Investigating the Endophyte Actinomycetota sp. JW0824 Strain as a Potential Bioinoculant to Enhance the Yield, Nutritive Value, and Chemical Composition of Different Cultivars of Anise (Pimpinella anisum L.) Seeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Molecular Identification
2.3. Plant Materials and Experimental Setup
2.4. Nutrient Analyses
2.5. Vitamins Analysis
2.6. Determination of Total Phenolic Content
2.7. Determination of the Levels and Metabolism of the Essential Oils
2.8. Essential Oil Analysis by GC-MS
2.9. Determination of DAHPS
2.10. Determination of PAL
2.11. Statistical Analysis
3. Results and Discussion
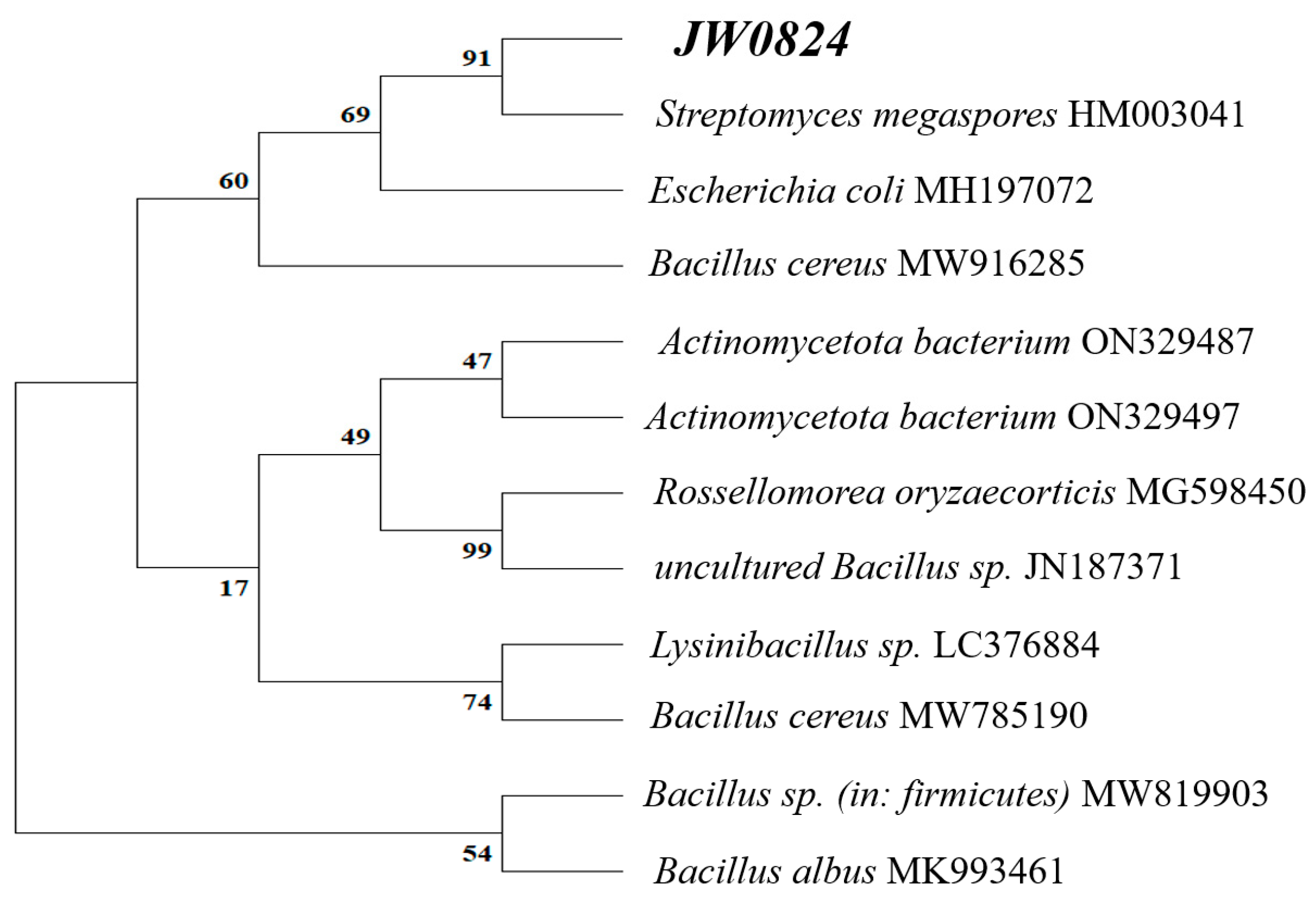
3.1. Molecular Identification of the Isolate
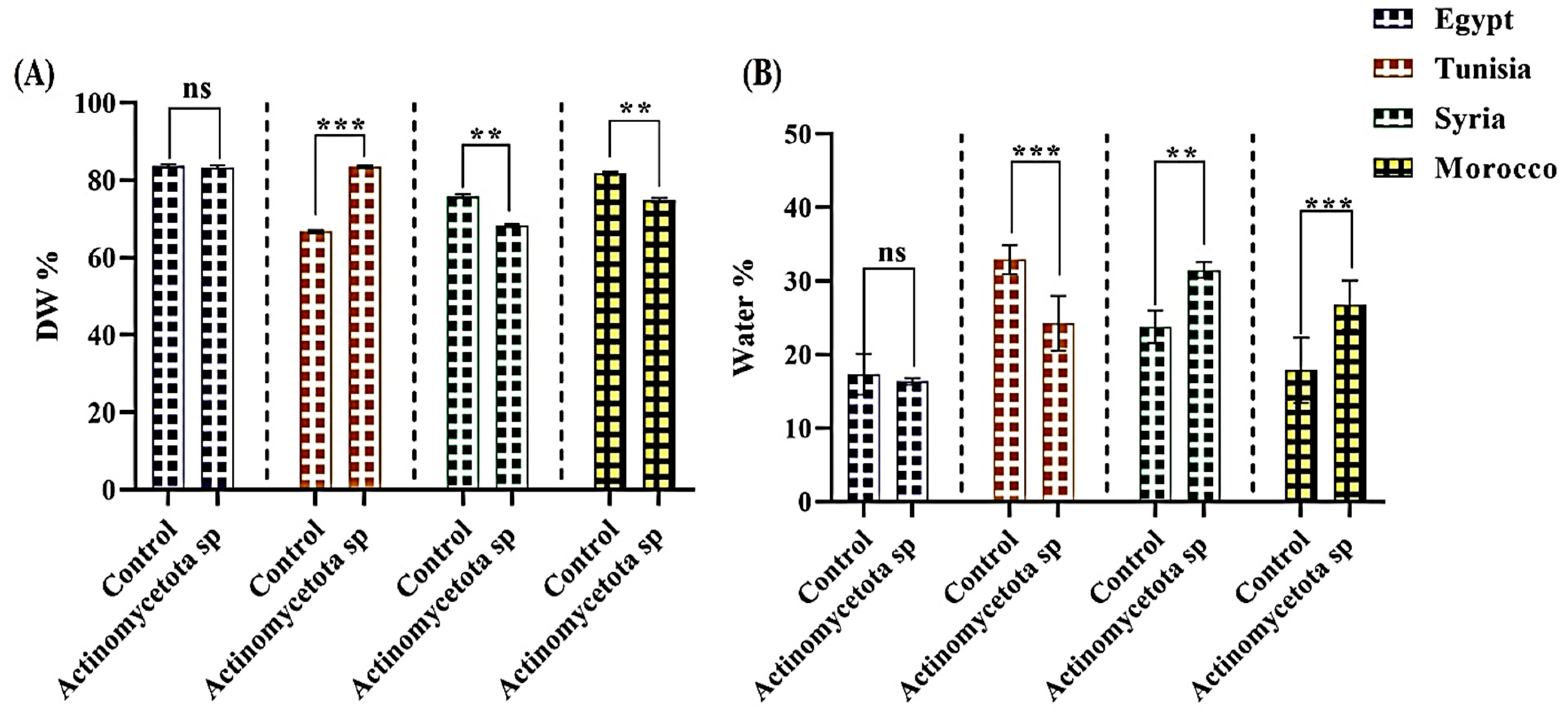
3.2. Seed Yield (Dry Weight and Water Content)
3.3. The Impact on Primary and Secondary Metabolites
3.4. Improved Essential Oil Levels and Metabolism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahrajabian, M.H.; Sun, W. Five Important Seeds in Traditional Medicine, and Pharmacological Benefits. Seeds 2023, 2, 290–308. [Google Scholar] [CrossRef]
- Aćimović, M.; Korać, J.; Jaćimović, G.; Oljača, S.; Đukanović, L.; Vuga-Janjatov, V. Influence of ecological conditions on seeds traits and essential oil contents in anise (Pimpinella anisum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 232–238. [Google Scholar]
- El-Sayed, S.M.; Ahmed, N.; Selim, S.; Al-Khalaf, A.A.; El Nahhas, N.; Abdel-Hafez, S.H.; Sayed, S.; Emam, H.M.; Ibrahim, M.A. Acaricidal and antioxidant activities of anise oil (Pimpinella anisum) and the oil’s effect on protease and acetylcholinesterase in the two-spotted spider mite (Tetranychus urticae Koch). Agriculture 2022, 12, 224. [Google Scholar] [CrossRef]
- Schiavone, V.; Romasco, T.; Di Pietrantonio, N.; Garzoli, S.; Palmerini, C.; Di Tomo, P.; Pipino, C.; Mandatori, D.; Fioravanti, R.; Butturini, E. Essential Oils from Mediterranean Plants Inhibit In Vitro Monocyte Adhesion to Endothelial Cells from Umbilical Cords of Females with Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 7225. [Google Scholar] [CrossRef] [PubMed]
- Cabuk, M.; Alcicek, A.; Bozkurt, M.; Imre, N. Antimicrobial properties of the essential oils isolated from aromatic plants and using possibility as alternative feed additives. Natl. Anim. Nutr. Congr. 2003, 18, 184–187. [Google Scholar]
- Soliman, K.M.; Badeaa, R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Tabanca, N.; Bedir, E.; Kirimer, N.; Baser, K.H.C.; Khan, S.I.; Jacob, M.R.; Khan, I.A. Antimicrobial compounds from Pimpinella species growing in Turkey. Planta Medica 2003, 69, 933–938. [Google Scholar]
