Transcriptome Analysis of Transiently Reversible Cell Vacuolization Caused by Excessive Serum Concentration in Scophthalmus maximus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. cDNA Library Construction
2.3. RNA Sequencing
2.4. Bioinformatics Methods
2.5. Validation by qPCR Analysis
3. Results
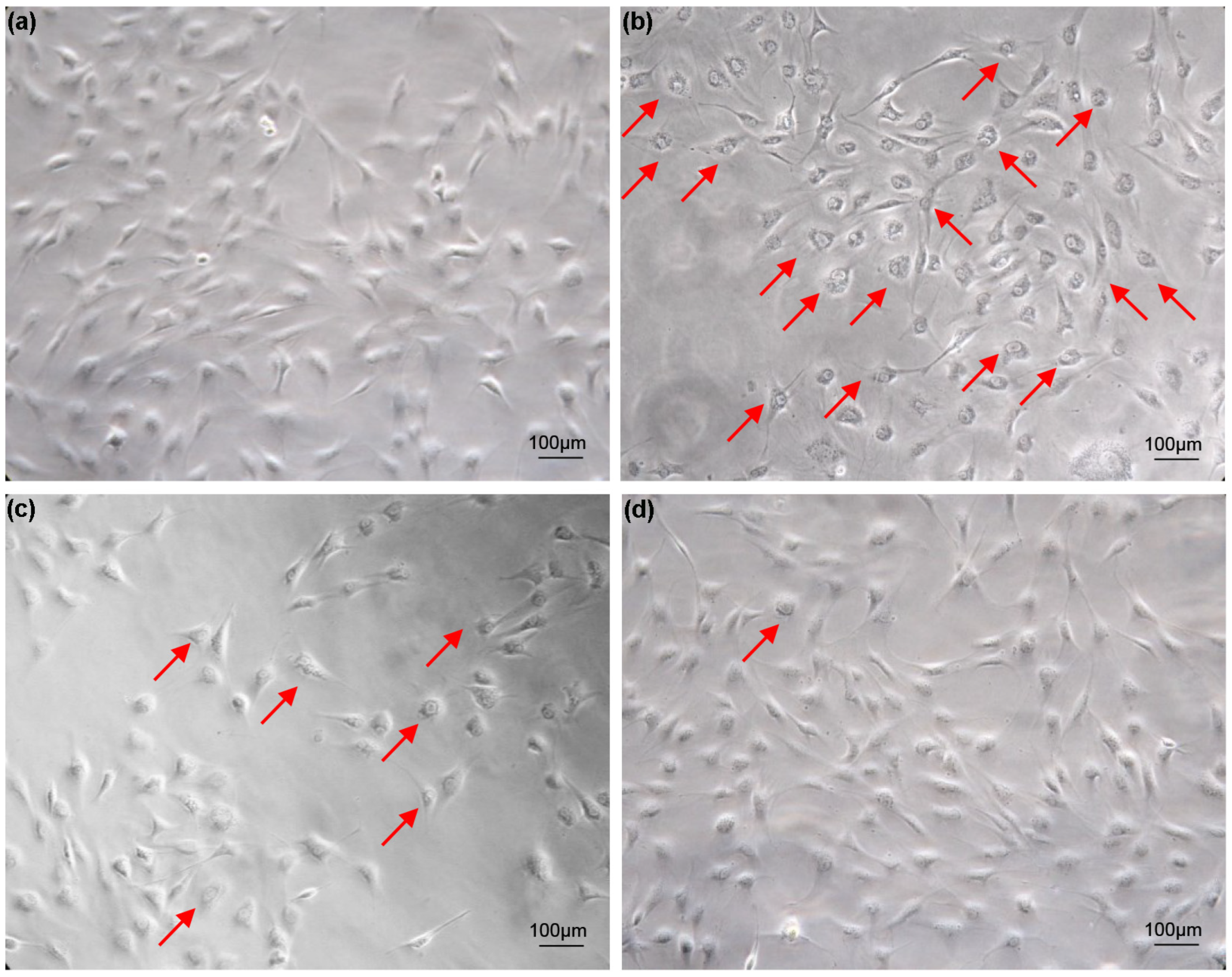
3.1. Effects of FBS Concentration and Trypsin Digestion on Cell Growth
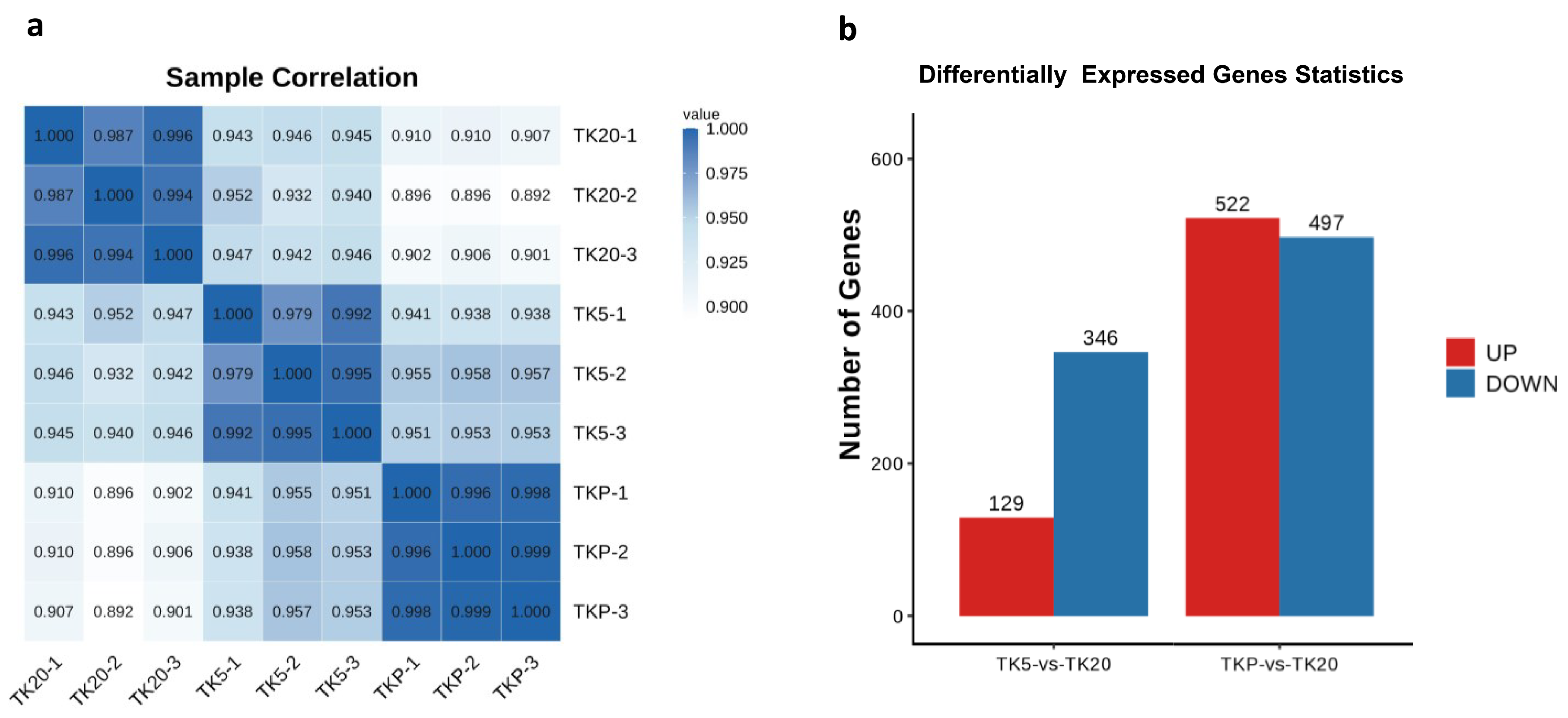
3.2. Transcriptome Sequencing and Identification of DEGs
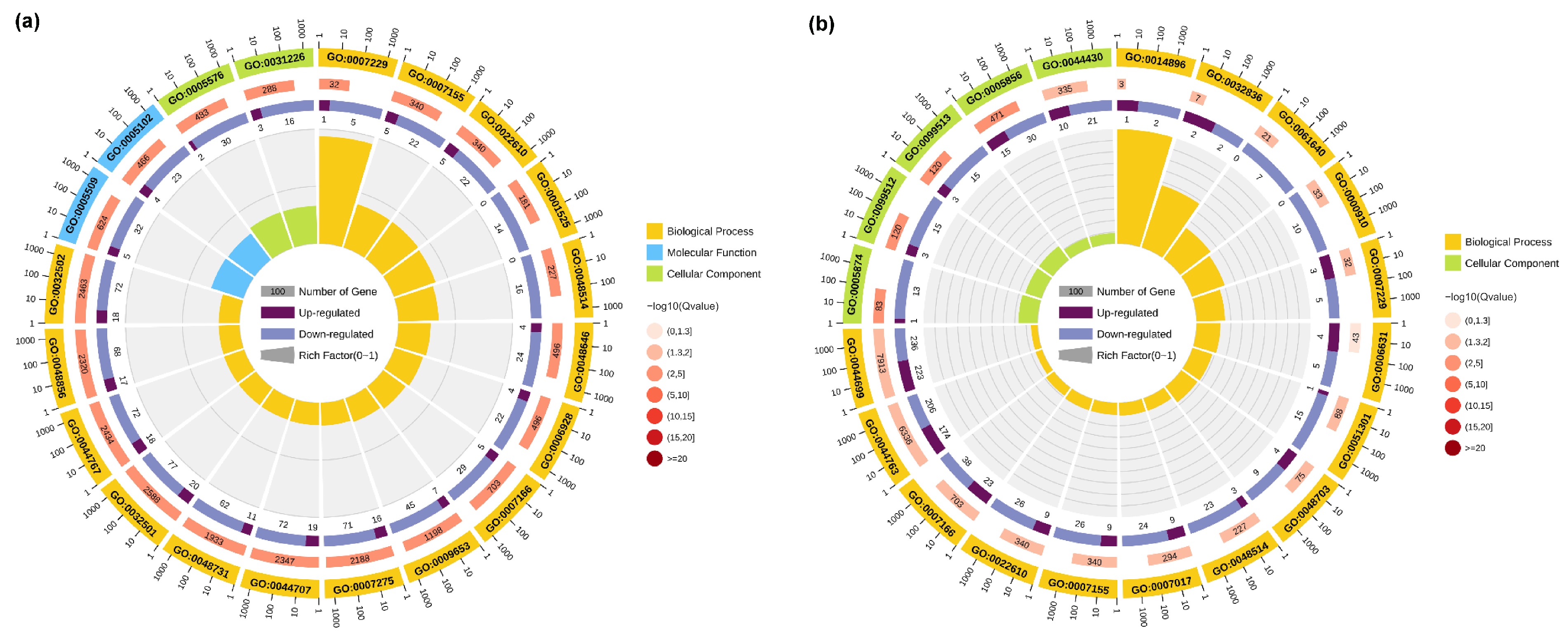
3.3. GO Enrichment Analysis
3.4. KEGG Enrichment Analysis
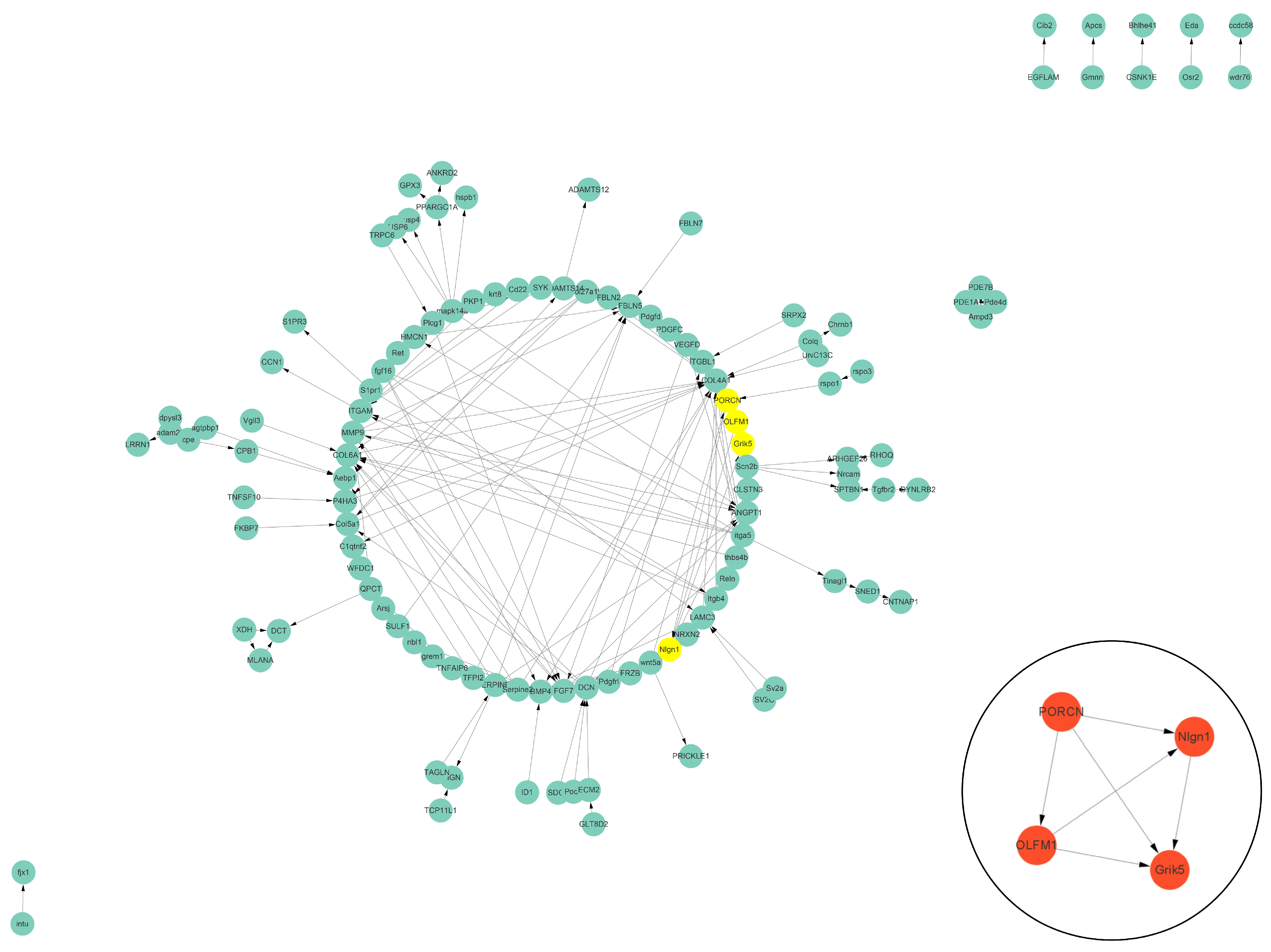
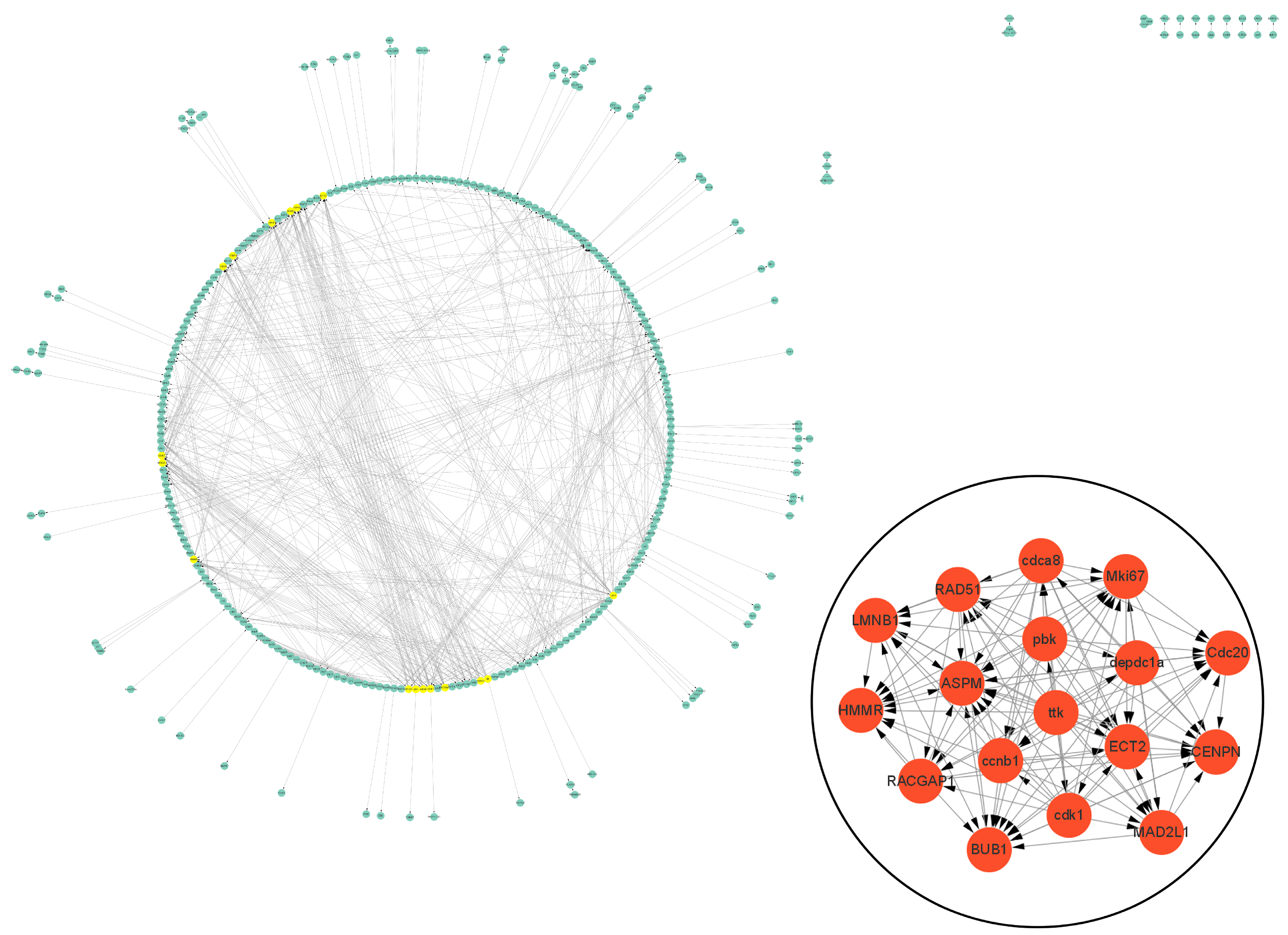
3.5. Protein–Protein Interaction (PPI) Analysis of DEGs Related to Cell Vacuolation
3.6. Bioinformatics Analysis of Interesting DEGs
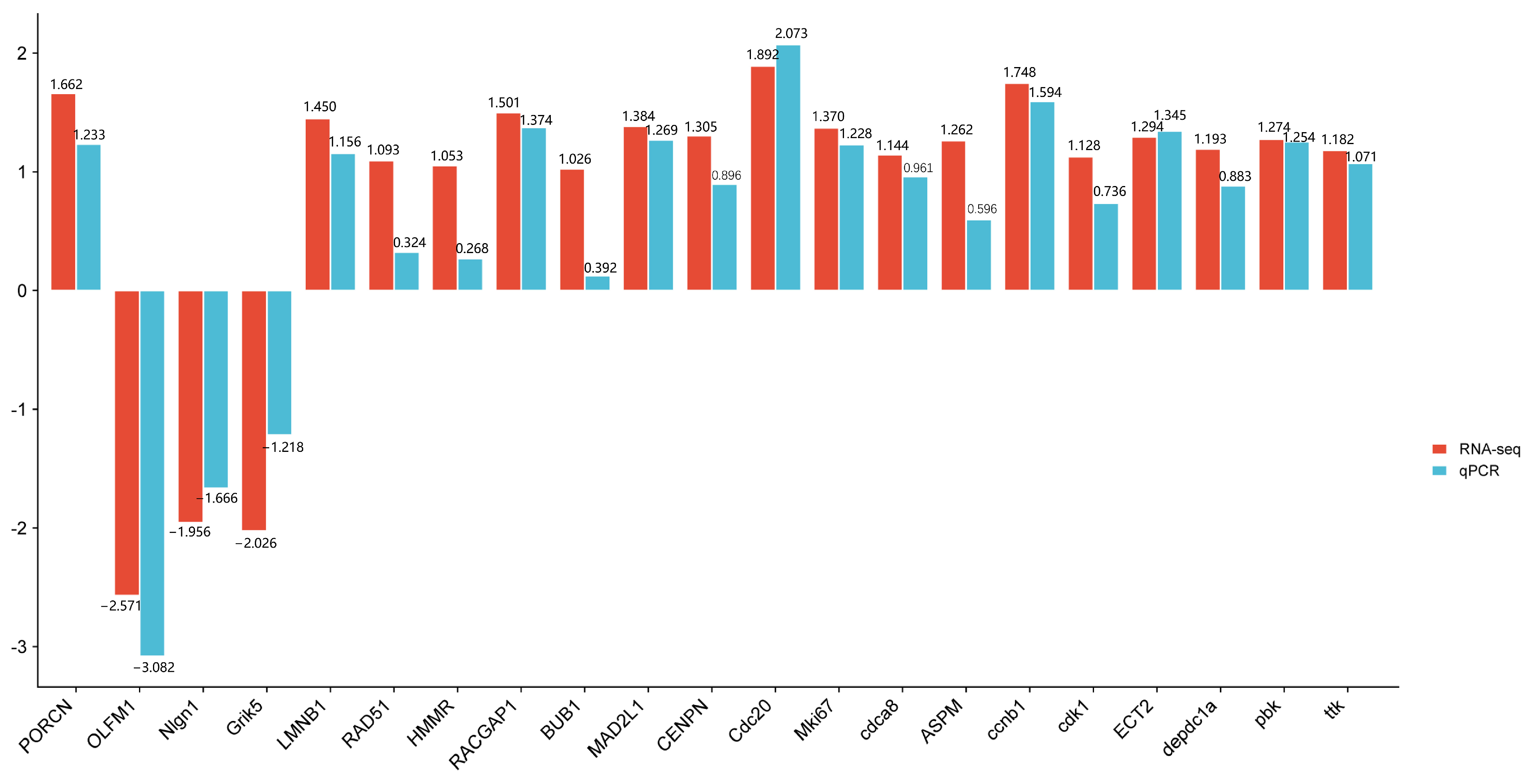
3.7. Validation of Select Gene Expression Changes by qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.P.; Chen, T.S.; Sun, L.; Cai, J.Y.; Wu, M.Q.; Mok, M. Live morphological analysis of taxol-induced cytoplasmic vacuoliazation in human lung adenocarcinoma cells. Micron 2008, 39, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Kugel, B.; Hoffman, R.W.; Friess, A. Effects of low pH on the chorion of rainbow trout, Oncorhynchus mykiss, and brown trout, Salmo trutta f. fario. J. Fish Biol. 1990, 37, 301–310. [Google Scholar] [CrossRef]
- Pinto, R.M.; Bosch, A.; Bishop, D.H. Structures associated with the expression of rabies virus structural genes in insect cells. Virus Res. 1994, 31, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863. [Google Scholar] [CrossRef] [PubMed]
