Enhanced Soil Fertility and Carbon Sequestration in Urban Green Spaces through the Application of Fe-Modified Biochar Combined with Plant Growth-Promoting Bacteria
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Soil Samples, Biochar, Bacillus clausii, and Ryegrass Seeds
2.2. Bacterial Inoculum Preparation
2.3. Experimental Design
2.4. Samples Analysis
2.4.1. Characterization of Bacillus clausii and Biochar Samples
2.4.2. Analysis of Plant and Soil Samples
2.4.3. Analytic Method for Bacterial Community Analysis
2.5. Statistical Analysis
3. Results
3.1. Changes in Characteristics of Fe-Modified Biochar
3.2. Plant Growth-Promoting Characteristics and Biomineralization of Bacillus clausii
3.3. Changes in Soil Physicochemical Properties and Fertility of FeB Combined with BC
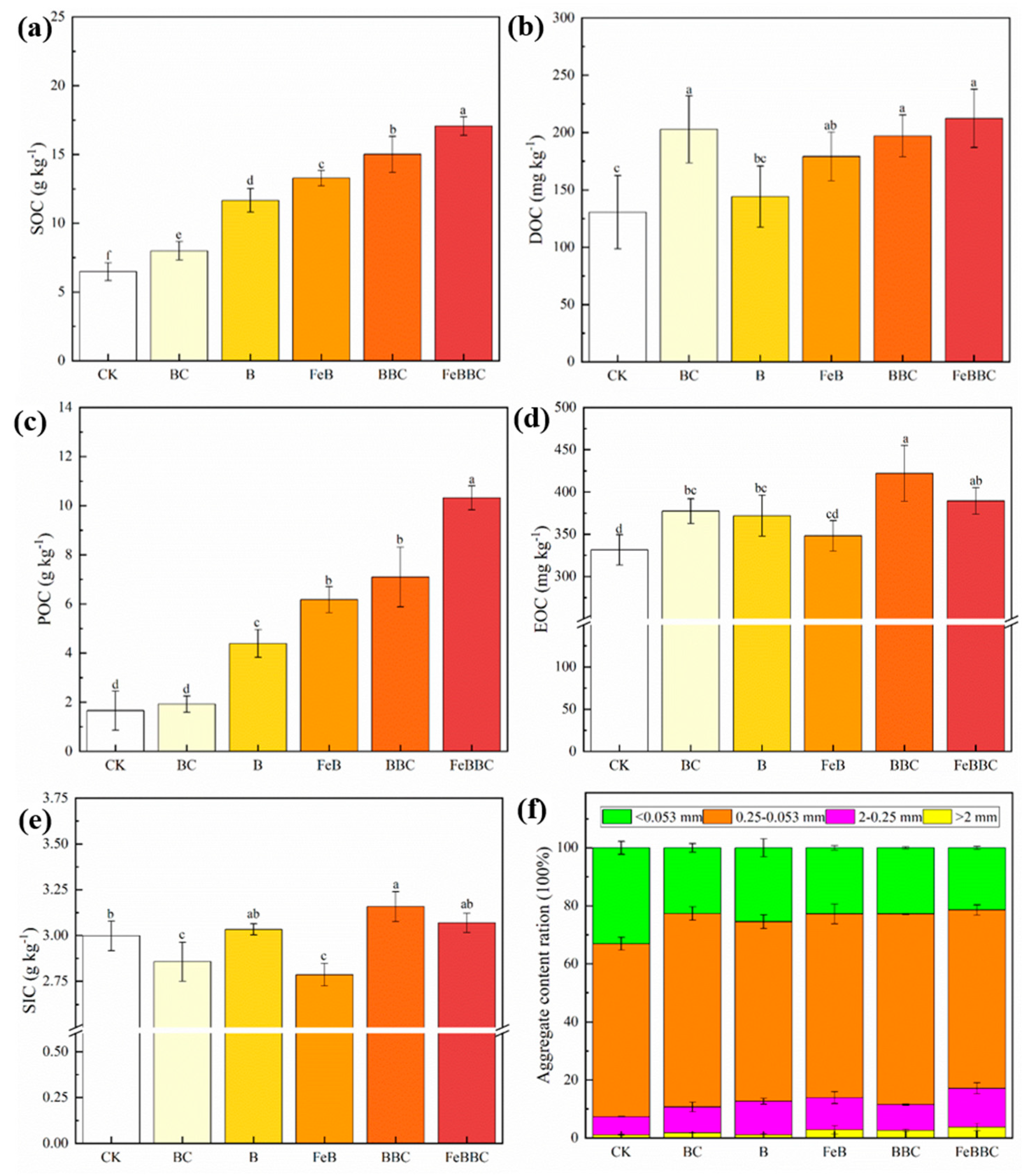
3.4. Changes in Soil Carbon Components for FeB Combined with BC
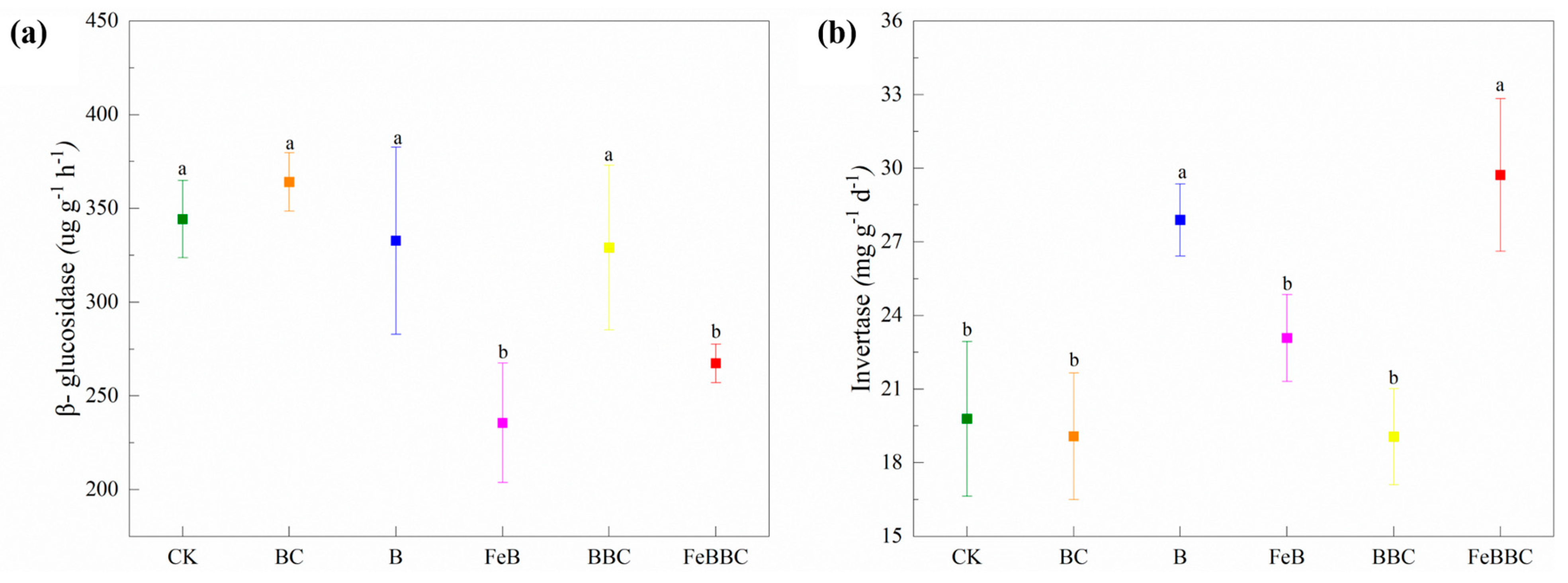
3.5. Changes in Soil C-Cycling-Related Enzyme Activity and Soil Bacterial Diversity for FeB Combined with BC
3.6. Changes in Bacterial Community Composition for FeB Combined with BC
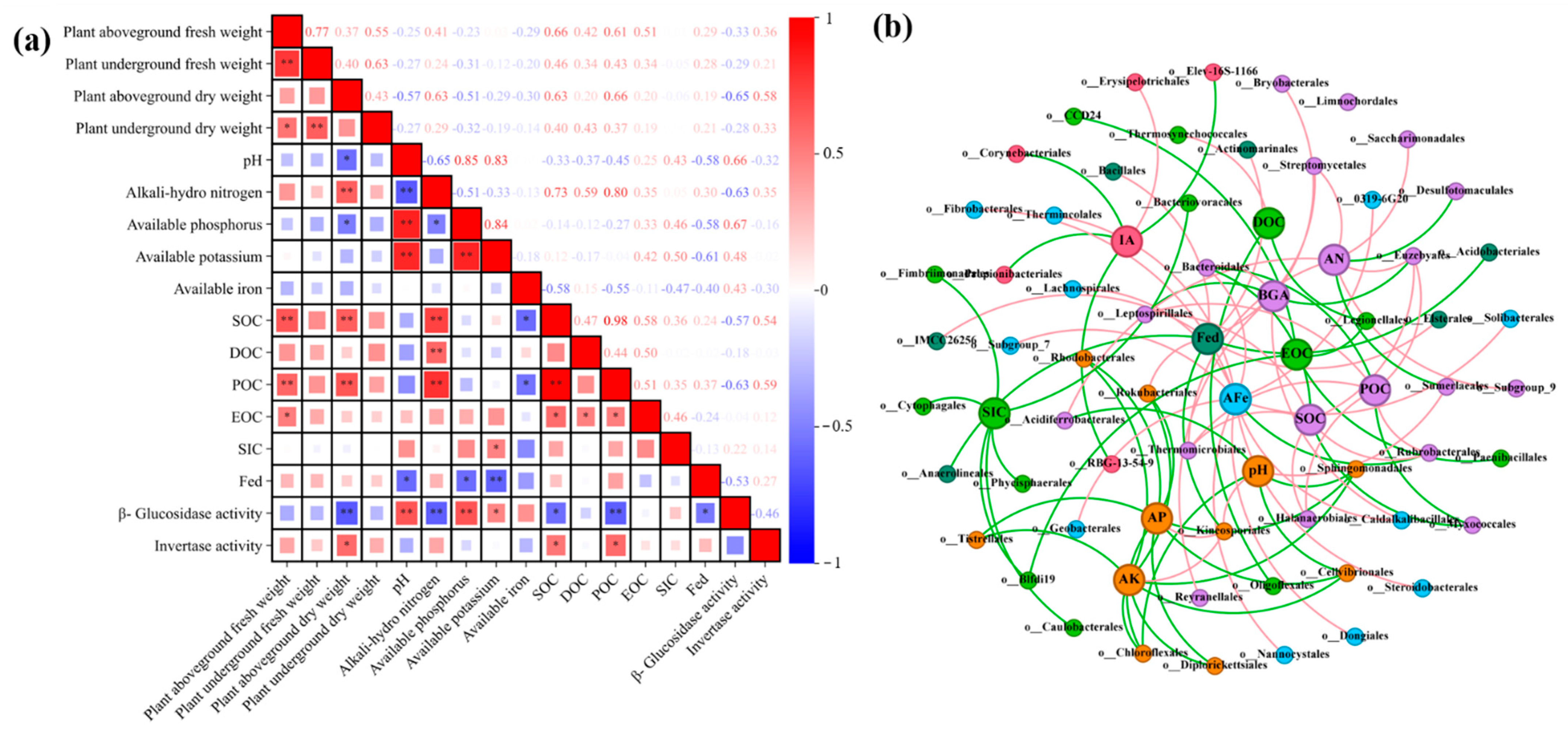
3.7. Exploring the Correlations between Environmental Factors and Bacterial Communities
4. Discussion
4.1. Characteristics of Biochar and Fe-Modified Biochar
4.2. Characteristics of Bacillus clausii
4.3. Effect of FeB Combined with BC Improves Soil Physicochemical Properties and Fertility
4.4. Effect of FeB Combined with BC Improves Soil Carbon Sequestration
4.5. Effect of FeB Combined with BC Improves C-Cycling-Related Enzyme Activity and Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feng, X.J.; Sun, X.Y.; Li, S.Y.; Zhang, J.D.; Hu, N. Relationship study among soils physicochemical properties and bacterial communities in urban green space and promotion of its composition and network analysis. Agron. J. 2021, 113, 515–526. [Google Scholar] [CrossRef]
- O’Riordan, R.; Davies, J.; Stevens, C.; Quinton, J.N.; Boyko, C. The ecosystem services of urban soils: A review. Geoderma 2021, 395, 115076. [Google Scholar] [CrossRef]
