Retrospective Single-Center Case Study of Clinical Variables and the Degree of Actinic Elastosis Associated with Rare Skin Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Inclusion Criteria
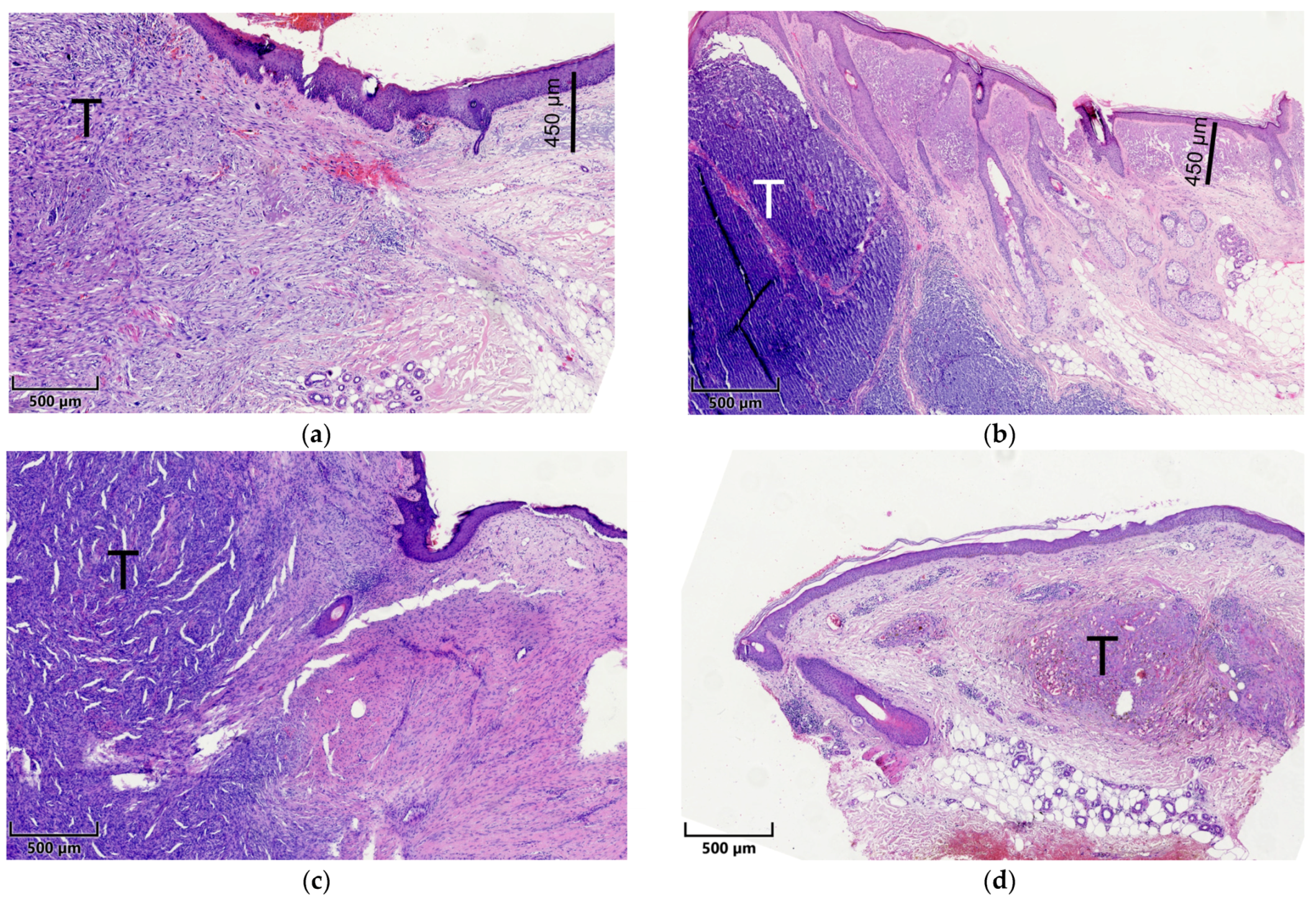
2.2. Histopathological Assessment
2.3. Microscopy and Visual Illustration
2.4. Statistical Analysis
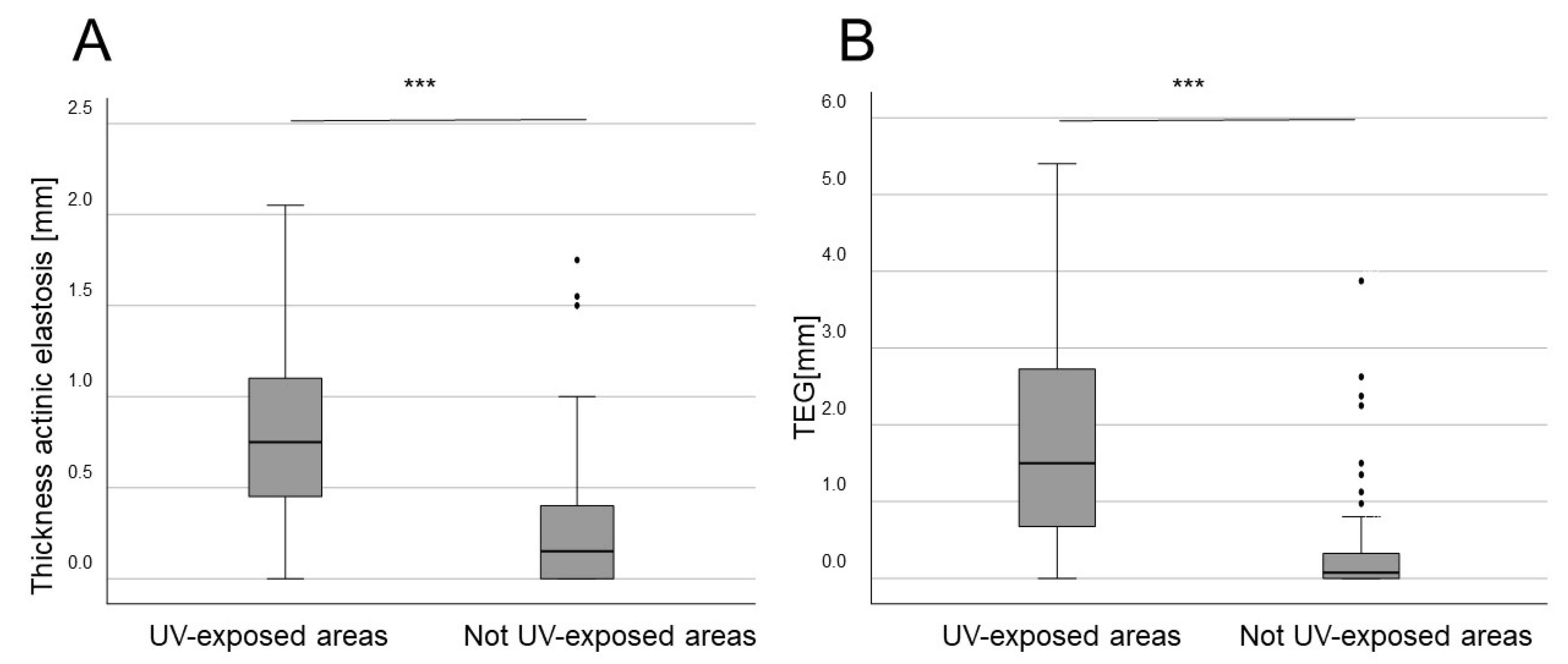
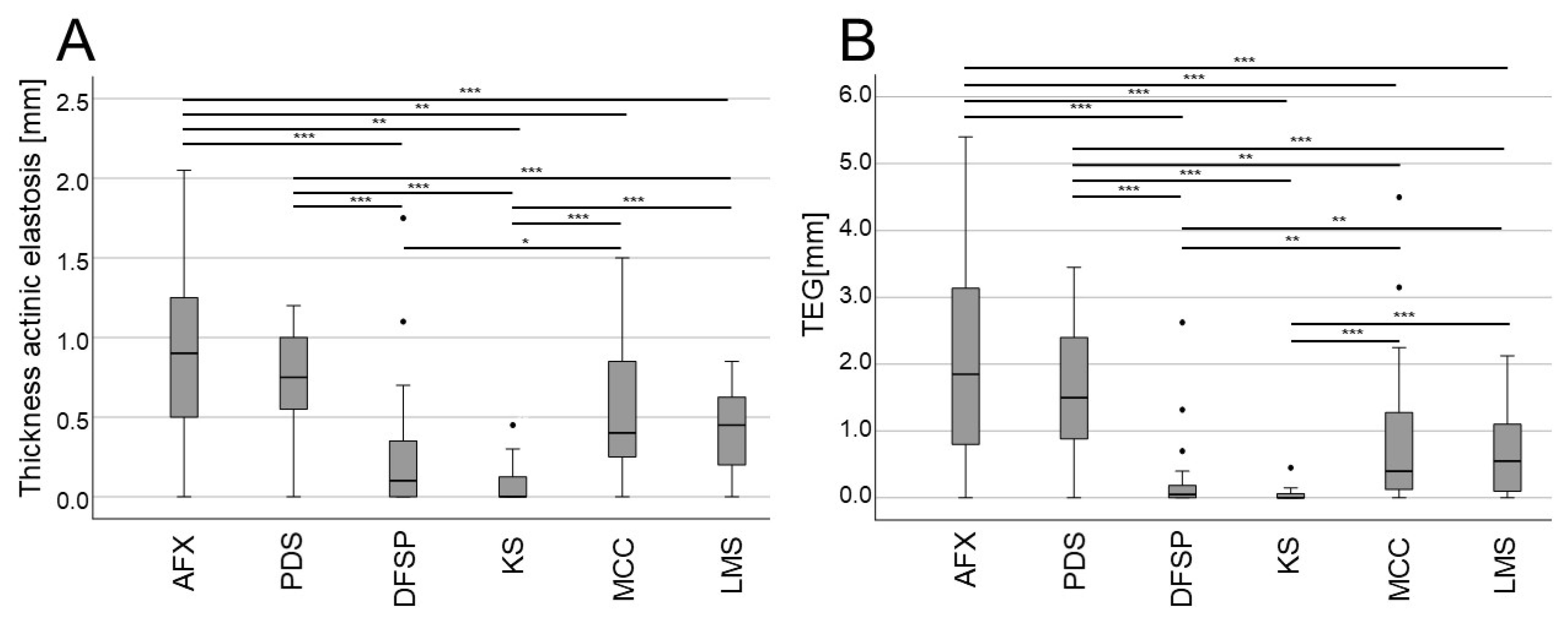
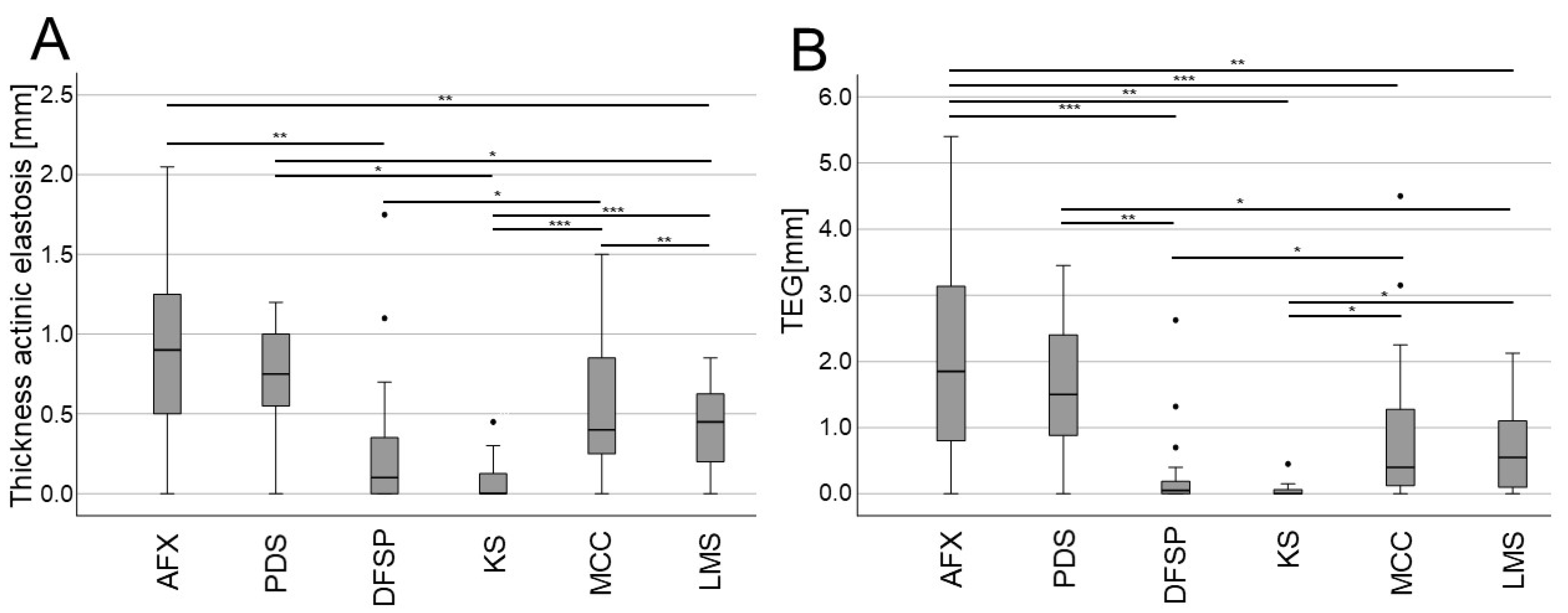
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Liopyris, K.; Kyrmanidou, E.; Fotiadou, C.; Sgouros, D.; Patsatsi, A.; Trakatelli, M.-G.; Kalloniati, E.; Lallas, A.; Lazaridou, E. Clinical and Dermoscopic Characteristics of Cutaneous Sarcomas: A Literature Review. Diagnostics 2023, 13, 1822. [Google Scholar] [CrossRef]
- Bolognia, J.; Jorizzo, J.L.; Schaffer, J.V. (Eds.) Dermatology, 3rd ed.; Elsevier/Saunders: Edinburgh, UK, 2012. [Google Scholar]
- Hunt, J.P.; Florell, S.R.; Buchmann, L.O. Rare skin malignancies of the head and neck: A review. Facial Plast. Surg. 2013, 29, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef]
- Schadendorf, D.; Lebbé, C.; zur Hausen, A.; Avril, M.-F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.; Schwertner, B.; Haerteis, S.; Aung, T.; Berneburg, M.; Geissler, E.K.; Mycielska, M.E.; Haferkamp, S. The Role of Citrate Homeostasis in Merkel Cell Carcinoma Pathogenesis. Cancers 2022, 14, 3425. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, J.; Steimle-Grauer, S.A.; Hein, R. Cutaneous sarcomas. J. Dtsch. Dermatol. Ges. 2017, 15, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Koch, A.; Hansel, G.; Schönlebe, J.; Kittner, T.; Pabst, F.; Haroske, G.; Nowak, A. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int. J. Dermatol. 2013, 52, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, P.; Fletcher, C.D.M.; Devesa, S.S.; Toro, J.R. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: An analysis of 12,114 cases. Cancer 2008, 113, 616–627. [Google Scholar] [CrossRef]
