Methanogenic Community Characteristics and Its Influencing Factors in Reservoir Sediments on the Northeastern Qinghai Plateau
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
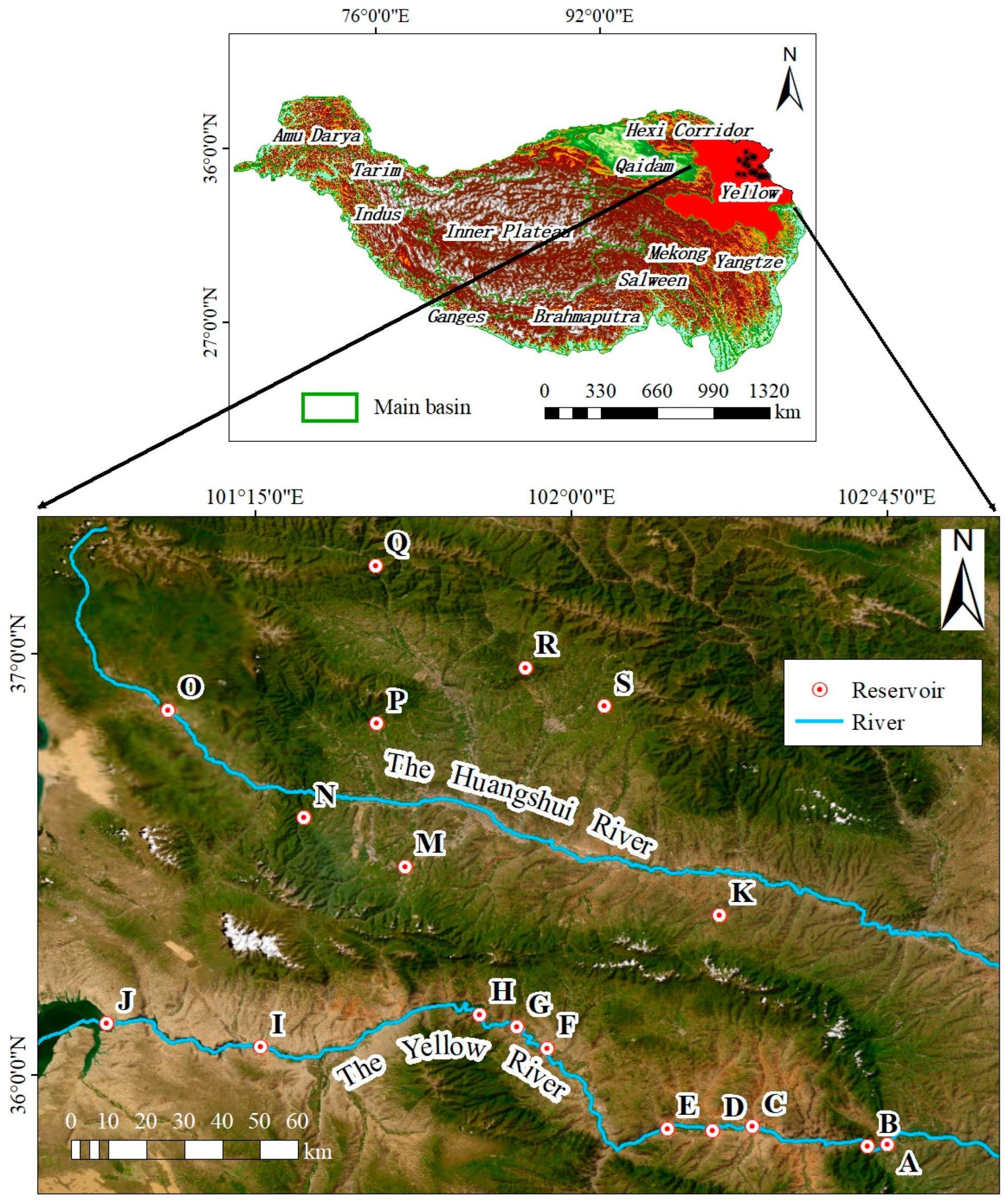
2.1. Sampling Site
2.2. Sample Collection and Preservation
2.3. Environmental Data Collection and Determination