- Vasenev, V.; Kuzyakov, Y. Urban soils as hot spots of anthropogenic carbon accumulation: Review of stocks, mechanisms and driving factors. Land Degrad. Dev. 2018, 29, 1607–1622. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Ren, Y.; Luo, X.S.; Zhu, Y.G. Urban soil and human health: A review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef]
- Ungaro, F.; Maienza, A.; Ugolini, F.; Lanini, G.M.; Baronti, S.; Calzolari, C. Assessment of joint soil ecosystem services supply in urban green spaces: A case study in Northern Italy. Urban For. Urban Green. 2022, 67, 127455. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Mishra, R.; Lo, S.-L.; Kumar, S. A green approach towards sorption of CO2 on waste derived biochar. Environ. Res. 2022, 214, 113954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Palviainen, M.; Koster, K.; Berninger, F.; Bruckman, V.J.; Pumpanen, J. Effects of Biochar on Fluxes and Turnover of Carbon in Boreal Forest Soils. Soil Sci. Soc. Am. J. 2019, 83, 126–136. [Google Scholar] [CrossRef]
- Nath, H.; Sarkar, B.; Mitra, S.; Bhaladhare, S. Biochar from Biomass: A Review on Biochar Preparation Its Modification and Impact on Soil Including Soil Microbiology. Geomicrobiol. J. 2022, 39, 373–388. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wen, E.; Yang, X.; Chen, H.; Shaheen, S.M.; Sarkar, B.; Xu, S.; Song, H.; Liang, Y.; Rinklebe, J.; Hou, D.; et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J. Hazard. Mater. 2021, 407, 124344. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, C.; Chen, G.; Zhou, J.; Chen, Z.; Li, Z.; Zhu, J.; Feng, T.; Chen, Y. Response of soil microbial communities to additions of straw biochar, iron oxide, and iron oxide–modified straw biochar in an arsenic-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 23761–23768. [Google Scholar] [CrossRef]
