Evidence of Potential Anammox Activities from Rice Paddy Soils in Microaerobic and Anaerobic Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
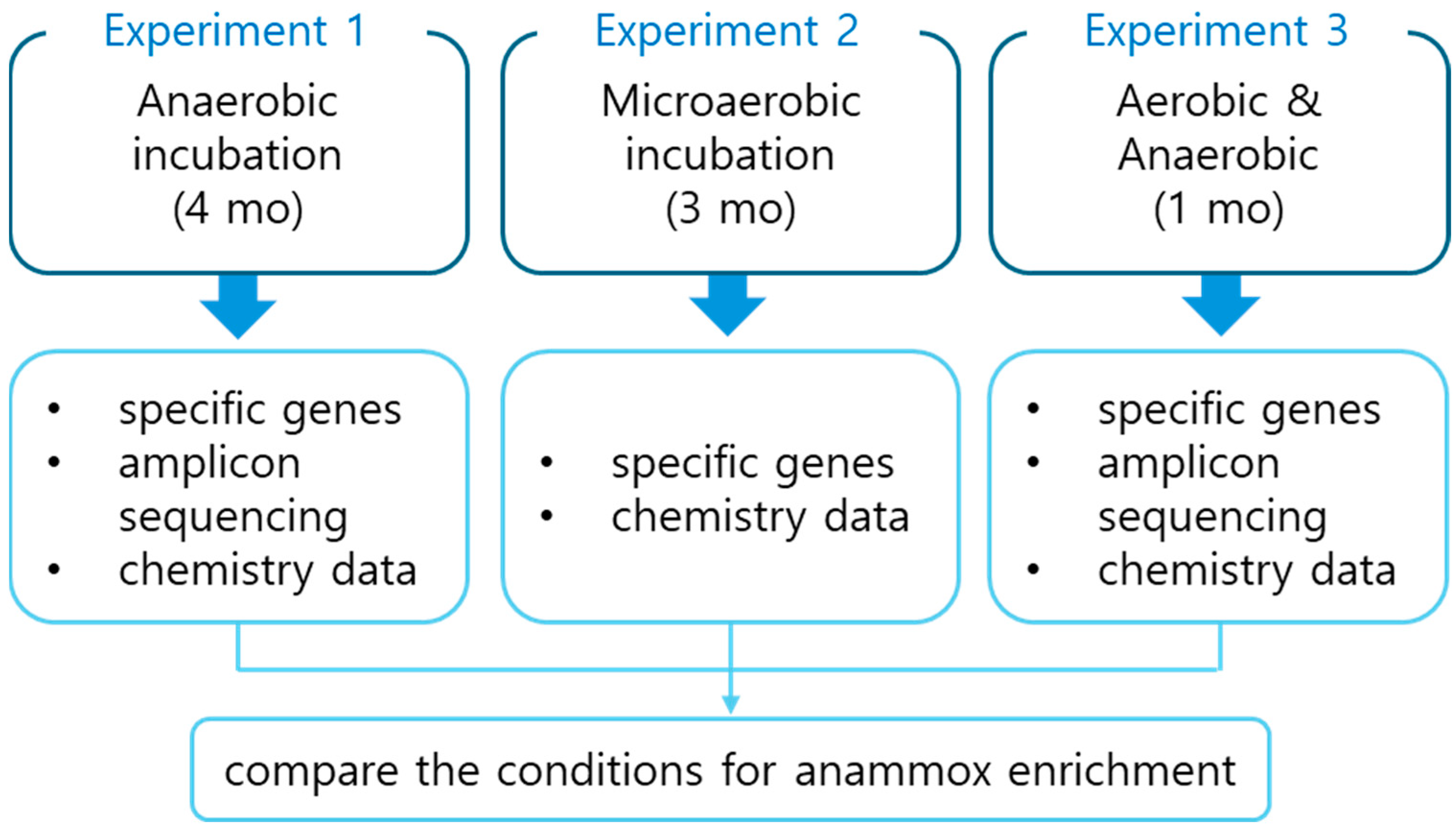
2. Materials and Methods
2.1. Experimental Setups
2.1.1. Experiment 1: Anaerobic Batch
2.1.2. Experiment 2: Microaerobic Column Batch
2.1.3. Experiment 3: Comparison of Aerobic and Anaerobic Incubations
3. Results and Discussion
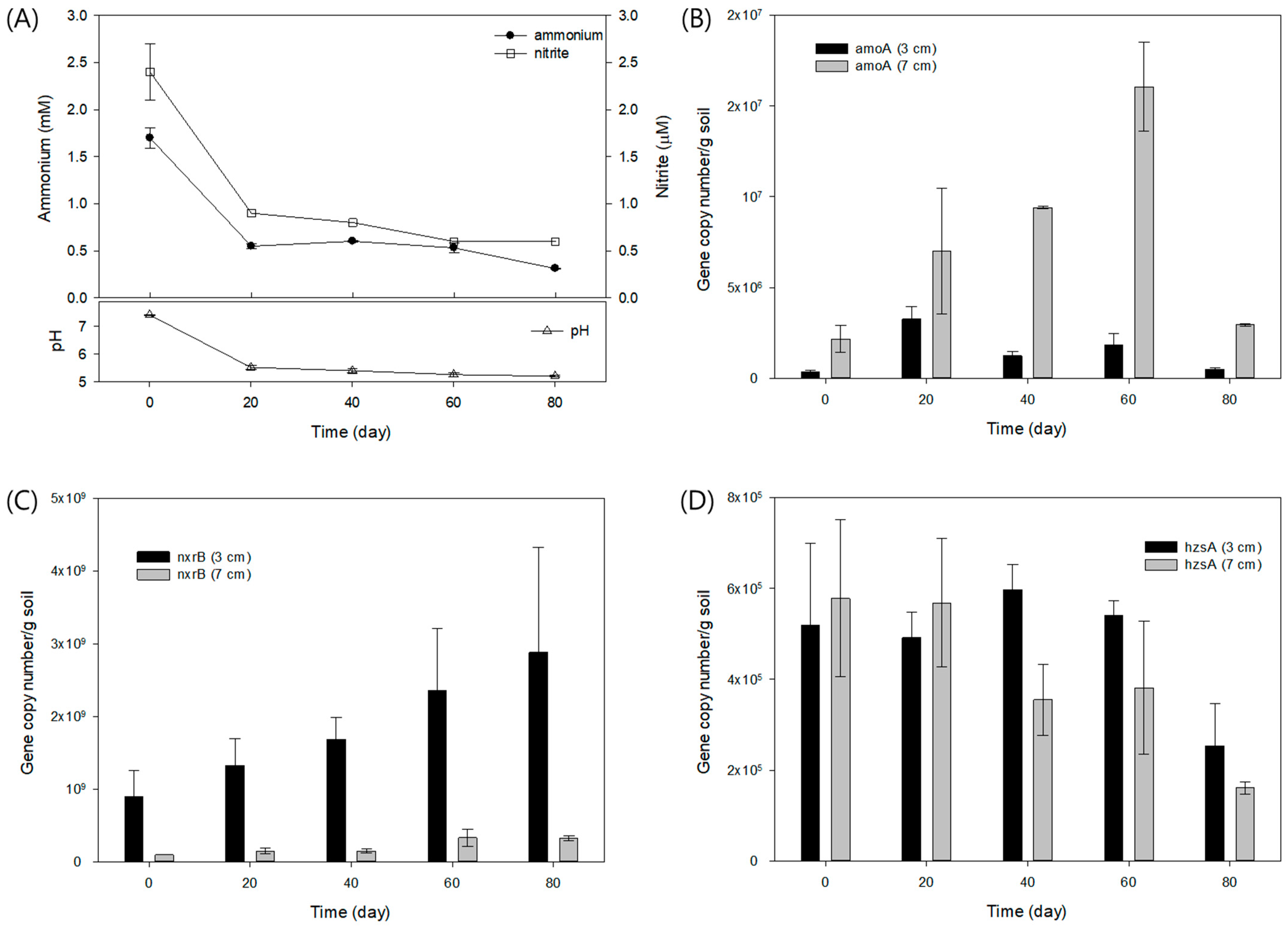
3.1. Anaerobic Batch Culture of the Rice Paddy Soil (Experiment 1)
3.2. Microaerobic Column Batch (Experiment 2)
3.3. Comparison of Aerobic and Anaerobic Incubations (Experiment 3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.A.; Naz, M.Y.; Shukrullah, S.; Sulaiman, S.A.; Ghaffar, A.; AbdEl-Salam, N.M. Nitrogen pollution impact and remediation through low cost starch based biodegradable polymers. Sci. Rep. 2020, 10, 5927. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shang, F.; Lu, X.; Huang, X.; Song, Y.; Liu, B.; Zhang, Q.; Liu, X.; Cao, J.; Xu, T.; et al. Unexpected response of nitrogen deposition to nitrogen oxide controls and implications for land carbon sink. Nat. Commun. 2022, 13, 3126. [Google Scholar] [CrossRef] [PubMed]
- Mosley, O.E.; Gios, E.; Close, M.; Weaver, L.; Daughney, C.; Handley, K.M. Nitrogen cycling and microbial cooperation in the terrestrial subsurface. ISME J. 2022, 16, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Rajta, A.; Bhatia, R.; Setia, H.; Pathania, P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J. Appl. Microbiol. 2020, 128, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, Y.; Zhu, J.; Li, C.; Chen, G. A comprehensive review on wastewater nitrogen removal and its recovery processes. Int. J. Environ. Res. Public Health 2023, 20, 3429. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B.; Tegetmeyer, H.E.; Sharma, R.; Klotz, M.G.; Ferdelman, T.G.; Hettich, R.L.; Geelhoed, J.S.; Strous, M. The environmental controls that govern the end product of bacterial nitrate respiration. Science 2014, 345, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Seruga, P.; Krzywonos, M.; Pyżanowska, J.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedźwiecki, Ł. Removal of ammonia from the municipal waste treatment effluents using natural minerals. Molecules 2019, 24, 3633. [Google Scholar] [CrossRef]