2.4. DNA Extraction and PCR Amplification of Methanogens
2.5. Statistical Analysis
3. Results
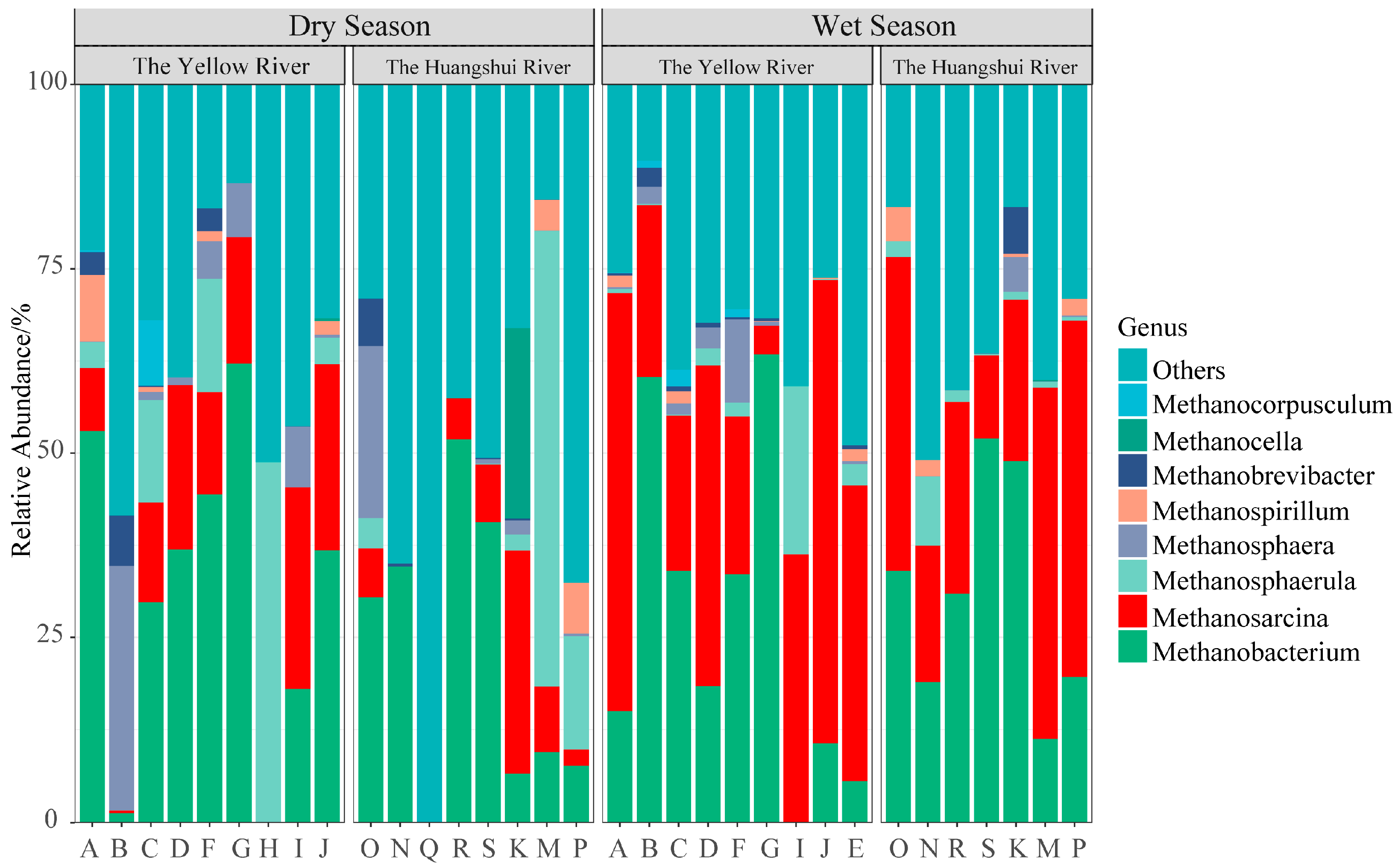
3.1. Composition of Methanogens
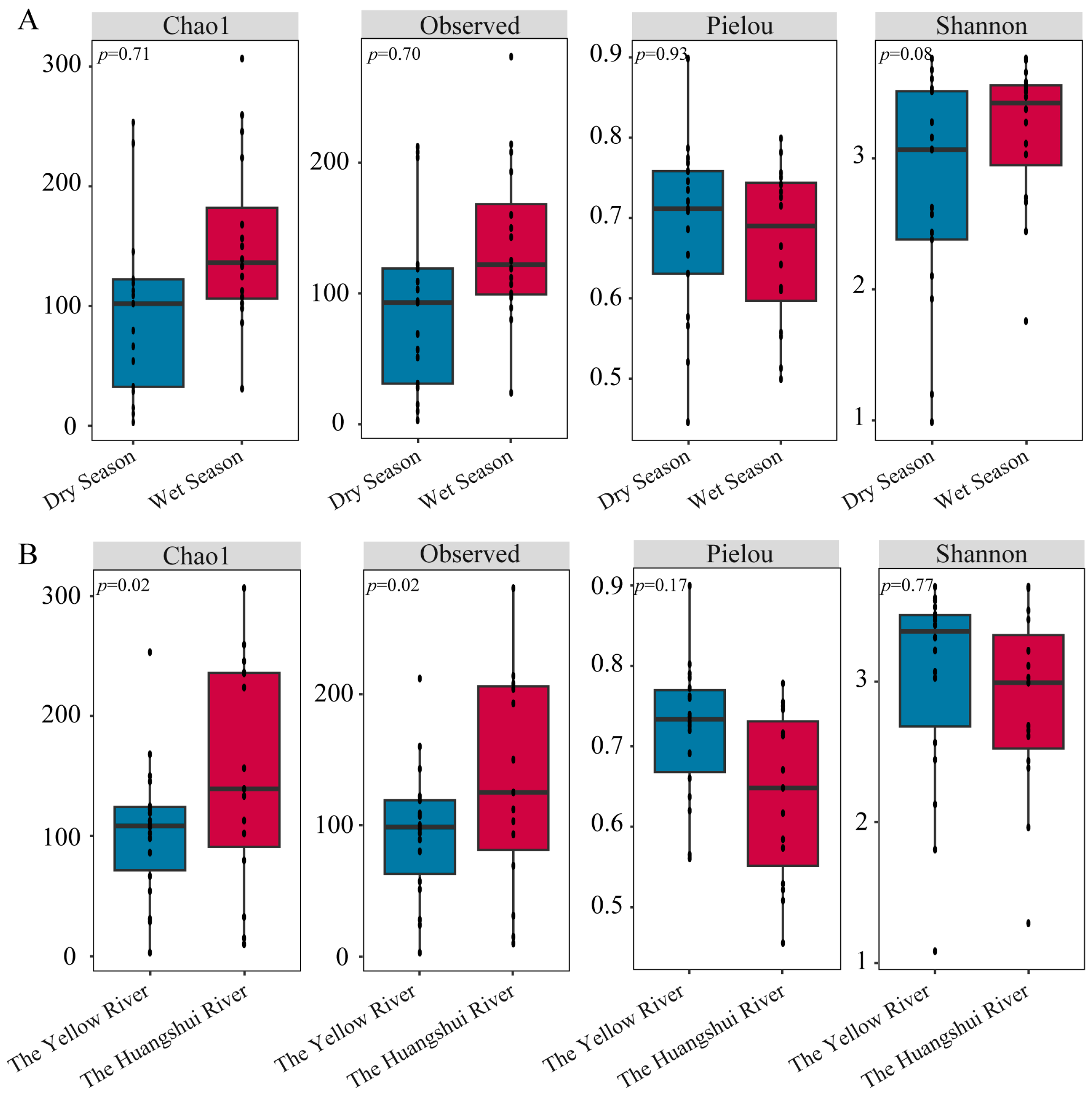
3.2. Diversity of Methanogens
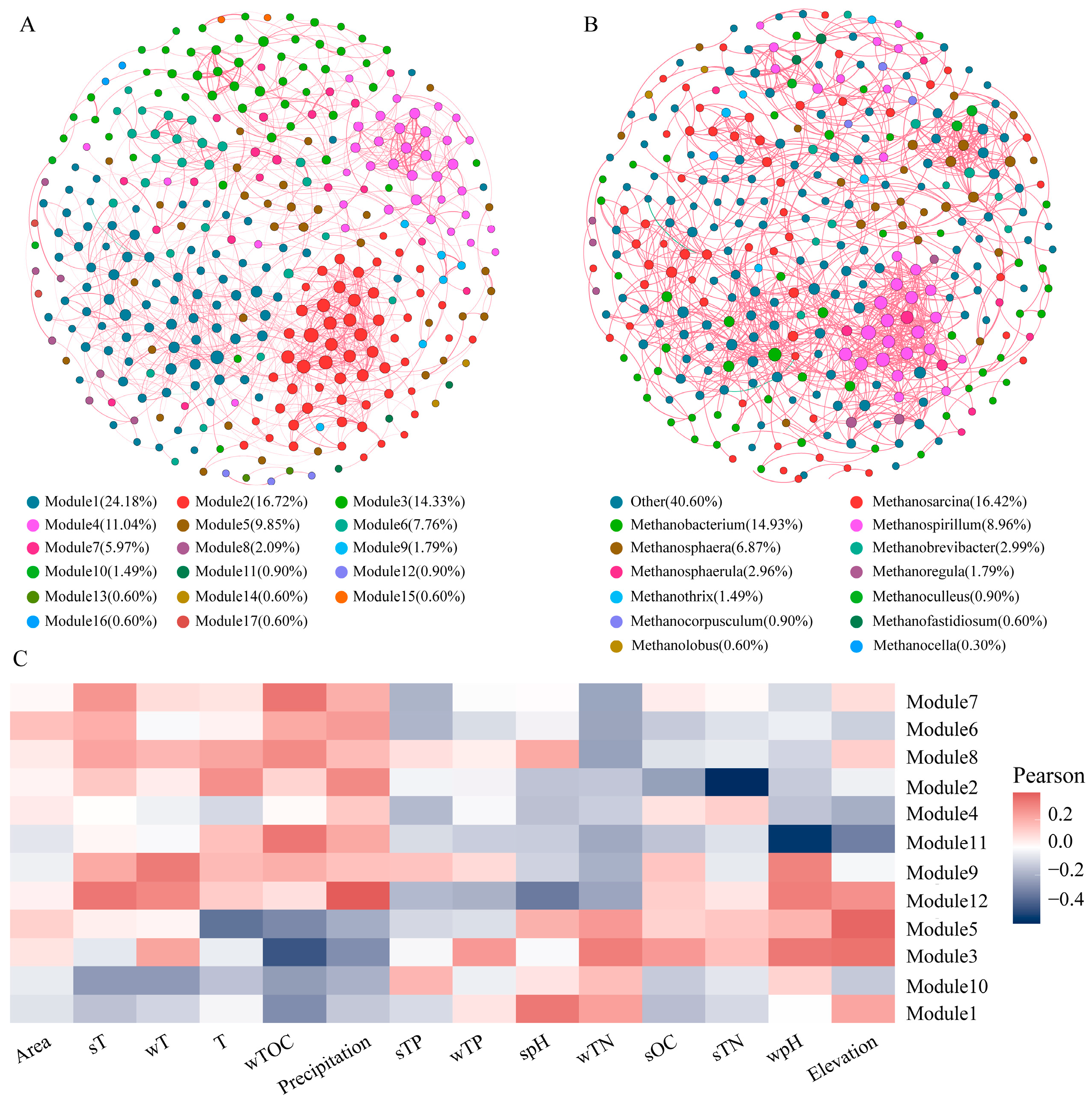