- Bao, Z.; Shi, C.; Tu, W.; Li, L.; Li, Q. Recent developments in modification of biochar and its application in soil pollution control and ecoregulation. Environ. Pollut. 2022, 313, 120184. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Colo, J.; Hajnal-Jafari, T.I.; Duric, S.; Stamenov, D.; Hamidovic, S. Plant Growth Promotion Rhizobacteria in Onion Production. Pol. J. Microbiol. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wang, H.; Cai, Q. Identification of An Alkalophilic Strain and Activities of Its Extracelluar Polysaccharide. Pharm. Biotechnol. 2008, 15, 370–374. [Google Scholar]
- Oulebsir-Mohandkaci, H.; Benzina-Tihar, F.; Hadjouti, R. Exploring biofertilizer potential of plant growth-promoting rhizobacteria Bacillus clausii strain B8 (MT305787) on Brassica napus and Medicago sativa. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12484. [Google Scholar] [CrossRef]
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F.; Besharati, H.; Khalaj, M.A. The effects of biochar on soil nutrients status, microbial activity and carbon sequestration potential in two calcareous soils. Biochar 2021, 3, 105–116. [Google Scholar] [CrossRef]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Ben Fekih, I.; Ditta, A.; Xing, S. Role of biochar and plant growth promoting rhizobacteria to enhance soil carbon sequestration—A review. Environ. Monit. Assess. 2019, 191, 251. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Chen, H.; Zhou, J.; Lv, C. Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability 2022, 14, 10922. [Google Scholar] [CrossRef]
- Liu, S.; Kong, F.; Li, Y.; Jiang, Z.; Xi, M.; Wu, J. Mineral-ions modified biochars enhance the stability of soil aggregate and soil carbon sequestration in a coastal wetland soil. Catena 2020, 193, 104618. [Google Scholar] [CrossRef]
- Srivastava, S.; Bharti, R.K.; Verma, P.K.; Thakur, I.S. Cloning and expression of gamma carbonic anhydrase from Serratia sp. ISTD04 for sequestration of carbon dioxide and formation of calcite. Bioresour. Technol. 2015, 188, 209–213. [Google Scholar] [CrossRef]