- Sarkar, D.; Christopher, A.; Shetty, K. Phenolic bioactives from plant-based foods for glycemic control. Front. Endocrinol. 2022, 12, 727503. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Fouad, S.; El Shebini, S.M.; Abdel-Moaty, M.; Ahmed, N.H.; Hussein, A.M.S.; Essa, H.A.; Tapozada, S.T. Menopause Anxiety and Depression; How Food Can Help? Open Access Maced. J. Med. Sci. 2021, 9, 64–71. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical characters and antioxidant, sugar, and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L.) cultivars and hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.; Laware, S.; Auti, S. Analysis of nutritional diversity and antioxidant activity of finger millet landraces. Indian J. Agric. Res. 2022, 56, 1–6. [Google Scholar] [CrossRef]
- Balkhyour, M.A.; Hassan, A.H.; Halawani, R.F.; Summan, A.S.; AbdElgawad, H. Effect of elevated CO2 on seed yield, essential oil metabolism, nutritive value, and biological activity of Pimpinella anisum L. accessions at different seed maturity stages. Biology 2021, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Michelsen, P.; Percy, N.; Conn, V.; Listiana, E.; Moll, S.; Loria, R.; Coombs, J. Actinobacterial endophytes for improved crop performance. Australas. Plant Pathol. 2007, 36, 524–531. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; El-Sawah, A.M.; Kobae, Y.; Basit, F.; Holford, P.; Yang, H.; El-Keblawy, A.; Abdel-Fattah, G.G.; Wang, S.; Araus, J.L.; et al. The effects of microbial fertilizers application on growth, yield and some biochemical changes in the leaves and seeds of guar (Cyamopsis tetragonoloba L.). Food Res. Int. 2023, 172, 113122. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; El-Sawah, A.M. The Mode of Integration Between Azotobacter and Rhizobium Affect Plant Growth, Yield, and Physiological Responses of Pea (Pisum sativum L.). J. Soil Sci. Plant Nutr. 2022, 22, 1238–1251. [Google Scholar] [CrossRef]
- Nader, A.A.; Hauka, F.I.A.; Afify, A.H.; El-Sawah, A.M. Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.). Microorganisms 2024, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Carril, P.; Ngendahimana, E.; Tenreiro, R.; Cruz, C. Root inoculation with Herbaspirillum seropedicae RAM10 reveals structural and functional shifts in the wheat seed-borne microbiota associated with improved plant phenotype. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Suleman, M.; Zaheer, A.; Reitz, T.; Tarkka, M.T.; Islam, E.; Mirza, M.S. Glucose dehydrogenase gene containing phosphobacteria for biofortification of Phosphorus with growth promotion of rice. Microbiol. Res. 2019, 223, 1–12. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X.-H.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef]