- Anderson, H.L.; Joseph, A.K. A pilot feasibility study of a rare skin tumor database. Dermatol. Surg. 2007, 33, 693–696. [Google Scholar] [CrossRef]
- Bowe, C.M.; Godhania, B.; Whittaker, M.; Walsh, S. Pleomorphic dermal sarcoma: A clinical and histological review of 49 cases. Br. J. Oral. Maxillofac. Surg. 2021, 59, 460–465. [Google Scholar] [CrossRef]
- Acosta, A.E.; Vélez, C.S. Dermatofibrosarcoma Protuberans. Curr. Treat. Opt. Oncol. 2017, 18, 56. [Google Scholar] [CrossRef]
- Patel, K.U.; Szabo, S.S.; Hernandez, V.S.; Prieto, V.G.; Abruzzo, L.V.; Lazar, A.J.F.; López-Terrada, D. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum. Pathol. 2008, 39, 184–193. [Google Scholar] [CrossRef]
- Hao, X.; Billings, S.D.; Wu, F.; Stultz, T.W.; Procop, G.W.; Mirkin, G.; Vidimos, A.T. Dermatofibrosarcoma Protuberans: Update on the Diagnosis and Treatment. J. Clin. Med. 2020, 9, 1752. [Google Scholar] [CrossRef]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Primers 2019, 5, 9. [Google Scholar] [CrossRef]
- Fu, L.; Tian, T.; Wang, B.; Lu, Z.; Gao, Y.; Sun, Y.; Lin, Y.-F.; Zhang, W.; Li, Y.; Zou, H. Global patterns and trends in Kaposi sarcoma incidence: A population-based study. Lancet Glob. Health 2023, 11, e1566–e1575. [Google Scholar] [CrossRef]
- Kazlouskaya, V.; Lai, Y.C.; Khachemoune, A. Leiomyosarcoma of the skin: Review of the literature with an emphasis on prognosis and management. Int. J. Dermatol. 2020, 59, 165–172. [Google Scholar] [CrossRef]
- Stam, H.; Lohuis, P.J.F.M.; Zupan-Kajcovski, B.; Wouters, M.W.J.M.; van der Hage, J.A.; Visser, O. Increasing incidence and survival of a rare skin cancer in the Netherlands. A population-based study of 2220 cases of skin adnexal carcinoma. J. Surg. Oncol. 2013, 107, 822–827. [Google Scholar] [CrossRef]