- Zheng, T.; Qian, C. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Y.; Liu, P.; Li, Y.; Huang, F.; Zeng, L.; Liang, Y.; Li, S.; Hou, B. Iron-montmorillonite treated corn straw biochar: Interfacial chemical behavior and stability. Sci. Total Environ. 2020, 708, 134773. [Google Scholar] [CrossRef]
- Khalil, H.M.A.; Hassan, R.M. Raising the Productivity and Fiber Quality of Both White and Colored Cotton Using Eco-Friendly Fertilizers and Rice Straw. Int. J. Plants Res. 2015, 5, 122–135. [Google Scholar]
- Ren, H.; Lv, C.; Fernández-García, V.; Huang, B.; Yao, J.; Ding, W. Biochar and PGPR amendments influence soil enzyme activities and nutrient concentrations in a eucalyptus seedling plantation. Biomass Convers. Biorefin. 2019, 11, 1865–1874. [Google Scholar] [CrossRef]
- Hu, J.; Dong, H.; Xu, Q.; Ling, W.; Qu, J.; Qiang, Z. Impacts of water quality on the corrosion of cast iron pipes for water distribution and proposed source water switch strategy. Water Res. 2018, 129, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Manirakiza, N.; Şeker, C.; Negiş, H. Effects of Woody Compost and Biochar Amendments on Biochemical Properties of the Wind Erosion Afflicted a Calcareous and Alkaline Sandy Clay Loam Soil. Commun. Soil Sci. Plant Anal. 2021, 52, 487–498. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Chen, M.; Fei, C.; Zhang, W.; Li, Y.; Ding, X. Fe-modified biochar combined with mineral fertilization promotes soil organic phosphorus mineralization by shifting the diversity of phoD-harboring bacteria within soil aggregates in saline-alkaline paddy soil. J. Soils Sediments 2022, 23, 619–633. [Google Scholar] [CrossRef]
- Cai, L.; Yang, Y.; Chong, Y.; Xiong, J.; Wu, J.; Ai, X.; Guo, Q.; Yuan, Y.; Li, Z. Higher Soil Aggregate Stability in Subtropical Coniferous Plantations Than Natural Forests Due to Microbial and Aggregate Factors. Forests 2022, 13, 2110. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, Y.; Wu, H.; Li, M.; Hu, Z.; Zhang, J. Enhanced removal of phenanthrene and nutrients in wetland sediment with metallic biochar: Performance and mechanisms. Chemosphere 2023, 327, 138523. [Google Scholar] [CrossRef]
