and ATP synthase. Environ. Pollut. 2021, 271, 116377. [Google Scholar] [CrossRef] [PubMed]
- Wade-Gueye, N.M.; Delissen, O.; Gourmelon, P.; Aigueperse, J.; Dublineau, I.; Souidi, M. Chronic exposure to natural uranium via drinking water affects bone in growing rats. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ghali, O.; Lencel, P.; Broux, O.; Chauveau, C.; Devedjian, J.; Hardouin, P.; Magne, D. TNF-α and IL-1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009, 84, 499–504. [Google Scholar] [CrossRef]
- Qin, Y.; Han, L.; Yang, D.; Wei, H.; Liu, Y.; Xu, J.; Autrup, H.; Deng, F.; Guo, X. Silver nanoparticles increase connexin43-mediated gap junctional intercellular communication in HaCaT cells through activation of reactive oxygen species and mitogen-activated protein kinase signal pathway. J. Appl. Toxicol. 2018, 38, 564–574. [Google Scholar] [CrossRef]
- Totland, M.Z.; Rasmussen, N.L.; Knudsen, L.M.; Leithe, E. Regulation of gap junction intercellular communication by connexin ubiquitination: Physiological and pathophysiological implications. Cell. Mol. Life Sci. 2020, 77, 573–591. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Sun, J.; Zhao, Y.; Yuan, Y.; Gu, J.; Bian, J.; Zou, H.; Liu, Z. Gap junction intercellular communication mediates cadmium-induced apoptosis in hepatocytes via the Fas/FasL pathway. Environ. Toxicol. 2022, 37, 2692–2702. [Google Scholar] [CrossRef]
- Wiesner, M.; Berberich, O.; Hoefner, C.; Blunk, T.; Bauer-Kreisel, P. Gap junctional intercellular communication in adipose-derived stromal/stem cells is cell density-dependent and positively impacts adipogenic differentiation. J. Cell. Physiol. 2018, 233, 3315–3329. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Q.; Quan, Y.; Wang, W.; Mei, T.; Li, J.; Yang, G.; Ren, X.; Xue, J.; Wang, Y. Repair rates of DNA double-strand breaks under different doses of proton and γ-ray irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 276, 1–6. [Google Scholar] [CrossRef]
- Rouach, N.; Avignone, E.; Même, W.; Koulakoff, A.; Venance, L.; Blomstrand, F.; Giaume, C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol. Cell 1995, 94, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Bresson, C.; Ansoborlo, E.; Vidaud, C. Radionuclide speciation: A key point in the field of nuclear toxicology studies. J. Anal. At. Spectrom. 2011, 26, 593–601. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Qiu, T.; Zhang, H.; Liao, L.; Su, X. Epigenetic therapy targeting bone marrow mesenchymal stem cells for age-related bone diseases. Stem Cell Res. Ther. 2022, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Mirto, H.; Hengé-Napoli, M.H.; Gibert, R.; Ansoborlo, E.; Fournier, M.; Cambar, J. Intracellular behaviour of uranium (VI) on renal epithelial cell in culture (LLC-PK1): Influence of uranium speciation. Toxicol. Lett. 1999, 104, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Carrière, M.; Gouget, B.; Gallien, J.-P.; Avoscan, L.; Gobin, R.; Verbavatz, J.-M.; Khodja, H. Cellular distribution of uranium after acute exposure of renal epithelial cells: SEM, TEM and nuclear microscopy analysis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 231, 268–273. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.; Wang, B.; Jin, Z.; Hou, Y.; Ma, S.; Liu, X. The role of lysosome in cell death regulation. Tumor Biol. 2016, 37, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Pierrefite-Carle, V.; Santucci-Darmanin, S.; Breuil, V.; Gritsaenko, T.; Vidaud, C.; Creff, G.; Solari, P.L.; Pagnotta, S.; Al-Sahlanee, R.; Auwer, C.D.; et al. Effect of natural uranium on the UMR-106 osteoblastic cell line: Impairment of the autophagic process as an underlying mechanism of uranium toxicity. Arch. Toxicol. 2017, 91, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Huang, Y.-S.; Hosokawa, M.; Miyashita, K.; Hu, M.-L. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem. Interact. 2009, 182, 165–172. [Google Scholar] [CrossRef]
- Gava, F.; Rigal, L.; Mondesert, O.; Pesce, E.; Ducommun, B.; Lobjois, V. Gap junctions contribute to anchorage-independent clustering of breast cancer cells. BMC Cancer 2018, 18, 221. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta (BBA) Biomembr. 2005, 1711, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014, 588, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Autsavapromporn, N.; Ahmad, T.A.F.T.; Oikawa, M.; Homma-Takeda, S.; Furusawa, Y.; Wang, J.; Konishi, T. Bystander WI-38 Cells Modulate DNA Double-Strand Break Repair in Microbeam-Targeted A549 Cells through Gap Junction Intercellular Communication. Radiat. Prot. Dosim. 2019, 183, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lin, Z.; Cheng, X.; Chu, J.; Li, X.; Chen, C.; Zhu, T.; Li, W.; Lin, W.; Tang, W. Thorium inhibits human respiratory chain complex IV (cytochrome c oxidase). J. Hazard. Mater. 2022, 424, 127546. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Das Sarma, S.; Babu, S.; Pal, D.; Das Sarma, J. Interaction of arsenic with gap junction protein connexin 43 alters gap junctional intercellular communication. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2018, 1865, 1423–1436. [Google Scholar] [CrossRef]
- Van Campenhout, R.; Cooreman, A.; Leroy, K.; Rusiecka, O.M.; Van Brantegem, P.; Annaert, P.; Muyldermans, S.; Devoogdt, N.; Cogliati, B.; Kwak, B.R.; et al. Non-canonical roles of connexins. Prog. Biophys. Mol. Biol. 2020, 153, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Pogoda, K. The role of connexin 43 and pannexin 1 during acute inflammation. Front. Physiol. 2020, 11, 594097. [Google Scholar] [CrossRef]
- Lee, S.T.; Chang, Y.; Venton, B.J. Pannexin1 channels regulate mechanically stimulated but not spontaneous adenosine release. Anal. Bioanal. Chem. 2022, 414, 3781–3789. [Google Scholar] [CrossRef]
- Yang, D.; Chen, M.; Yang, S.; Deng, F.; Guo, X. Connexin hemichannels and pannexin channels in toxicity: Recent advances and mechanistic insights. Toxicology 2023, 488, 153488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, Y.; Yu, X. The Cytotoxic Effects of Human Mesenchymal Stem Cells Induced by Uranium. Biology 2024, 13, 525. https://doi.org/10.3390/biology13070525
Quan Y, Yu X. The Cytotoxic Effects of Human Mesenchymal Stem Cells Induced by Uranium. Biology. 2024; 13(7):525. https://doi.org/10.3390/biology13070525
Chicago/Turabian StyleQuan, Yi, and Xiaofang Yu. 2024. "The Cytotoxic Effects of Human Mesenchymal Stem Cells Induced by Uranium" Biology 13, no. 7: 525. https://doi.org/10.3390/biology13070525
APA StyleQuan, Y., & Yu, X. (2024). The Cytotoxic Effects of Human Mesenchymal Stem Cells Induced by Uranium. Biology, 13(7), 525. https://doi.org/10.3390/biology13070525