- Kuntz, T.; Siebdrath, J.; Hofmann, S.C.; Baltaci, M.; Schaller, J.; Hellmich, M.; von Goltzheim, L.S.; Assaf, C.; Oellig, F.; Michalowitz, A.-L.; et al. Increase of atypical fibroxanthoma and pleomorphic dermal sarcoma: A retrospective analysis of four German skin cancer centers. J. Dtsch. Dermatol. Ges. 2022, 20, 1581–1588. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Elder, D.E.; Massi, D.; Scolyer, R.A. (Eds.) WHO Classification of Skin Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Kurz, B.; Berneburg, M.; Singer, S. Sonnenschutz der menschlichen Haut: Grundlagen. Hautarzt 2022, 73, 251–256. [Google Scholar] [CrossRef]
- Oyasiji, T.; Tan, W.; Kane, J.; Skitzki, J.; Francescutti, V.; Salerno, K.; Khushalani, N.I. Malignant adnexal tumors of the skin: A single institution experience. World J. Surg. Oncol. 2018, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Kleibert, M.; Płachta, I.; Czarnecka, A.M.; Spałek, M.J.; Szumera-Ciećkiewicz, A.; Rutkowski, P. Treatment of Malignant Adnexal Tumors of the Skin: A 12-Year Perspective. Cancers 2022, 14, 998. [Google Scholar] [CrossRef]
- Waqas, O.; Faisal, M.; Haider, I.; Amjad, A.; Jamshed, A.; Hussain, R. Retrospective study of rare cutaneous malignant adnexal tumors of the head and neck in a tertiary care cancer hospital: A case series. J. Med. Case Rep. 2017, 11, 67. [Google Scholar] [CrossRef]
- Agelli, M.; Clegg, L.X. Epidemiology of primary Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2003, 49, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Pena, N.; Martínez-Campayo, N.; López-Solache, L. Relación entre fibroxantoma atípico y sarcoma pleomórfico dérmico: Histopatología de ambos y revisión de la literatura. Actas Dermosifiliogr. Engl. Ed. 2021, 112, 392–405. [Google Scholar] [CrossRef]
- Dei Tos, A.P.; Maestro, R.; Doglioni, C.; Gasparotto, D.; Boiocchi, M.; Laurino, L.; Fletcher, C.D. Ultraviolet-induced p53 mutations in atypical fibroxanthoma. Am. J. Pathol. 1994, 145, 11–17. [Google Scholar]