- He, C.; Wang, X.; Wang, D.; Zhao, Z.; Wang, F.; Cheng, L.; Feng, H.; Zhang, P. Impact of Spartina alterniflora invasion on soil bacterial community and associated greenhouse gas emission in the Jiuduansha wetland of China. Appl. Soil Ecol. 2021, 168, 104168. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, A.; Xia, C.L.; Lam, S.S.; Khan, A.A.; Tripathi, S.; Kumar, R.; Gupta, V.K.; Nadda, A.K. Enzyme mediated transformation of CO2 into calcium carbonate using purified microbial carbonic anhydrase. Environ. Res. 2022, 212, 113538. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef]
- Khan, M.N.; Li, D.; Shah, A.; Huang, J.; Zhang, L.; Núñez-Delgado, A.; Han, T.; Du, J.; Ali, S.; Sial, T.A.; et al. The impact of pristine and modified rice straw biochar on the emission of greenhouse gases from a red acidic soil. Environ. Res. 2022, 208, 112676. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, D.; Liao, X.; Miao, Y.; Li, Y.; Li, J.; Yuan, J.; Chen, Z.; Ding, W. Field-aged biochar enhances soil organic carbon by increasing recalcitrant organic carbon fractions and making microbial communities more conducive to carbon sequestration. Agric. Ecosyst. Environ. 2022, 340, 108177. [Google Scholar] [CrossRef]
- Ji, C.; Liu, Z.; Hao, L.; Song, X.; Wang, C.; Liu, Y.; Li, H.; Li, C.; Gao, Q.; Liu, X. Effects of Enterobacter cloacae HG-1 on the Nitrogen-Fixing Community Structure of Wheat Rhizosphere Soil and on Salt Tolerance. Front. Plant Sci. 2020, 11, 111094. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Guo, L.; Wu, F.; Liu, D.; Zhang, H.; Zou, S.; Xing, S.; Mao, Y. Field-applied biochar-based MgO and sepiolite composites possess CO2 capture potential and alter organic C mineralization and C-cycling bacterial structure in fertilized soils. Sci. Total Environ. 2022, 813, 152495. [Google Scholar] [CrossRef]