- Qu, H.; Wang, Y.; Wang, B.; Li, C. Pulsed electric field treatment of seeds modulates the endophytic bacterial community and promotes early growth of roots in buckwheat. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Darzi, N.M.T. Effects of the Application of Vermicompost and Phosphate Solubilizing Bacterium on the Morphological Traits and Seed Yield of Anise (Pimpinella anisum L.). J. Med. Plant Res. 2012, 6, 215–219. [Google Scholar]
- Hoseini, A.; Salehi, A.; Sayyed, R.Z.; Balouchi, H.; Moradi, A.; Piri, R.; Fazeli-Nasab, B.; Poczai, P.; Ansari, M.J.; Obaid, S.A.; et al. Efficacy of Biological Agents and Fillers Seed Coating in Improving Drought Stress in Anise. Front. Plant Sci. 2022, 13, 955512. [Google Scholar] [CrossRef] [PubMed]
- Sallam, D. phytochemical studies on anise (Pimpinella anisum L.) plant under using chemical fertilization, biofertilizer and thidiazuron treatments. Al-Azhar J. Pharm. Sci. 2015, 52, 38–56. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plantz Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.E.; De Beuf, K.; Vekeman, B.; Willems, A. A large diversity of non-rhizobial endophytes found in legume root nodules in Flanders (Belgium). Soil Biol. Biochem. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shafiq, M.; Tariq, M.R.; Sami, A.; Nawaz-Ul-Rehman, M.S.; Bhatti, M.H.T.; Haider, M.S.; Sadiq, S.; Abbas, M.T.; Hussain, M.; et al. Interaction between Bacterial Endophytes and Host Plants. Front. Plant Sci. 2023, 13, 1092105. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Gu, M.; Yu, S.; Shi, J.; Cheng, L.; Jin, J.; Lu, P.; Zhang, J.; Li, H.; Cao, P. The Beneficial Endophytic Microbes Enhanced Tobacco Defense System to Resist Bacterial Wilt Disease. Chem. Biol. Technol. Agric. 2024, 11, 21. [Google Scholar] [CrossRef]
- Devi, K.A.; Pandey, G.; Rawat, A.K.S.; Sharma, G.D.; Pandey, P. The Endophytic Symbiont—Pseudomonas aeruginosa Stimulates the Antioxidant Activity and Growth of Achyranthes aspera L. Front. Microbiol. 2017, 8, 1897. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Li, Q.; Han, L.; Jiang, C. Molecular phylogenetic identification of Actinobacteria. In Actinobacteria-Basics and Biotechnological Applications; Dharumadurai, D., Jiang, Y., Eds.; Intechopen: London, UK, 2016; pp. 141–174. [Google Scholar]