- Saleh, J.S.; Whittington, C.P.; Bresler, S.C.; Patel, R.M. Pleomorphic Dermal Sarcoma. Surg. Pathol. Clin. 2024, 17, 153–158. [Google Scholar] [CrossRef]
- Soleymani, T.; Aasi, S.Z.; Novoa, R.; Hollmig, S.T. Atypical Fibroxanthoma and Pleomorphic Dermal Sarcoma: Updates on Classification and Management. Dermatol. Clin. 2019, 37, 253–259. [Google Scholar] [CrossRef]
- Stoneham, S.; Hunter, A.; Raahimi, M.; Livesey, A.; Mitchell, C.D.; Keohane, S. Cutaneous sarcoma: A review and practical approach to management. Clin. Exp. Dermatol. 2023, 48, 866–872. [Google Scholar] [CrossRef]
- Klein, S.; Quaas, A.; Noh, K.-W.; Cartolano, M.; Abedpour, N.; Mauch, C.; Quantius, J.; Reinhardt, H.C.; Buettner, R.; Peifer, M.; et al. Integrative Analysis of Pleomorphic Dermal Sarcomas Reveals Fibroblastic Differentiation and Susceptibility to Immunotherapy. Clin. Cancer Res. 2020, 26, 5638–5645. [Google Scholar] [CrossRef]
- Goh, G.; Walradt, T.; Markarov, V.; Blom, A.; Riaz, N.; Doumani, R.; Stafstrom, K.; Moshiri, A.; Yelistratova, L.; Levinsohn, J.; et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016, 7, 3403–3415. [Google Scholar] [CrossRef]
- Peng, C.; Jian, X.; Xie, Y.; Li, L.; Ouyang, J.; Tang, L.; Zhang, X.; Su, J.; Zhao, S.; Liu, H.; et al. Genomic alterations of dermatofibrosarcoma protuberans revealed by whole-genome sequencing. Br. J. Dermatol. 2022, 186, 997–1009. [Google Scholar] [CrossRef]
- Iglesias-Pena, N.; López-Solache, L.; Martínez-Campayo, N.; Meilán-Sánchez, I.; Yebra-Pimentel, M.T.; Balboa-Barreiro, V.; Paradela, S.; Fonseca, E. Incidence rate and clinicopathological features of 62 atypical fibroxanthomas in a North-Western Spanish population. Australas. J. Dermatol. 2020, 61, e22–e27. [Google Scholar] [CrossRef]
- Bitel, A.; Schönlebe, J.; Krönert, C.; Wollina, U. Atypical fibroxanthoma: An analysis of 105 tumors. Dermatol. Ther. 2020, 33, e13962. [Google Scholar] [CrossRef]