- Shivaji, S.; Ray, M.K.; Shyamala Rao, N.; Saisree, L.; Jagannadham, M.V.; Seshu Kumar, G.; Reddy, G.S.N.; Bhargava, P.M. Sphingobacterium antarcticus sp. nov., a Psychrotrophic Bacterium from the Soils of Schirmacher Oasis, Antarctica. Int. J. Syst. Evol. Microbiol. 1992, 42, 102–106. [Google Scholar] [CrossRef]
- Nicolitch, O.; Feucherolles, M.; Churin, J.L.; Fauchery, L.; Turpault, M.P.; Uroz, S. A microcosm approach highlights the response of soil mineral weathering bacterial communities to an increase of K and Mg availability. Sci. Rep. 2019, 9, 14403. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Zhang, H.; Guo, L.; Chen, Y.; Heiling, M.; Zhou, B.; Mao, Y. Biochar interaction with chemical fertilizer regulates soil organic carbon mineralization and the abundance of key C-cycling-related bacteria in rhizosphere soil. Eur. J. Soil Biol. 2021, 106, 103350. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z.; Chen, H.Y.H. Soil aggregate-associated bacterial metabolic activity and community structure in different aged tea plantations. Sci. Total Environ. 2019, 654, 1023–1032. [Google Scholar] [CrossRef]
- Lin, W.; Fan, F.; Xu, G.; Gong, K.; Cheng, X.; Yuan, X.; Zhang, C.; Gao, Y.; Wang, S.; Ng, H.Y.; et al. Microbial community assembly responses to polycyclic aromatic hydrocarbon contamination across water and sediment habitats in the Pearl River Estuary. J. Hazard. Mater. 2023, 457, 131762. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y. Cultivation of Uncultured Chloroflexi Subphyla: Significance and Ecophysiology of Formerly Uncultured Chloroflexi ‘Subphylum I’ with Natural and Biotechnological Relevance. Microbes Environ. 2009, 24, 205–216. [Google Scholar] [CrossRef]

| Samples | Biochar (%) | Bacillus clausii Suspension (1 × 108 CFU mL−1) (mL) | Lolium perenne L. |
|---|---|---|---|
| CK | × | × | √ |
| BC | × | 10 | √ |
| B | 2 | × | √ |
| FeB | 2 | × | √ |
| BBC | 2 | 10 | √ |
| FeBBC | 2 | 10 | √ |
| Soil (g) | 1200 | ||
| Lolium perenne L. (Number) | 50 | ||
| Incubation cycle (d) | 56 | ||
| Samples | pH | AP (mg kg−1) | AN (mg kg−1) | AK (mg kg−1) | AFe (mg kg−1) | Fed (mg kg−1) |
|---|---|---|---|---|---|---|