- Farris, M.; Olson, J. Detection of Actinobacteria cultivated from environmental samples reveals bias in universal primers. Lett. Appl. Microbiol. 2007, 45, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, C.; Kobara, Y. Determination of glucose by a modification of Somogyi-Nelson method. Agric. Biol. Chem. 1980, 44, 2943–2949. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Hozzein, W.N.; Saleh, A.M.; Habeeb, T.H.; Wadaan, M.A.; AbdElgawad, H. CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem. 2020, 308, 125661. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S.; Hassan, A.H.; Abdel-Mawgoud, M.; Khamis, G.; Selim, S.; Al Jaouni, S.K.; AbdElgawad, H. Laser light as a promising approach to improve the nutritional value, antioxidant capacity and anti-inflammatory activity of flavonoid-rich buckwheat sprouts. Food Chem. 2021, 345, 128788. [Google Scholar] [CrossRef] [PubMed]
- Okla, M.K.; El-Tayeb, M.A.; Qahtan, A.A.; Abdel-Maksoud, M.A.; Elbadawi, Y.B.; Alaskary, M.K.; Balkhyour, M.A.; Hassan, A.H.; AbdElgawad, H. Laser light treatment of seeds for improving the biomass photosynthesis, chemical composition and biological activities of lemongrass sprouts. Agronomy 2021, 11, 478. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, S.; Wang, Y.; Liu, J.; Ma, H.; Wang, Y.; Tian, Y.; Hou, W. Effects of light irradiation on essential oil biosynthesis in the medicinal plant Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim) Kitag. PLoS ONE 2020, 15, e0237952. [Google Scholar]
- Habeeb, T.H.; Abdel-Mawgoud, M.; Yehia, R.S.; Khalil, A.M.A.; Saleh, A.M.; AbdElgawad, H. Interactive impact of arbuscular mycorrhizal fungi and elevated CO2 on growth and functional food value of Thymus vulgare. J. Fungi 2020, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, R.A.; Bahrami, Y.; Kakaei, E. Identification and Antibacterial Evaluation of Endophytic Actinobacteria from Luffa cylindrica. Sci. Rep. 2022, 12, 18236. [Google Scholar] [CrossRef]
- Maryam, H.; Fazel, P.; Mostafa, N. Isolation and Screening of Antibacterial Activity of Actinomycetota from the Medicinal Plant, Anthemis pseudocotula boiss. Arch. Razi Inst. 2023, 78, 1638–1646. [Google Scholar]
- Banchio, E.; Bogino, P.C.; Santoro, M.; Torres, L.; Zygadlo, J.; Giordano, W. Systemic induction of monoterpene biosynthesis in Origanum × majoricum by soil bacteria. J. Agric. Food Chem. 2010, 58, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Arshad, M.; Frankenberger, W.T. Plant Growth Promoting Rhizobacteria: Applications and Perspectives in Agriculture. Adv. Agron. 2003, 81, 97–168. [Google Scholar] [CrossRef]