- Ak, M.; Kahraman, A.; Arnold, F.M.; Turko, P.; Levesque, M.P.; Zoche, M.; Ramelyte, E.; Dummer, R. Clinicopathological and Genomic Profiles of Atypical Fibroxanthoma and Pleomorphic Dermal Sarcoma Identify Overlapping Signatures with a High Mutational Burden. Genes 2021, 12, 974. [Google Scholar] [CrossRef]
- Helbig, D.; Dippel, E.; Erdmann, M.; Frisman, A.; Kage, P.; Leiter, U.; Mentzel, T.; Seidel, C.; Weishaupt, C.; Ziemer, M.; et al. S1-guideline cutaneous and subcutaneous leiomyosarcoma. J. Dtsch. Dermatol. Ges. 2023, 21, 555–563. [Google Scholar] [CrossRef]
- Klein, S.; Persa, O.-D.; Mauch, C.; Noh, K.-W.; Pappesch, R.; Wagener-Ryczek, S.; Buettner, R.; Quaas, A.; Helbig, D. First report on two cases of pleomorphic dermal sarcoma successfully treated with immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1665977. [Google Scholar] [CrossRef]
- Malouf, G.G.; Lu, X.; Mouawad, R.; Spano, J.-P.; Grange, P.; Yan, F.; Aractingi, S.; Su, X.; Dupin, N. Genetic landscape of indolent and aggressive Kaposi sarcomas. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2343–2351. [Google Scholar] [CrossRef]
- Miller, T.I.; Zoumberos, N.A.; Johnson, B.; Rhodes, D.R.; Tomlins, S.A.; Chan, M.P.; Andea, A.A.; Lucas, D.R.; McHugh, J.B.; Smith, N.; et al. A genomic survey of sarcomas on sun-exposed skin reveals distinctive candidate drivers and potentially targetable mutations. Hum. Pathol. 2020, 102, 60–69. [Google Scholar] [CrossRef]
- Koelsche, C.; Schrimpf, D.; Stichel, D.; Sill, M.; Sahm, F.; Reuss, D.E.; Blattner, M.; Worst, B.; Heilig, C.E.; Beck, K.; et al. Sarcoma classification by DNA methylation profiling. Nat. Commun. 2021, 12, 498. [Google Scholar] [CrossRef]
- Mashima, E.; Sawada, Y. Epigenetics of Cutaneous Sarcoma. Int. J. Mol. Sci. 2021, 23, 422. [Google Scholar] [CrossRef]