- van de Graaf, A.A.; de Bruijn, P.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef]
- Date, Y.; Isaka, K.; Ikuta, H.; Sumino, T.; Kaneko, N.; Yoshie, S.; Tsuneda, S.; Inamori, Y. Microbial diversity of anammox bacteria enriched from different types of seed sludge in an anaerobic continuous-feeding cultivation reactor. J. Biosci. Bioeng. 2009, 107, 281–286. [Google Scholar] [CrossRef]
- Du, R.; Horn, H.; Cao, S. Maximizing anammox in mainstream wastewater treatment: An integrated nitrite producing approach. Chem. Eng. J. 2023, 468, 143696. [Google Scholar] [CrossRef]
- Fan, N.-S.; Bai, Y.-H.; Wu, J.; Zhang, Q.; Fu, J.-J.; Zhou, W.-L.; Huang, B.-C.; Jin, R.-C. A two-stage anammox process for the advanced treatment of high-strength ammonium wastewater: Microbial community and nitrogen transformation. J. Clean. Prod. 2020, 261, 121148. [Google Scholar] [CrossRef]
- Fu, Y.; Wen, X.; Huang, J.; Sun, D.; Jin, L. Advances in the efficient enrichment of anammox bacteria. Water 2023, 15, 2556. [Google Scholar] [CrossRef]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Niederdorfer, R.; Hausherr, D.; Palomo, A.; Wei, J.; Magyar, P.; Smets, B.F.; Joss, A.; Bürgmann, H. Temperature modulates stress response in mainstream anammox reactors. Commun. Biol. 2021, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, M.; Wang, C.; Qi, W.; Peng, Y. Enrichment of anammox bacteria using anammox sludge as a primer combined with ordinary activated sludge. Sustainability 2023, 15, 12123. [Google Scholar] [CrossRef]
- Ren, Z.-Q.; Wang, H.; Zhang, L.-G.; Du, X.-N.; Huang, B.-C.; Jin, R.-C. A review of anammox-based nitrogen removal technology: From microbial diversity to engineering applications. Bioresour. Technol. 2022, 363, 127896. [Google Scholar] [CrossRef]
- Lu, Y.; Natarajan, G.; Nguyen, T.Q.N.; Thi, S.S.; Arumugam, K.; Seviour, T.; Williams, R.B.H.; Wuertz, S.; Law, Y. Controlling anammox speciation and biofilm attachment strategy using N-biotransformation intermediates and organic carbon levels. Sci. Rep. 2022, 12, 21720. [Google Scholar] [CrossRef]
