Raphanus sativus Linne Protects Human Nucleus Pulposus Cells against H2O2-Induced Damage by Inhibiting TREM2
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing of NP Cells
2.2. Preparation of RSL Extracts
2.3. RSL or H2O2 Treatment
2.4. Cell Viability Assays
2.5. LDH Assay
2.6. Live–Dead Assay
2.7. Western Blotting
2.8. Real-Time PCR
2.9. Apoptosis
2.10. Immunocytochemistry
2.11. Statistical Analyses
3. Results
3.1. RSL Extracts Protect Human NP Cells against H2O2 Treatment
3.2. RSL Extracts Inhibit Apoptosis in H2O2-Treated NP Cells
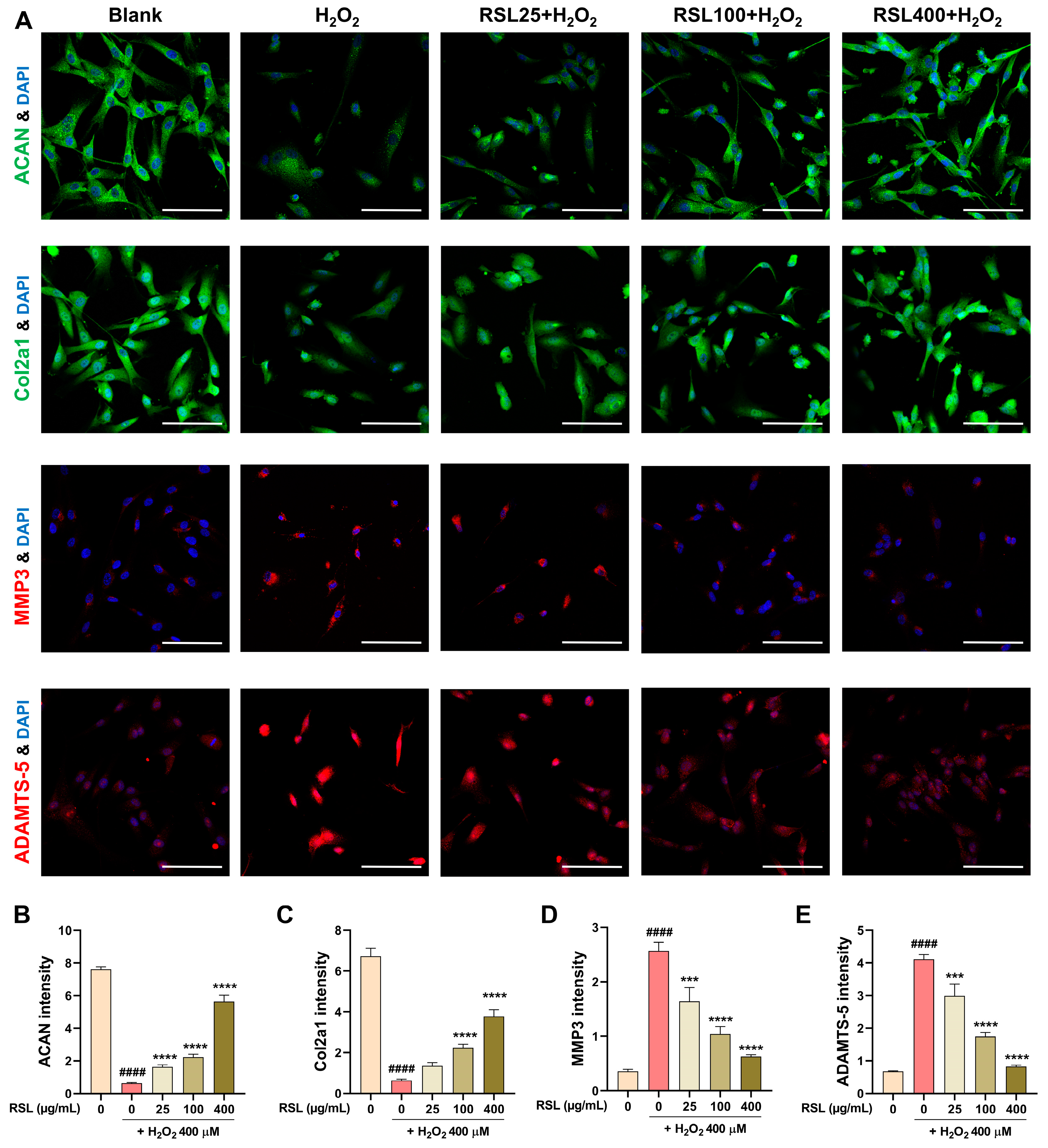
3.3. RSL Inhibits H2O2-Induced Degeneration in NP Cells by Regulating the Expression of Key Matrix Components and Enzyme-Related mRNAs
3.4. RSL Inhibits H2O2-Induced Degeneration in NP Cells by Regulating the Expression of Key Matrix Components and Enzyme-Related Proteins
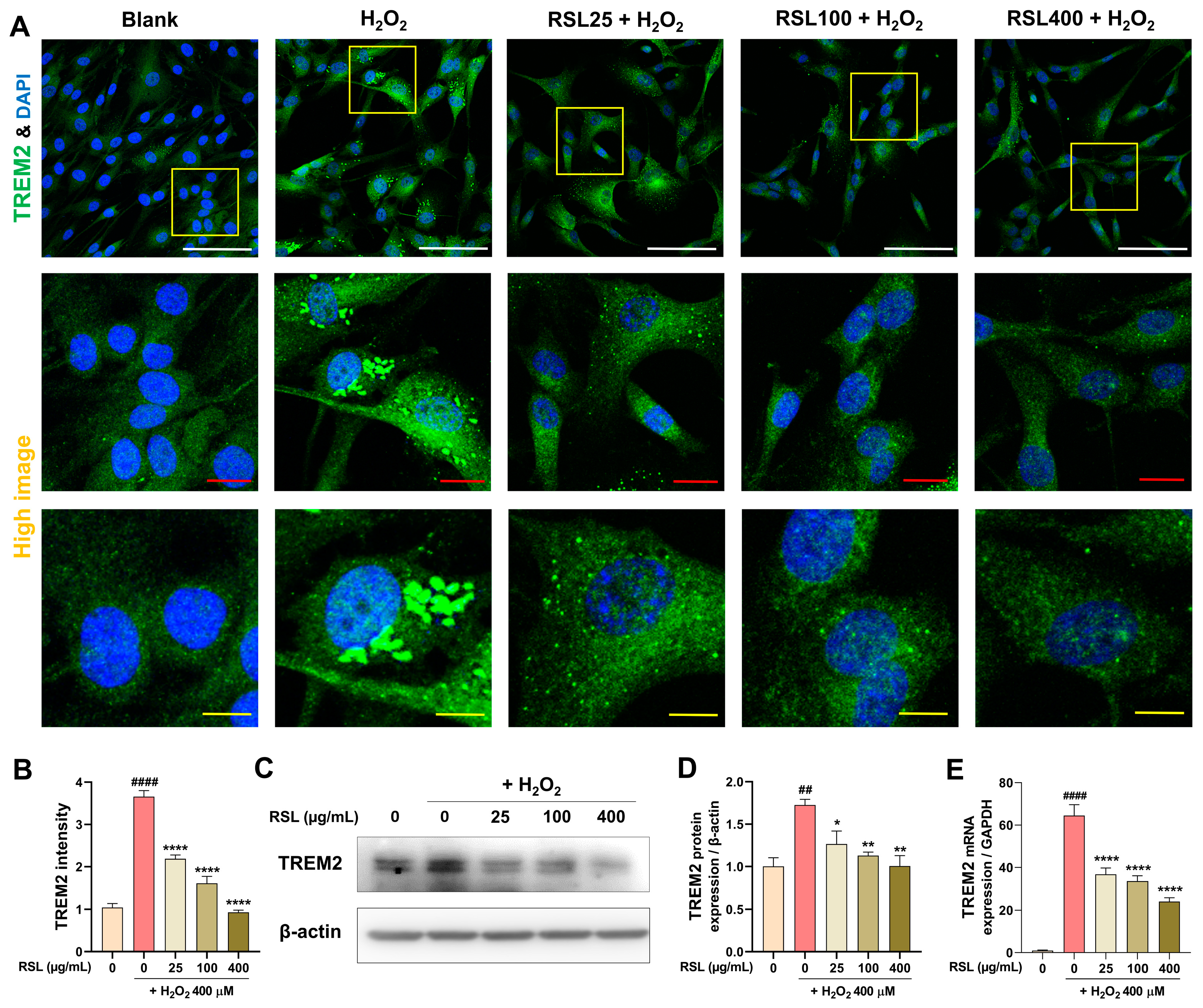
3.5. RSL Regulates TREM2 Expression in H2O2-Treated NP Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatchel, R.J. The Continuing and Growing Epidemic of Chronic Low Back Pain. Healthcare 2015, 3, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef]
- Samanta, A.; Lufkin, T.; Kraus, P. Intervertebral disc degeneration-Current therapeutic options and challenges. Front. Public Health 2023, 11, 1156749. [Google Scholar] [CrossRef]
- Mern, D.S.; Thome, C. Identification and characterization of human nucleus pulposus cell specific serotypes of adeno-associated virus for gene therapeutic approaches of intervertebral disc disorders. BMC Musculoskelet. Disord. 2015, 16, 341. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Liu, M.Q.; Chen, H.W.; Wu, Z.L.; Gao, Y.C.; Ma, Z.J.; He, X.G.; Kang, X.W. NF-kappaB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021, 54, e13057. [Google Scholar] [CrossRef] [PubMed]
- Cassinelli, E.H.; Hall, R.A.; Kang, J.D. Biochemistry of intervertebral disc degeneration and the potential for gene therapy applications. Spine J. 2001, 1, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, P.; Li, Y.; Yao, L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthr. Cartil. 2016, 24, 206–212. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Futterman, B. Anatomy, Back, Intervertebral Discs. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gansau, J.; Buckley, C.T. Incorporation of Collagen and Hyaluronic Acid to Enhance the Bioactivity of Fibrin-Based Hydrogels for Nucleus Pulposus Regeneration. J. Funct. Biomater. 2018, 9, 43. [Google Scholar] [CrossRef]