- Spurgeon, M.E.; Lambert, P.F. Merkel cell polyomavirus: A newly discovered human virus with oncogenic potential. Virology 2013, 435, 118–130. [Google Scholar] [CrossRef]
- Starrett, G.J.; Marcelus, C.; Cantalupo, P.G.; Katz, J.P.; Cheng, J.; Akagi, K.; Thakuria, M.; Rabinowits, G.; Wang, L.C.; Symer, D.E.; et al. Merkel Cell Polyomavirus Exhibits Dominant Control of the Tumor Genome and Transcriptome in Virus-Associated Merkel Cell Carcinoma. mBio 2017, 8, e02079-16. [Google Scholar] [CrossRef]
- Brazel, D.; Kumar, P.; Doan, H.; Pan, T.; Shen, W.; Gao, L.; Moyers, J.T. Genomic Alterations and Tumor Mutation Burden in Merkel Cell Carcinoma. JAMA Netw. Open 2023, 6, e2249674. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Engels, E.A.; Frisch, M.; Goedert, J.J.; Biggar, R.J.; Miller, R.W. Merkel cell carcinoma and HIV infection. Lancet 2002, 359, 497–498. [Google Scholar] [CrossRef]
- Thomas, N.E.; Kricker, A.; From, L.; Busam, K.; Millikan, R.C.; Ritchey, M.E.; Armstrong, B.K.; Lee-Taylor, J.; Marrett, L.D.; Anton-Culver, H.; et al. Associations of cumulative sun exposure and phenotypic characteristics with histologic solar elastosis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2932–2941. [Google Scholar] [CrossRef]
- Pain, S.; Berthélémy, N.; Naudin, C.; Degrave, V.; André-Frei, V. Understanding Solar Skin Elastosis-Cause and Treatment. J. Cosmet. Sci. 2018, 69, 175–185. [Google Scholar]
- Drexler, K.; Zenderowski, V.; Schreieder, L.; Koschitzki, K.; Karrer, S.; Berneburg, M.; Haferkamp, S.; Niebel, D. Subtypes of Melanomas Associated with Different Degrees of Actinic Elastosis in Conventional Histology, Irrespective of Age and Body Site, Suggesting Chronic Ultraviolet Light Exposure as Driver for Lentigo Maligna Melanoma and Nodular Melanoma. Cancers 2023, 16, 1. [Google Scholar] [CrossRef]