- Henics, T.; Wheatley, D.N. Vacuolar cytoplasmic phase separation in cultured mammalian cells involves the microfilament network and reduces motional properties of intracellular water. Int. J. Exp. Pathol. 1997, 78, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Henics, T.; Wheatley, D.N. Cytoplasmic vacuolation, adaptation and cell death: A view on new perspectives and features. Biol. Cell 1999, 91, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Nara, A.; Uemura, K. Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biol. Toxicol. 2012, 28, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Soomro, M.A.; Pavase, T.R.; Hu, G. Role of pattern recognition receptors in teleost fish: Recent advances. Int. J. Fish. Aquat. Stud. 2021, 9, 136–151. [Google Scholar]
- Chen, S.L.; Ren, G.C.; Sha, Z.X.; Hong, Y. Development and characterization of a continuous embryonic cell line from turbot (Scophthalmus maximus). Aquaculture 2005, 249, 63–68. [Google Scholar] [CrossRef]
- Fan, T.J.; Ren, B.X.; Geng, X.F.; Yu, Q.T.; Wang, L.Y. Establishment of a turbot fin cell line and its susceptibility to turbot reddish body iridovirus. Cytotechnology 2010, 62, 217–223. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.L.; Sha, Z.X.; Tian, Y.S.; Chen, S.L. Development and characterization of a new marine fish cell line from turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2010, 36, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Liu, Y.; Ge, X.; Li, C. Establishment, Characterization and Expression Pattern of a Spleen Cell Line, SMSP, Derived from Turbot (Scophthalmus maximus). J. Ocean Univ. 2023, 22, 1403–1411. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, D.; Guo, R.; Zheng, L.; An, X.; Zeng, Z. In vivo and in vitro Paralichthys olivaceus and toxicities of diethyl phthalate to flounder fish and its gill cell line (FG cells). J. Environ. Sci. 2018, 39, 73–81. [Google Scholar] [CrossRef]
- Borchardt, R. The application of cell culture systems in drug discovery and development. J. Drug Target. 1995, 3, 179–182. [Google Scholar] [CrossRef]
- Masters, J.R. Human cancer cell lines: Fact and fantasy. Nat. Rev. Mol. Cell Biol. 2000, 1, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Ohta, T.; Ozaki, Y.; Tanaka, H.; Miura, T. Trypsin is a multifunctional factor in spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 20972–20977. [Google Scholar] [CrossRef]
- Pirolo, J.S.; Hutchins, G.M.; Moore, G.W. Myocyte vacuolization in infarct border zones is reversible. Am. J. Pathol. 1985, 121, 444. [Google Scholar]
- Tasdemir, E.; Galluzzi, L.; Maiuri, M.C.; Criollo, A.; Vitale, I.; Hangen, E.; Modjtahedi, N.; Kroemer, G. Methods for assessing autophagy and autophagic cell death. Methods Mol. Biol. 2018, 445, 29–76. [Google Scholar]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Z.; Chen, J.; Ma, T.; Wang, L.; Huang, T.; Sheng, Y.; Huang, W.; Lu, C. PORCN promotes hepatocellular carcinogenesis via Wnt/β-catenin-dependent manner. Transl. Cancer Res. 2023, 12, 1703. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Donnelly, L.; Elghobashi-Meinhardt, N.; Long, T.; Zhou, R.W.; Sun, Y.; Wang, B.; Li, X. Mechanisms and inhibition of Porcupine-mediated Wnt acylation. Nature 2022, 607, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Virshup, D.M. Functional regulation of Wnt protein through post-translational modifications. Biochem. Soc. Trans. 