- Hwang, P.Y.; Chen, J.; Jing, L.; Hoffman, B.D.; Setton, L.A. The role of extracellular matrix elasticity and composition in regulating the nucleus pulposus cell phenotype in the intervertebral disc: A narrative review. J. Biomech. Eng. 2014, 136, 021010. [Google Scholar] [CrossRef]
- Dou, Y.; Sun, X.; Ma, X.; Zhao, X.; Yang, Q. Intervertebral Disk Degeneration: The Microenvironment and Tissue Engineering Strategies. Front. Bioeng. Biotechnol. 2021, 9, 592118. [Google Scholar] [CrossRef]
- Peng, B.; Li, Y. Concerns about cell therapy for intervertebral disc degeneration. NPJ Regen. Med. 2022, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Rider, S.M.; Mizuno, S.; Kang, J.D. Molecular Mechanisms of Intervertebral Disc Degeneration. Spine Surg. Relat. Res. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zheng, B.; Zhang, B.; Ma, T.; Hao, L.; Zhang, Y. The role of ageing and oxidative stress in intervertebral disc degeneration. Front. Mol. Biosci. 2022, 9, 1052878. [Google Scholar] [CrossRef]
- Zielinska, N.; Podgorski, M.; Haladaj, R.; Polguj, M.; Olewnik, L. Risk Factors of Intervertebral Disc Pathology-A Point of View Formerly and Today-A Review. J. Clin. Med. 2021, 10, 409. [Google Scholar] [CrossRef]
- Stokes, I.A.; Iatridis, J.C. Mechanical conditions that accelerate intervertebral disc degeneration: Overload versus immobilization. Spine 2004, 29, 2724–2732. [Google Scholar] [CrossRef]
- Neidlinger-Wilke, C.; Mietsch, A.; Rinkler, C.; Wilke, H.J.; Ignatius, A.; Urban, J. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J. Orthop. Res. 2012, 30, 112–121. [Google Scholar] [CrossRef]
- Costachescu, B.; Niculescu, A.G.; Teleanu, R.I.; Iliescu, B.F.; Radulescu, M.; Grumezescu, A.M.; Dabija, M.G. Recent Advances in Managing Spinal Intervertebral Discs Degeneration. Int. J. Mol. Sci. 2022, 23, 6460. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.; Mangamoori, L.N.; Gowda, B.B. Polyphenolics profile and antioxidant properties of Raphanus sativus L. Nat. Prod. Res. 2012, 26, 557–563. [Google Scholar] [CrossRef]
- Kook, S.H.; Choi, K.C.; Lee, Y.H.; Cho, H.K.; Lee, J.C. Raphanus sativus L. seeds prevent LPS-stimulated inflammatory response through negative regulation of the p38 MAPK-NF-kappaB pathway. Int. Immunopharmacol. 2014, 23, 726–734. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, H.; Duan, Y.; Chen, G. Protective effects of radish (Raphanus sativus L.) leaves extract against hydrogen peroxide-induced oxidative damage in human fetal lung fibroblast (MRC-5) cells. Biomed. Pharmacother. 