- Drexler, K.; Drexler, H.; Karrer, S.; Landthaler, M.; Haferkamp, S.; Zeman, F.; Berneburg, M.; Niebel, D. Degree of Actinic Elastosis Is a Surrogate of Exposure to Chronic Ultraviolet Radiation and Correlates More Strongly with Cutaneous Squamous Cell Carcinoma than Basal Cell Carcinoma. Life 2023, 13, 811. [Google Scholar] [CrossRef]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institue of Pathology; McGraw Hill: New York, NY, USA, 1968. [Google Scholar]
- Beer, T.W.; Drury, P.; Heenan, P.J. Atypical fibroxanthoma: A histological and immunohistochemical review of 171 cases. Am. J. Dermatopathol. 2010, 32, 533–540. [Google Scholar] [CrossRef]
- Massi, D.; Franchi, A.; Alos, L.; Cook, M.; Di Palma, S.; Enguita, A.B.; Ferrara, G.; Kazakov, D.V.; Mentzel, T.; Michal, M.; et al. Primary cutaneous leiomyosarcoma: Clinicopathological analysis of 36 cases. Histopathology 2010, 56, 251–262. [Google Scholar] [CrossRef]
- Kransdorf, M.J. Malignant soft-tissue tumors in a large referral population: Distribution of diagnoses by age, sex, and location. AJR Am. J. Roentgenol. 1995, 164, 129–134. [Google Scholar] [CrossRef]
- Kreicher, K.L.; Kurlander, D.E.; Gittleman, H.R.; Barnholtz-Sloan, J.S.; Bordeaux, J.S. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol. Surg. 2016, 42 (Suppl. 1), S24–S31. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Chen, Y.Q.; Kopp, J.B.; Fisher, L.; Brown, D.B.; Hahn, P.J.; Robey, F.A.; Lakkakorpi, J.; Uitto, J. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J. Am. Acad. Dermatol. 1996, 34, 209–218. [Google Scholar] [CrossRef]
| AFX (n = 81) | PDS (n = 36) | DFSP (n = 27) | KS (n = 20) | MCC (n = 27) | LMS (n = 19) | ||
|---|---|---|---|---|---|---|---|
| Sex | Male | 63 (77.8%) | 31 (86.1%) | 14 (51.9%) | 17 (85%) | 18 (66.7%) | 4 (21.1%) |
| Female | 18 (22.2%) | 5 (13.9%) | 13 (48.1%) | 3 (15%) | 9 (33.3%) | 15 (78.9%) | |
| Age at diagnosis (y) | 66.7 ± 17.2 | 70.9 ± 11.4 | 63.9 ± 17.1 | 66.1 ± 15.6 | 68.4 ± 17.5 | 66.2 ± 16.3 | |
| UV-exposed body site | 95 (96%) | 35 (97.2%) | 5 (18.5%) | 1 (5%) | 10 (37%) | 11 (57.9%) | |