- Santoro, M.V.; Zygadlo, J.; Giordano, W.; Banchio, E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol. Biochem. 2011, 49, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Warkentin, T.D. Biofortification of pulse crops: Status and future perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, D.L.; Maldonado, R.A.; Bianchi, J.S.; Kurth, D.; Gramajo, H.; Chiesa, M.A.; Rodríguez, E. Streptomyces N2A, an endophytic actinobacteria that promotes soybean growth and increases yield and seed quality under field conditions. Plant Sci. 2024, 343, 112073. [Google Scholar] [CrossRef]
- Liatukas, Ž.; Suproniene, S.; Ruzgas, V.; Leistrumaitė, A. Effects of organic seed treatment methods on spring barley seed quality, crop, productivity and disease incidence. Zemdirb.-Agric. 2019, 106, 241–248. [Google Scholar] [CrossRef]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.-H.I.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K.A. Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Zong, Y.; Li, F.Y.; Han, Y.; Hao, X. Photosynthesis and metabolite responses of Isatis indigotica Fortune to elevated [CO2]. Crop J. 2017, 5, 345–353. [Google Scholar] [CrossRef]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Baset, M.M.; Shamsuddin, Z.; Wahab, Z.; Marziah, M. Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured'musa'plantlets under nitrogen-free hydroponics condition. Aust. J. Crop Sci. 2010, 4, 85–90. [Google Scholar]
- Sreevidya, M.; Gopalakrishnan, S.; Kudapa, H.; Varshney, R. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz. J. Microbiol. 2016, 47, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.; Al-Shihi, B.I.; Baqi, Y. Date palm tree (Phoenix dactylifera L.): Natural products and therapeutic options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Hatami, M.; Kariman, K.; Abbaszadeh Dahaji, P. Phytochemical variations and enhanced efficiency of antioxidant and antimicrobial ingredients in Salvia officinalis as inoculated with different rhizobacteria. Chem. Biodivers. 2016, 13, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Sarma, B.K.; Singh, D.P. Effect of plant growth-promoting rhizobacteria and culture filtrate of Sclerotium rolfsii on phenolic and salicylic acid contents in chickpea (Cicer arietinum). Curr. Microbiol. 2003, 46, 0131–0140. [Google Scholar] [CrossRef] [PubMed]
- Solecka, J.; Zajko, J.; Postek, M.; Rajnisz, A. Biologically active secondary metabolites from Actinomycetes. Open Life Sci. 2012, 7, 373–390. [Google Scholar] [CrossRef]
- Kutchan, T.M. Ecological arsenal and developmental dispatcher. The paradigm of secondary metabolism. Plant Physiol. 2001, 125, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, E.; Leniarska, U. Production of B-group vitamins by mycorrhizal fungi and actinomycetes isolated from the root zone of pine (Pinus sylvestris L.). Plant Soil 1985, 96, 387–394. [Google Scholar] [CrossRef]