2022, 50, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Hu, Q.; Elghobashi-Meinhardt, N.; Long, T.; Chen, H.; Li, X. Molecular basis of Wnt biogenesis, secretion, and Wnt7-specific signaling. Cell 2023, 186, 5028–5040. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Sogabe, T.; Hayashi, A.; Motohashi, J.; Miura, E.; Arai, I.; Yuzaki, M. In vivo nanoscopic landscape of neurexin ligands underlying anterograde synapse specification. Neuron 2022, 110, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, X.; Yang, Y.; Chi, P.; Huang, J.; Qiu, S.; Zheng, X.; Chen, X. Upregulated NLGN1 predicts poor survival in colorectal cancer. BMC Cancer 2021, 21, 884. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhou, Y.; Tang, C.; He, C.; Zuo, Z. Developmental exposure to mepanipyrim induces locomotor hyperactivity in zebrafish (Danio rerio) larvae. Chemosphere 2020, 256, 127106. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Aramaki, T.; Sakai, M.; Ninomiya, H.; Tashiro, N.; Iwata, N.; Ozaki Norio Fukumaki, Y. Association study of polymorphisms in the GluR7, KA1 and KA2 kainate receptor genes (GRIK3, GRIK4, GRIK5) with schizophrenia. Psychiatry Res. 2006, 141, 39–51. [Google Scholar] [CrossRef]
- Chew, L.J.; Yuan, X.; Scherer, S.E.; Qie, L.; Huang, F.; Hayes, W.P.; Gallo, V. Characterization of the rat GRIK5 kainate receptor subunit gene promoter and its intragenic regions involved in neural cell specificity. J. Biol. Chem. 2001, 276, 42162–42171. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Garg, S.; He, L.; Jagdhane, P.; Carsten, M.T.; Caroline, P. PS980 Grik5 drives self renewal pathways in human acute myeloid leukemia cells. HemaSphere 2019, 3, 440–441. [Google Scholar] [CrossRef]