2018, 103, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K.; et al. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 2018, 48, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Magno, L.; Bunney, T.D.; Mead, E.; Svensson, F.; Bictash, M.N. TREM2/PLCgamma2 signalling in immune cells: Function, structural insight, and potential therapeutic modulation. Mol. Neurodegener. 2021, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Yaghmoor, F.; Noorsaeed, A.; Alsaggaf, S.; Aljohani, W.; Scholtzova, H.; Boutajangout, A.; Wisniewski, T. The Role of TREM2 in Alzheimer’s Disease and Other Neurological Disorders. J. Alzheimers Dis. Parkinsonism 2014, 4, 160. [Google Scholar] [CrossRef]
- Bai, M.; Yin, H.P.; Zhao, J.; Li, Y.; Wu, Y.M. Roles of TREM2 in degeneration of human nucleus pulposus cells via NF-kappaB p65. J. Cell Biochem. 2018, 119, 8784–8796. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, F. Targeting TREM2 for Parkinson’s Disease: Where to Go? Front. Immunol. 2021, 12, 795036. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Wang, H.; Yang, K.; Luan, J.; Wang, S. TREM2: Potential therapeutic targeting of microglia for Alzheimer’s disease. Biomed. Pharmacother. 2023, 165, 115218. [Google Scholar] [CrossRef]
- Liu, A.H.; Chu, M.; Wang, Y.P. Up-Regulation of Trem2 Inhibits Hippocampal Neuronal Apoptosis and Alleviates Oxidative Stress in Epilepsy via the PI3K/Akt Pathway in Mice. Neurosci. Bull. 2019, 35, 471–485. [Google Scholar] [CrossRef]
- Hou, Q.; Cymbalyuk, E.; Hsu, S.C.; Xu, M.; Hsu, Y.T. Apoptosis modulatory activities of transiently expressed Bcl-2: Roles in cytochrome C release and Bax regulation. Apoptosis 2003, 8, 617–629. [Google Scholar] [CrossRef]
- Jurgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shao, Z.W.; Xiong, L.M. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Yu, X.H.; Wang, C.; Yang, W.; He, W.S.; Zhang, S.J.; Yan, Y.G.; Zhang, J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta 2015, 448, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, M.; Wei, F.; Liang, J.; Deng, W.; Dai, X.; Zhou, G.; Zou, X. Shock absorbing function study on denucleated intervertebral disc with or without hydrogel injection through static and dynamic biomechanical tests in vitro. Biomed. Res. Int. 2014, 2014, 461724. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, X.; Zhang, P.; Guo, J.; Rong, K.; Wang, X.; Cao, X.; Zhou, T.; Zhao, J. Engeletin Alleviates the Inflammation and Apoptosis in Intervertebral Disc Degeneration via Inhibiting the NF-kappaB and MAPK Pathways. J. Inflamm. Res. 2022, 15, 5767–5783. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, L.; Deng, X.; Shi, D.; Wu, F.; Liang, H.; Huang, D.; Shao, Z. Mesenchymal Stem Cells Protect Nucleus Pulposus Cells from Compression-Induced Apoptosis by Inhibiting the Mitochondrial Pathway. Stem Cells Int. 2017, 2017, 9843120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, X.; Shen, H.; Zhang, C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int. J. Mol. Med. 2016, 37, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Ranganathan, V.; Farnsworth, M.L.; Kavallaris, M.; Lock, R.B. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000, 7, 102–111. [Google Scholar] [CrossRef]
- Lin, S.S.; Niu, C.C.; Yuan, L.J.; Tsai, T.T.; Lai, P.L.; Chong, K.Y.; Wei, K.C.; Huang, C.Y.; Lu, M.L.; Yang, C.Y.; et al. Mir-573 regulates cell proliferation and apoptosis by targeting Bax in human degenerative disc cells following hyperbaric oxygen treatment. J. Orthop. Surg. Res. 2021, 16, 16. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.M.; Kim, H.H.; Ha, K.T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Bagci, E.Z.; Vodovotz, Y.; Billiar, T.R.; Ermentrout, G.B.; Bahar, I. Bistability in apoptosis: Roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys. J. 2006, 90, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wen, B.; Sun, D. miR-573 regulates cell proliferation and apoptosis by targeting Bax in nucleus pulposus cells. Cell Mol. Biol. Lett. 2019, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Lee, T.S.; Zhou, R.H.; Chin, J.; Lotz, J.C.; Mayoux-Benhamou, M.A.; Barbet, J.P.; Chevrot, A.; Shyy, J.Y. Intervertebral disc degeneration: The role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am. J. Pathol. 2004, 164, 915–924. [Google Scholar] [CrossRef]
- Sudo, H.; Minami, A. Regulation of apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2 overexpression. J. Orthop. Res. 2010, 28, 1608–1613. [Google Scholar] [CrossRef]
- Zhang, X.B.; Hu, Y.C.; Cheng, P.; Zhou, H.Y.; Chen, X.Y.; Wu, D.; Zhang, R.H.; Yu, D.C.; Gao, X.D.; Shi, J.T.; et al. Targeted therapy for intervertebral disc degeneration: Inhibiting apoptosis is a promising treatment strategy. Int. J. Med. Sci. 2021, 18, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, J.; Suo, M.; Huang, H.; Liu, X.; Wang, J.; Li, Z. Can extracellular vesicles be considered as a potential frontier in the treatment of intervertebral disc disease? Ageing Res. Rev. 2023, 92, 102094. [Google Scholar] [CrossRef]