- Mozafar, A.; Oertli, J. Uptake of a microbially-produced vitamin (B12) by soybean roots. Plant Soil 1992, 139, 23–30. [Google Scholar] [CrossRef]
- Yousaf, A.; Qadir, A.; Anjum, T.; Ahmad, A. Identification of microbial metabolites elevating vitamin contents in barley seeds. J. Agric. Food Chem. 2015, 63, 7304–7310. [Google Scholar] [CrossRef]
- Sathya, A.; Vijayabharathi, R.; Srinivas, V.; Gopalakrishnan, S. Plant growth-promoting actinobacteria on chickpea seed mineral density: An upcoming complementary tool for sustainable biofortification strategy. 3 Biotech 2016, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.; Selim, S.; Zinta, G.; Mousa, A.S.; Hozzein, W.N. Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 2020, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Angami, T.; Wangchu, L.; Debnath, P.; Sarma, P.; Singh, B.; Singh, A.K.; Singh, S.; Hazarika, B.; Singh, M.C.; Chhetri, A. Determination of nutritional content of Spondias species from the eastern Himalaya. Int. Res. J. Pure Appl. Chem. 2020, 21, 12–17. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Konishi, Y.; Bruno, M.; Valoy, M.; Prado, F.E. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J. Sci. Food Agric. 2012, 92, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Hinkaew, J.; Aursalung, A.; Sahasakul, Y.; Tangsuphoom, N.; Suttisansanee, U. A comparison of the nutritional and biochemical quality of date palm fruits obtained using different planting techniques. Molecules 2021, 26, 2245. [Google Scholar] [CrossRef] [PubMed]
- Zand, A.; Darzi, M.T.; Hadi, M. Effects of phosphate solubilizing microorganisms and plant density on seed yield and essential oil content of anise (Pimpinella anisum). Middle-East J. Sci. Res. 2013, 14, 940–946. [Google Scholar]
- del Rosario Cappellari, L.; Santoro, M.V.; Nievas, F.; Giordano, W.; Banchio, E. Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2013, 70, 16–22. [Google Scholar] [CrossRef]
- Ullah, H.; Honermeier, B. Fruit yield, essential oil concentration and composition of three anise cultivars (Pimpinella anisum L.) in relation to sowing date, sowing rate and locations. Ind. Crops Prod. 2013, 42, 489–499. [Google Scholar] [CrossRef]
- Alraey, D.A.; Haroun, S.A.; Omar, M.N.; Abd-ElGawad, A.M.; El-Shobaky, A.M.; Mowafy, A.M. Fluctuation of essential oil constituents in Origanum syriacum subsp. sinaicum in response to plant growth promoting bacteria. J. Essent. Oil Bear. Plants 2019, 22, 1022–1033. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Sangwan, N.; Farooqi, A.; Shabih, F.; Sangwan, R. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Lim, J.-H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]