- Hui, L.; Yang, N.; Yang, H.; Guo, X.; Jang, X. Identification of biomarkers with a tumor stage-dependent expression and exploration of the mechanism involved in laryngeal squamous cell carcinoma. Oncol. Rep. 2015, 34, 2627–2635. [Google Scholar] [CrossRef]
- Davenport, J.; Harris, L.D.; Goorha, R. Spindle checkpoint function requires Mad2-dependent Cdc20 binding to the Mad3 homology domain of BubR1. Exp. Cell Res. 2006, 312, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, H.; Yang, S.; Feng, T.; Jie, M.; Chen, H.; Jiang, H. Bub1 and Bub3 regulate metabolic adaptation via macrolipophagy in Drosophila. Cell Reports 2023, 42, 112343. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kim, H.; Ji, Z.; Zhang, T.; Chen, B.; Ge, Y.; Hu, Y.; Feng, X.; Han, X.; Xu, H.; et al. The BUB3-BUB1 complex promotes telomere DNA replication. Mol. Cell 2018, 70, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Meng, L.B.; Su, J.Z.; Fan, B.; Zhao, S.B.; Wang, H.Y.; Li, T.; Wang, T.Y.; Zhang, A.L.; Ni, X.C. Plk1 as one novel target for the poor prognosis of bladder cancer: An observational study. Medicine 2022, 101, e30723. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.M.; Sabu, A.S.; Hussain, A.; Kombarakkaran, D.P.; Lakshmi, R.B.; Manna, T.K. E3-ubiquitin ligase, FBXW7 regulates mitotic progression by targeting BubR1 for ubiquitin-mediated degradation. Cell. Mol. Life Sci. 2023, 80, 374. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dai, Y.; Hu, W.; Zhang, Y.; Cao, Z.; Pei, W.; Liu, N.; Nie, J.; Wu, A.; Mao, W.; et al. The long noncoding RNA CRYBG3 induces aneuploidy by interfering with spindle assembly checkpoint via direct binding with Bub3. Oncogene 2021, 40, 1821. [Google Scholar] [CrossRef]
- Dreesen, O.; Chojnowski, A.; Ong, P.F.; Zhao, T.Y.; Common, J.E.; Lunny, D.; Lane, E.B.; Lee, S.J.; Vardy, L.A.; Stewart, C.L.; et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Buchwalter, A.; Kaneshiro, J.M.; Hetzer, M.W. Coaching from the sidelines: The nuclear periphery in genome regulation. Nat. Rev. Genet. 2019, 20, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Erdos, M.R.; Ried, T. The role of lamin B1 for the maintenance of nuclear structure and function. Nucleus 2015, 6, 8–14. [Google Scholar] [CrossRef]
- Li, J.; Sun, Z.; Cui, Y.; Qin, L.; Wu, F.; Li, Y.; Du, N.; Li, X. Knockdown of LMNB1 inhibits the proliferation of lung adenocarcinoma cells by inducing DNA damage and cell senescence. Front. Oncol. 2022, 12, 913740. [Google Scholar] [CrossRef]
- Yang, M.; Chen, B.; Kong, L.; Chen, X.; Ouyang, Y.; Bai, J.; Yu, D.; Zhang, H.; Li, X.; Zhang, D. HMMR promotes peritoneal implantation of gastric cancer by increasing cell–cell interactions. Discov. Oncol. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Kawaguchi, A.; Luyt, L.G.; Turley, E. Design of peptide mimetics to block pro-inflammatory functions of HA fragments. Matrix Biol. 2019, 78, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Missinato, M.A.; Tobita, K.; Romano, N.; Carroll, J.A.; Tsang, M. Extracellular component hyaluronic acid and its receptor Hmmr are required for epicardial EMT during heart regeneration. Cardiovasc. Res. 2015, 107, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, B.; Hengel, S.R.; Grundy, M.K.; Bernstein, K.A. RAD51 gene family structure and function. Annu. Rev. Genet. 