| CK | 8.15 ± 0.02 b | 7.36 ± 0.82 c | 17.97 ± 1.07 d | 81.31 ± 2.75 c | 13.54 ± 0.50 b | 5.63 ± 0.13 ab |
| BC | 8.12 ± 0.02 c | 7.59 ± 0.23 bc | 20.77 ± 0.81 bc | 93.02 ± 2.53 b | 19.15 ± 1.35 a | 4.40 ± 0.68 c |
| B | 8.33 ± 0.01 a | 8.73 ± 0.60 a | 18.67 ± 2.65 cd | 124.34 ± 9.21 a | 12.64 ± 0.13b cd | 4.64 ± 0.55 c |
| FeB | 7.86 ± 0.02 d | 6.14 ± 0.48 d | 22.63 ± 1.46 b | 77.77 ± 3.80 c | 11.94 ± 0.70 cd | 5.65 ± 0.46 ab |
| BBC | 8.32 ± 0.02 a | 8.35 ± 0.26 ab | 21.93 ± 0.40 b | 117.46 ± 6.10 a | 11.31 ± 0.75 d | 4.98 ± 0.21 bc |
| FeBBC | 7.82 ± 0.01 e | 6.75 ± 0.13 cd | 25.43 ± 1.07 a | 80.51 ± 7.34 c | 12.76 ± 0.42 bc | 5.86 ± 0.14 a |
| Sample | ASVs | Ace | Chao | Goods Coverage (%) | Shannon | Simpson |
|---|---|---|---|---|---|---|
| CK | 3916 | 3994.39 | 3937.67 | 99.59 | 7.47 | 0.0012 |
| BC | 3690 | 3734.58 | 3702.95 | 99.73 | 7.59 | 0.0009 |
| B | 3536 | 3556.61 | 3538.84 | 99.87 | 7.52 | 0.0009 |
| FeB | 3453 | 3453.00 | 3453.00 | 100.00 | 7.47 | 0.0011 |
| BBC | 3424 | 3461.45 | 3431.95 | 99.78 | 7.41 | 0.0012 |
| FeBBC | 4077 | 4113.06 | 4081.85 | 99.80 | 7.61 | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, G.; He, C.; Mao, S.; Chen, Z.; Ma, Y.; Zhu, Y. Enhanced Soil Fertility and Carbon Sequestration in Urban Green Spaces through the Application of Fe-Modified Biochar Combined with Plant Growth-Promoting Bacteria. Biology 2024, 13, 611. https://doi.org/10.3390/biology13080611
Niu G, He C, Mao S, Chen Z, Ma Y, Zhu Y. Enhanced Soil Fertility and Carbon Sequestration in Urban Green Spaces through the Application of Fe-Modified Biochar Combined with Plant Growth-Promoting Bacteria. Biology. 2024; 13(8):611. https://doi.org/10.3390/biology13080611
Chicago/Turabian StyleNiu, Guoyao, Chiquan He, Shaohua Mao, Zongze Chen, Yangyang Ma, and Yi Zhu. 2024. "Enhanced Soil Fertility and Carbon Sequestration in Urban Green Spaces through the Application of Fe-Modified Biochar Combined with Plant Growth-Promoting Bacteria" Biology 13, no. 8: 611. https://doi.org/10.3390/biology13080611