3.3. Co-Occurrence Network of Methanogens
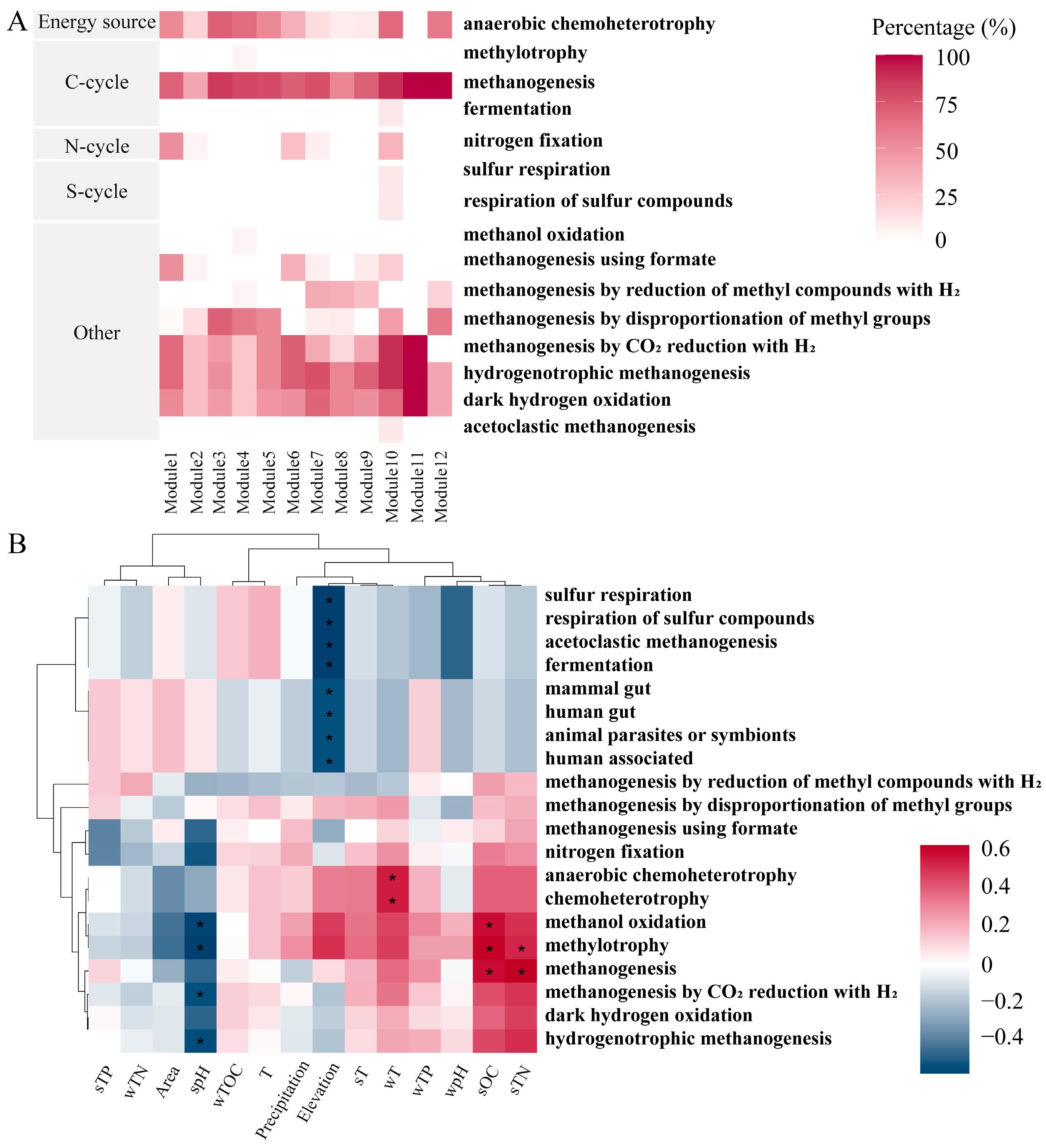
3.4. FAPROTAX Ecological Function Prediction of Methanogens
4. Discussion
4.1. Temporal and Spatial Characteristics of Methanogenic Composition and Diversity
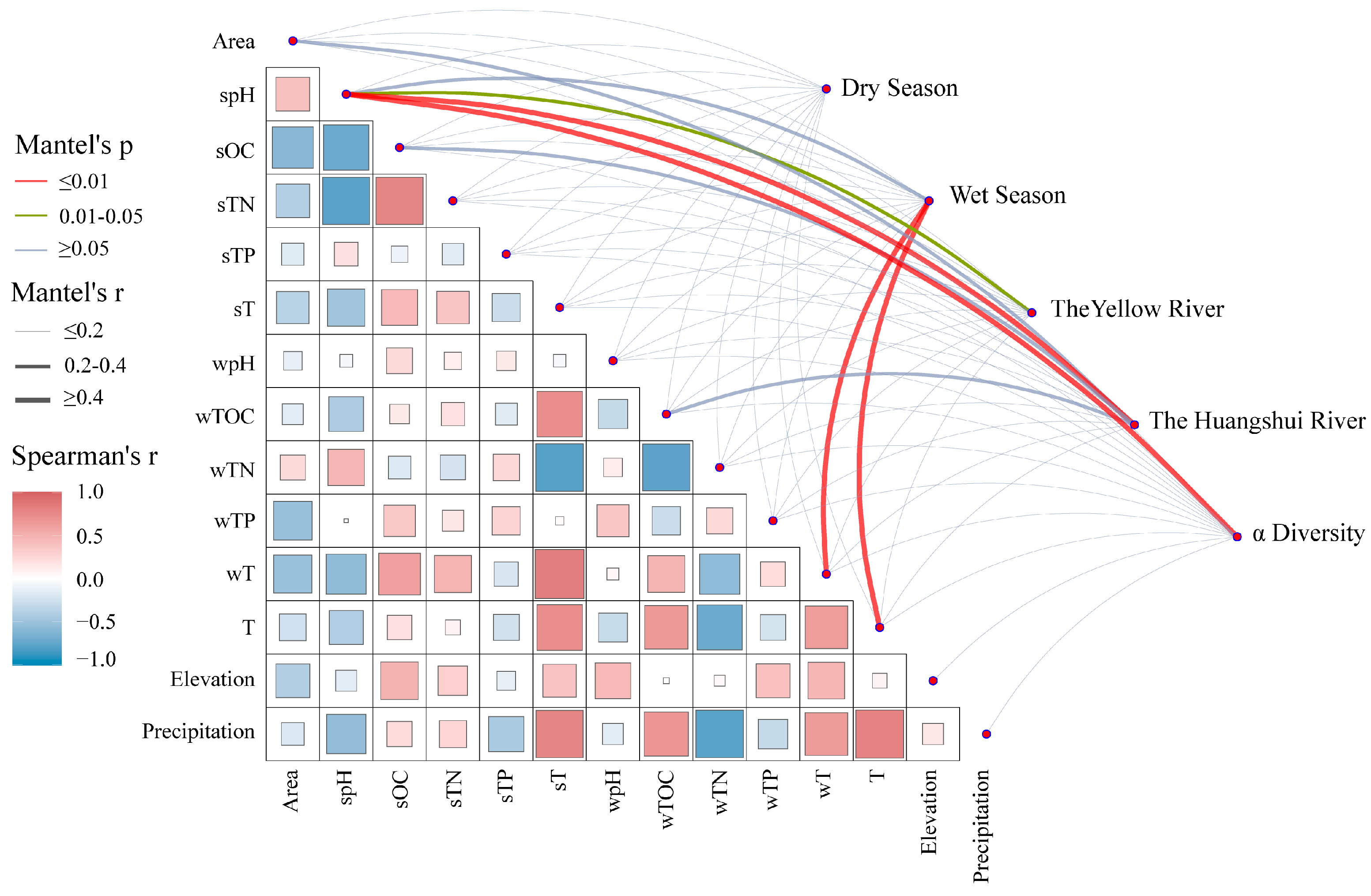
4.2. Influencing Factors on the Composition and Diversity of Methanogens
4.3. Hydrogenotrophic Methanogenesis Was the Dominant Pathway
5. Conclusions
- (1)
- The dominant genus of methanogens in the reservoir sediments on the northeastern Qinghai Plateau are Methanobacterium (28.87%) and Methanosarcina (21.67%). The hydrogenotrophic pathway using CO2/H2 as substrates predominates in the production of methane in reservoir sediments.
- (2)
- The α-diversity of methanogenic communities is higher during the wet season compared to the dry season (p > 0.05). Additionally, the diversity of methanogens is greater in large reservoirs than in small reservoirs (p < 0.05). β-diversity exhibits significant differences between different seasons and river basins (p < 0.05). Furthermore, temperature and pH have a significant impact on the composition and diversity of methanogenic communities in the reservoir sediments on the northeastern Qinghai Plateau (p < 0.05).