2020, 54, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Sanchez, A.; Ranjha, L.; Reginato, G.; Ceppi, I.; Acharya, A.; Anand, R.; Cejka, P. Double-stranded DNA binding function of RAD51 in DNA protection and its regulation by BRCA2. Mol. Cell 2022, 82, 3553–3565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Xie, Y.; Zheng, L.; Han, J.; Wang, H.; Tian, X.; Fang, W. Association study of germline variants in CCNB1 and CDK1 with breast cancer susceptibility, progression, and survival among Chinese Han women. PLoS ONE 2013, 8, e84489. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Pérez, T.; Hayward, D.; Holder, J.; Gruneberg, U.; Barr, F.A. MAD1-dependent recruitment of CDK1-CCNB1 to kinetochores promotes spindle checkpoint signaling. J. Cell Biol. 2019, 218, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Pomerening, J.R.; Ubersax, J.A.; Ferrell, J.E., Jr. Rapid cycling and precocious termination of G1 phase in cells expressing CDK1AF. Mol. Biol. Cell 2008, 19, 3426–3441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Slipicevic, A.; Førsund, M.; Trope, C.G.; Nesland, J.M.; Holm, R. Expression of CDK1Tyr15, pCDK1Thr161, Cyclin B1 (Total) and pCyclin B1Ser126 in vulvar squamous cell carcinoma and their relations with clinicopatological features and prognosis. PLoS ONE 2015, 10, e0121398. [Google Scholar] [CrossRef]