- Khanal, A.; Lee, J.-H. Functional diversity and abundance of nitrogen cycle-related genes in paddy soil. Appl. Biol. Chem. 2020, 63, 17. [Google Scholar] [CrossRef]
- Khanal, A.; Lee, S.; Lee, J.-H. Detection and potential abundances of anammox bacteria in the paddy soil. Korean J. Environ. Agric. 2020, 39, 26–35. [Google Scholar] [CrossRef]
- Kartal, B.; Keltjens, J.T. Anammox biochemistry: A tale of heme c proteins. Trends Biochem. Sci. 2016, 41, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Hatzenpichler, R. Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl. Environ. Microbiol. 2012, 78, 7501–7510. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lücker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.; Zhou, S.; Lin, X.; Ma, B.; Park, H.-D.; Yan, Y. Expression of the nirS, hzsA, and hdh genes in response to nitrite shock and recovery in Candidatus Kuenenia stuttgartiensis. Environ. Sci. Technol. 2016, 50, 6940–6947. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Kovaleva, O.L.; Merkel, A.Y.; Novikov, A.A.; Baslerov, R.V.; Toshchakov, S.V.; Bonch-Osmolovskaya, E.A. Tepidisphaera mucosa gen. nov., sp. nov., a moderately thermophilic member of the class Phycisphaerae in the phylum Planctomycetes, and proposal of a new family, Tepidisphaeraceae fam. nov., and a new order, Tepidisphaerales ord. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 549–555. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Kirkegaard, R.H.; Dueholm, M.S.; Fernando, E.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. Culture-independent analyses reveal novel Anaerolineaceae as abundant primary fermenters in anaerobic digesters treating waste activated sludge. Front. Microbiol. 2017, 8, 1134. [Google Scholar] [CrossRef] [PubMed]
- van Niftrik, L.; Geerts, W.J.C.; van Donselaar, E.G.; Humbel, B.M.; Webb, R.I.; Fuerst, J.A.; Verkleij, A.J.; Jetten, M.S.M.; Strous, M. Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria: Cell plan, glycogen storage, and localization of cytochrome c proteins. J. Bacteriol. 2008, 190, 708–717. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Zhang, X.; Zhu, Z.; Wu, Y.; Wang, C.; Cai, T.; Li, X.; Wu, P. Mainstream anammox driven by micro-oxygen nitrification and partial denitrification using step-feed for advanced nitrogen removal from municipal wastewater. J. Clean. Prod. 2022, 378, 134544. [Google Scholar] [CrossRef]