| Company | Product No. | Host | Reactivity | Application | Dilution | |
|---|---|---|---|---|---|---|
| Cleaved caspase-3 | CST | 9661 | Rabbit | H M R Mk | WB | 1:500 |
| Bax | CST | 2772 | Rabbit | H M R Mk | WB | 1:1000 |
| BCL-2 | Santa cruz | SC-7382 | Mouse | H M R | WB | 1:200 |
| TREM2 | Santa cruz | SC-373828 | Mouse | H | WB | 1:200 |
| β-actin | Santa cruz | SC-47778 | Mouse | H M R | WB | 1:3000 |
| Aggrecan | Proteintech | 13880-1-AP | Rabbit | H M R | ICC | 1:200 |
| Col2a1 | Invtirogen | PA1-26206 | Rabbit | B H R | ICC | 1:100 |
| Adamts5 | Invtirogen | PA5-27165 | Rabbit | H M | ICC | 1:100 |
| MMP3 | Abcam | Ab52915 | Rabbit | H M R | ICC | 1:100 |
| FITC Goat anti-rab IgG | Jackson | 112-095-003 | Goat | ICC | 1:300 | |
| Rhodamine Goat anti-rab IgG | Jackson | 111-295-045 | Goat | ICC | 1:300 | |
| Goat anti-rabbit IgG | Abcam | ab205718 | Goat | WB | 1:2500 | |
| Goat anti-mouse IgG | Abcam | ab205719 | Goat | WB | 1:2500 |
| Target | Forward (5′–3′) | Reverse (3′–5′) |
|---|---|---|
| ACAN | TGAAACCACCTCTGCATTCCA | GACGCCTCGCCTTCTTGAA |
| Col2a1 | GTCACAGAAGACCTCACGCCTC | TCCACACCGAATTCCTGCTC |
| Adamts-4 | ACTGGTGGTGGCAGATGACA | TCACTGTTAGCAGGTAGCGCTTT |
| Adamts-5 | GCTTCTATCGGGGCACAGT | CAGCAGTGGCTTTAGGGTGTAG |
| MMP3 | GCTGTTTTTGAAGAATTTGGGTTC | GCACAGGCAGGAGAAAACGA |
| MMP13 | CCAGGCATCACCATTCAAG | ATCATCTTCATCACCACCACTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Yeo, C.; Hong, J.Y.; Jeon, W.-J.; Kim, H.; Lee, J.; Lee, Y.J.; Baek, S.H.; Ha, I.-H. Raphanus sativus Linne Protects Human Nucleus Pulposus Cells against H2O2-Induced Damage by Inhibiting TREM2. Biology 2024, 13, 602. https://doi.org/10.3390/biology13080602
Kim H, Yeo C, Hong JY, Jeon W-J, Kim H, Lee J, Lee YJ, Baek SH, Ha I-H. Raphanus sativus Linne Protects Human Nucleus Pulposus Cells against H2O2-Induced Damage by Inhibiting TREM2. Biology. 2024; 13(8):602. https://doi.org/10.3390/biology13080602
Chicago/Turabian StyleKim, Hyunseong, Changhwan Yeo, Jin Young Hong, Wan-Jin Jeon, Hyun Kim, Junseon Lee, Yoon Jae Lee, Seung Ho Baek, and In-Hyuk Ha. 2024. "Raphanus sativus Linne Protects Human Nucleus Pulposus Cells against H2O2-Induced Damage by Inhibiting TREM2" Biology 13, no. 8: 602. https://doi.org/10.3390/biology13080602
APA StyleKim, H., Yeo, C., Hong, J. Y., Jeon, W.-J., Kim, H., Lee, J., Lee, Y. J., Baek, S. H., & Ha, I.-H. (2024). Raphanus sativus Linne Protects Human Nucleus Pulposus Cells against H2O2-Induced Damage by Inhibiting TREM2. Biology, 13(8), 602. https://doi.org/10.3390/biology13080602