| Egypt | Tunisia | Syria | Morocco | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | JW0824 | Control | JW0824 | Control | JW0824 | Control | JW0824 | ||
| Phenyl prostanoids | trans-anethole | 75.27 ± 2.15 | 81.13 ± 2.20 ns | 53.38 ± 1.1 | 75.97 ± 1.16 ** | 71.0 ± 0.09 | 61.3 ± 0.08 * | 50.6 ± 2.1 | 64 ± 0.09 ** |
| o-isoeugenol | 4.73 ± 3.15 | 6.8 ± 3.12 **** | 3.21 ± 2.11 | 5.53 ± 2.15 *** | 5.25 ± 1.11 | 5.26 ± 1.1 ns | 3.87 ± 0.07 | 4.3 ± 0.05 * | |
| Anisole | 3.22 ± 0.09 | 4.64 ± 1.11 *** | 3.68 ± 1.17 | 5.39 ± 2.12 *** | 3.57 ± 1.13 | 4.31 ± 2.15 * | 3.10 ± 0.06 | 3.6 ± 0.0 ns | |
| p-anisaldehyde | 0.45 ± 0.09 | 0.22 ± 0.001 ns | 0.64 ± 0.09 | 0.35 ± 0.002 ns | 0.30 ± 0.01 | 0.32 ± 0.1 ns | 0.20 ± 0.07 | 0.24 ± 0.08 ns | |
| Estragole | 12.68 ± 2.13 | 17.39 ± 1.17 ** | 8.51 ± 1.01 | 12.50 ± 2 *** | 13.6 ± 1.11 | 13.20 ± 1 ns | 9.37 ± 0.07 | 11.0 ± 0.09 * | |
| Monoterpene hydrocarbons | α-pinene | 0.07 ± 0.001 | 0.10 ± 0.003 ** | 0.07 ± 0.00 | 0.09 ± 0.001 ** | 61.3 ± 0.09 | 0.11 ± 0.0 ** | 0.07 ± 0.00 | 0.07 ± 0.00 ns |
| Limonene | 0.70 ± 0.13 | 0.75 ± 0.15 ns | 0.33 ± 0.07 | 0.42 ± 0.06 ns | 5.2 ± 0.09 | 0.4 ± 0.09 ns | 0.25 ± 0.07 | 0.28 ± 0.08 ns | |
| Myrcene | 1.53 ± 0.14 | 4.27 ± 1.16 ns | 1.61 ± 0.08 | 1.90 ± 0.07 ns | 4.3 ± 1.10 | 2.53 ± 1.1 ns | 1.86 ± 0.08 | 1.4 ± 0.09 ns | |
| Linalool | 2.54 ± 1.15 | 7.19 ± 3 **** | 2.79 ± 0.06 | 2.74 ± 0.05 ns | 0.32 ± 0.09 | 4.2 ± 0.00 ns | 3.11 ± 0.00 | 2.1 ± 0.04 ** | |
| Cis-β-ocimene | 0.35 ± 0.09 | 0.60 ± 0.01 ns | 0.36 ± 0.16 | 0.79 ± 0.10 ns | 13.2 ± 1.10 | 0.55 ± 2.1 ns | 0.40 ± 0.03 | 0.43 ± 0.06 ns | |
| Sabinene | 0.38 ± 0.03 | 0.82 ± 0.19 * | 0.52 ± 0.02 | 0.77 ± 0.01 ns | 61.3 ± 1.11 | 0.67 ± 1.1 ns | 0.48 ± 0.02 | 0.45 ± 0.0 ns | |
| p-cymene | 0.53 ± 0.017 | 0.90 ± 0.01 ** | 0.97 ± 0.17 | 0.94 ± 0.101 ns | 5.2 ± 0.00 | 0.86 ± 0.0 ** | 0.56 ± 0.08 | 0.46 ± 0.07 * | |
| Aα-phellandrene | 3.37 ± 0.03 | 4.81 ± 0.04 ** | 2.42 ± 0.02 | 4.85 ± 0.01 *** | 4.3 ± 0.08 | 3.14 ± 0.07 * | 2.99 ± 0.09 | 1.9 ± 0.0 *** | |
| Oxygenated monoterpenes | Fenchone | 5.82 ± 0.04 | 5.74 ± 0.03 ns | 5.81 ± 0.01 | 6.24 ± 0.02 ns | 5.25 ± 0.07 | 5.3 ± 0.08 ns | 3.65 ± 0.01 | 3.50 ± 0.05 ns |
| 1,8-cineole | 2.44 ± 0.08 | 2.91 ± 1.10 ns | 2.39 ± 1.15 | 2.50 ± 2.10 ns | 2.43 ± 1.01 | 2.3 ± 2.01 ns | 1.78 ± 0.07 | 0.9 ± 0.08 ** | |
| α-fenchyl acetate | 0.09 ± 0.04 | 0.15 ± 0.05 * | 0.09 ± 0.02 | 0.17 ± 0.03 * | 0.11 ± 0.06 | 0.25 ± 0.07 ** | 0.13 ± 0.05 | 0.12 ± 0.0 ns | |