- Hou, X.H.; Li, X.Y.; Zhu, X.R.; Li, W.Y.; Kao, C.K.; Peng, Y.Z. Advanced nitrogen removal from municipal wastewater through partial nitrification-denitrification coupled with anammox in step-feed continuous system. Bioresour. Technol. 2024, 391, 129967. [Google Scholar] [CrossRef]
- Brunk, C.F.; Avaniss-Aghajani, E.; Brunk, C.A. A computer analysis of primer and probe hybridization potential with bacterial small-subunit rRNA sequences. Appl. Environ. Microbiol. 1996, 62, 872–879. [Google Scholar] [CrossRef]
- Harhangi, H.R.; Le Roy, M.; van Alen, T.; Hu, B.L.; Groen, J.; Kartal, B.; Tringe, S.G.; Quan, Z.X.; Jetten, M.S.; Op den Camp, H.J. Hydrazine synthase, a unique phylomarker with which to study the presence and biodiversity of anammox bacteria. Appl. Environ. Microbiol. 2012, 78, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.P.R.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Lücker, S.; Wagner, M.; Maixner, F.; Pelletier, E.; Koch, H.; Vacherie, B.; Rattei, T.; Damsté, J.S.S.; Spieck, E.; Le Paslier, D.; et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 13479–13484. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, A.; Song, H.-G.; Cho, Y.-S.; Yang, S.-Y.; Kim, W.-S.; Joshi, A.; Min, J.; Lee, J.-H. Evidence of Potential Anammox Activities from Rice Paddy Soils in Microaerobic and Anaerobic Conditions. Biology 2024, 13, 548. https://doi.org/10.3390/biology13070548
Khanal A, Song H-G, Cho Y-S, Yang S-Y, Kim W-S, Joshi A, Min J, Lee J-H. Evidence of Potential Anammox Activities from Rice Paddy Soils in Microaerobic and Anaerobic Conditions. Biology. 2024; 13(7):548. https://doi.org/10.3390/biology13070548
Chicago/Turabian StyleKhanal, Anamika, Hyung-Geun Song, Yu-Sung Cho, Seo-Yeon Yang, Won-Seok Kim, Alpana Joshi, Jiho Min, and Ji-Hoon Lee. 2024. "Evidence of Potential Anammox Activities from Rice Paddy Soils in Microaerobic and Anaerobic Conditions" Biology 13, no. 7: 548. https://doi.org/10.3390/biology13070548