- Miyazaki, T.; Arai, S. Two distinct controls of mitotic cdk1/cyclin B1 activity requisite for cell growth prior to cell division. Cell Cycle 2007, 6, 1418–1424. [Google Scholar] [CrossRef]


| Sample | Raw Data | Clean Data | Q20 (%) | Q30 (%) | GC (%) | Total Mapped (%) |
|---|---|---|---|---|---|---|
| TK20-1 | 47,664,300 | 47,444,024 | 97.71% | 93.36% | 48.98% | 75.22% |
| TK20-2 | 41,142,298 | 40,955,680 | 97.49% | 92.83% | 48.97% | 74.54% |
| TK20-3 | 38,962,908 | 38,782,894 | 97.42% | 92.80% | 49.10% | 74.36% |
| TK5-1 | 42,437,616 | 42,266,802 | 97.80% | 93.52% | 48.95% | 72.10% |
| TK5-2 | 49,232,362 | 48,957,950 | 97.14% | 92.13% | 49.07% | 71.62% |
| TK5-3 | 51,970,388 | 51,665,498 | 97.32% | 92.62% | 48.78% | 71.43% |
| TKP-1 | 45,281,128 | 45,052,994 | 97.82% | 93.63% | 49.28% | 79.38% |
| TKP-2 | 43,641,442 | 43,389,930 | 97.28% | 92.47% | 49.47% | 79.22% |
| TKP-3 | 49,557,298 | 49,263,406 | 97.28% | 92.46% | 49.34% | 79.34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Shao, L.; Yu, X. Transcriptome Analysis of Transiently Reversible Cell Vacuolization Caused by Excessive Serum Concentration in Scophthalmus maximus. Biology 2024, 13, 545. https://doi.org/10.3390/biology13070545
Song Y, Shao L, Yu X. Transcriptome Analysis of Transiently Reversible Cell Vacuolization Caused by Excessive Serum Concentration in Scophthalmus maximus. Biology. 2024; 13(7):545. https://doi.org/10.3390/biology13070545
Chicago/Turabian StyleSong, Yuting, Lijun Shao, and Xiaoli Yu. 2024. "Transcriptome Analysis of Transiently Reversible Cell Vacuolization Caused by Excessive Serum Concentration in Scophthalmus maximus" Biology 13, no. 7: 545. https://doi.org/10.3390/biology13070545