| Sunburns | 52 (64.2%) | 27 (75.0%) | 12 (44.4%) | 3 (15%) | 15 (55.6%) | 9 (47.4%) | |
| Immunosuppression | 10 (12.3%) | 5 (13.9%) | 1 (3.7%) | 5 (25%) | 3 (11.1%) | 4 (21.1%) | |
| Tumor Localization | |||||||
| Head/Neck | 77 (95.1%) | 35 (97.2%) | 2 (7.4%) | 1 (5%) | 8 (29.6) | 10 (52.6%) | |
| Trunk | 2 (2.5%) | 1 (2.8%) | 18 (66.7%) | 3 (15%) | 8 (29.6%) | 2 (10.5%) | |
| Extremities | 2 (2.5%) | - | 7 (25.9%) | 16 (80%) | 11 (40.7%) | 7 (36.8%) | |
| Mean depth of AE (mm) | 0.88 ± 0.52 | 0.73 ± 0.32 | 0.24 ± 0.4 | 0.56 ± 0.42 | 0.56 ± 0.42 | 0.42 ± 0.27 | |
| TEG (mm) | 2.04 ± 1.4 | 1.61 ± 0.98 | 0.24 ± 0.55 | 0.88 ± 1.08 | 0.88 ± 1.08 | 0.67 ± 0.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drexler, K.; Bollmann, L.; Karrer, S.; Berneburg, M.; Haferkamp, S.; Niebel, D. Retrospective Single-Center Case Study of Clinical Variables and the Degree of Actinic Elastosis Associated with Rare Skin Cancers. Biology 2024, 13, 529. https://doi.org/10.3390/biology13070529
Drexler K, Bollmann L, Karrer S, Berneburg M, Haferkamp S, Niebel D. Retrospective Single-Center Case Study of Clinical Variables and the Degree of Actinic Elastosis Associated with Rare Skin Cancers. Biology. 2024; 13(7):529. https://doi.org/10.3390/biology13070529
Chicago/Turabian StyleDrexler, Konstantin, Lara Bollmann, Sigrid Karrer, Mark Berneburg, Sebastian Haferkamp, and Dennis Niebel. 2024. "Retrospective Single-Center Case Study of Clinical Variables and the Degree of Actinic Elastosis Associated with Rare Skin Cancers" Biology 13, no. 7: 529. https://doi.org/10.3390/biology13070529
APA StyleDrexler, K., Bollmann, L., Karrer, S., Berneburg, M., Haferkamp, S., & Niebel, D. (2024). Retrospective Single-Center Case Study of Clinical Variables and the Degree of Actinic Elastosis Associated with Rare Skin Cancers. Biology, 13(7), 529. https://doi.org/10.3390/biology13070529