| α-Terpinene | 0.09 ± 0.09 | 0.17 ± 0.05 ** | 0.10 ± 0.01 | 0.14 ± 0.05 ** | 0.12 ± 0.00 | 0.2 ± 0.18 *** | 0.10 ± 0.05 | 0.08 ± 0.00 * | |
| Sesquiterpene hydrocarbons | γ-himachalene | 0.12 ± 0.01 | 0.32 ± 0.03 ns | 0.22 ± 0.02 | 0.13 ± 0.04 ns | 0.15 ± 0.05 | 0.67 ± 0.07 ** | 0.11 ± 0.08 | 0.09 ± 0.0 ns |
| Isolongifolene | 0.38 ± 0.02 | 0.44 ± 0.01 ns | 0.39 ± 0.03 | 0.44 ± 0.01 ns | 0.37 ± 0.06 | 0.43 ± 0.0 ns | 0.31 ± 0.09 | 0.33 ± 0.0 ns | |
| β-Elemene | 0.65 ± 0.01 | 0.67 ± 0.02 ns | 0.74 ± 0.01 | 0.66 ± 0.07 ns | 0.60 ± 0.05 | 1.1 ± 0.01 * | 0.48 ± 0.01 | 0.40 ± 0.04 ns | |
| Zingiberene | 0.93 ± 0.09 | 1.47 ± 1.11 ** | 0.95 ± 0.18 | 1.25 ± 0.16 ns | 1.09 ± 0.01 | 1.5 ± 0.09 ns | 0.90 ± 0.07 | 0.96 ± 0.04 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.M.; Reyad, A.M.; Khalaf, M.H.; Sheteiwy, M.S.; Dawood, M.F.A.; El-Sawah, A.M.; Shaban Ahmed, E.; Malik, A.; Al-Qahtani, W.H.; Abdel-Maksoud, M.A.; et al. Investigating the Endophyte Actinomycetota sp. JW0824 Strain as a Potential Bioinoculant to Enhance the Yield, Nutritive Value, and Chemical Composition of Different Cultivars of Anise (Pimpinella anisum L.) Seeds. Biology 2024, 13, 553. https://doi.org/10.3390/biology13080553
Mahmoud AM, Reyad AM, Khalaf MH, Sheteiwy MS, Dawood MFA, El-Sawah AM, Shaban Ahmed E, Malik A, Al-Qahtani WH, Abdel-Maksoud MA, et al. Investigating the Endophyte Actinomycetota sp. JW0824 Strain as a Potential Bioinoculant to Enhance the Yield, Nutritive Value, and Chemical Composition of Different Cultivars of Anise (Pimpinella anisum L.) Seeds. Biology. 2024; 13(8):553. https://doi.org/10.3390/biology13080553
Chicago/Turabian StyleMahmoud, Ahmed M., Ahmed M. Reyad, Maha H. Khalaf, Mohamed S. Sheteiwy, Mona F. A. Dawood, Ahmed M. El-Sawah, Enas Shaban Ahmed, Abdul Malik, Wahidah H. Al-Qahtani, Mostafa A. Abdel-Maksoud, and et al. 2024. "Investigating the Endophyte Actinomycetota sp. JW0824 Strain as a Potential Bioinoculant to Enhance the Yield, Nutritive Value, and Chemical Composition of Different Cultivars of Anise (Pimpinella anisum L.) Seeds" Biology 13, no. 8: 553. https://doi.org/10.3390/biology13080553
APA StyleMahmoud, A. M., Reyad, A. M., Khalaf, M. H., Sheteiwy, M. S., Dawood, M. F. A., El-Sawah, A. M., Shaban Ahmed, E., Malik, A., Al-Qahtani, W. H., Abdel-Maksoud, M. A., Mousa, N. H. S., Alyafei, M., & AbdElgawad, H. (2024). Investigating the Endophyte Actinomycetota sp. JW0824 Strain as a Potential Bioinoculant to Enhance the Yield, Nutritive Value, and Chemical Composition of Different Cultivars of Anise (Pimpinella anisum L.) Seeds. Biology, 13(8), 553. https://doi.org/10.3390/biology13080553