- (3)
- The network stability of methanogens in the reservoir sediments on the northeastern Qinghai Plateau is robust, as indicated by a modularity index of 0.69. The main function within this network is cooperation, with a high positive correlation edge number of 99.82%.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knoblauch, C.; Beer, C.; Liebner, S.; Grigoriev, M.N.; Pfeiffer, E.-M. Methane production as key to the greenhouse gas budget of thawing permafrost. Nat. Clim. Chang. 2018, 8, 309–312. [Google Scholar] [CrossRef]
- Yang, M.; Hu, M.; Yang, T.; Zhang, L.; Wang, Q.; Yuan, S.; Ba, Z.; Xia, X. Greenhouse gas emissions from high-altitude hydropower reservoir—An example of the Zangmu reservoir on the Yarlung Tsangpo. Acta Sci. Circumstantiae 2022, 42, 188–194. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Y.; Cheng, L.; Zhou, Z.; Wu, Q.; Wang, Q.; Yuan, Q. Activity and structure of methanogenic microbial communities in sediments of cascade hydropower reservoirs, Southwest China. Sci. Total Environ. 2021, 786, 147515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xia, X. Influence of reservoir construction and operation on greenhouse gas emission. Acta Sci. Circumstantiae 2022, 42, 298–307. [Google Scholar] [CrossRef]
- Deemer, B.R.; Harrison, J.A.; Li, S.; Beaulieu, J.J.; Delsontro, T.; Barros, N.; Bezerra-Neto, J.F.; Powers, S.M.; dos Santos, M.A.; Vonk, J.A. Greenhouse Gas Emissions from reservoir water surfaces: A new global synthesis. BioScience 2016, 66, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Wang, J.; Guo, J.; Jin, Z.; Yu, P.; Ma, Z. In situ, high-resolution evidence of phosphorus release from sediments controlled by the reductive dissolution of iron-bound phosphorus in a deep reservoir, southwestern China. Sci. Total Environ. 2019, 666, 39–45. [Google Scholar] [CrossRef]
- Bastviken, D.; Cole, J.J.; Pace, M.L.; Van de Bogert, M.C. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J. Geophys. Res. Biogeosci. 2008, 113, G02024. [Google Scholar] [CrossRef]
- Hu, B.; Chang, D.; Wang, B.; Yu, Z. Recent advances in aerobic methanogenesis. Microbiol. China 2020, 47, 1935–1944. [Google Scholar] [CrossRef]
- Borrel, G.; Jézéquel, D.; Biderre-Petit, C.; Morel-Desrosiers, N.; Morel, J.-P.; Peyret, P.; Fonty, G.; Lehours, A.-C. Production and consumption of methane in freshwater lake ecosystems. Res. Microbiol. 2011, 162, 832–847. [Google Scholar] [CrossRef]
- Conrad, R. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: A mini review. Pedosphere 2020, 30, 25–39. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, J.; Liu, Y.; Duan, C.; Li, M. Genomic and transcriptomic insights into methanogenesis potential of novel methanogens from mangrove sediments. Microbiome 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Berberich, M.E.; Beaulieu, J.J.; Hamilton, T.L.; Waldo, S.; Buffam, I. Spatial variability of sediment methane production and methanogen communities within a eutrophic reservoir: Importance of organic matter source and quantity. Limnol. Oceanogr. 2020, 65, 1336–1358. [Google Scholar] [CrossRef] [PubMed]
- Nie, M. Hydrospheric methane emission and its microbiological mechanisms under climate warming. Microbiol. China 2020, 60, 1821–1833. [Google Scholar] [CrossRef]
- Hayes, N.M.; Deemer, B.R.; Corman, J.R.; Razavi, N.R.; Strock, K.E. Key differences between lakes and reservoirs modify climate signals: A case for a new conceptual model. Limnol. Oceanogr. Lett. 2017, 2, 47–62. [Google Scholar] [CrossRef]
- Knoll, L.B.; Vanni, M.J.; Renwick, W.H.; Kollie, S. Burial rates and stoichiometry of sedimentary carbon, nitrogen and phosphorus in Midwestern US reservoirs. Freshw. Biol. 2014, 59, 2342–2353. [Google Scholar] [CrossRef]
- Conrad, R.; Noll, M.; Claus, P.; Klose, M.; Bastos, W.R.; Enrich-Prast, A. Stable carbon isotope discrimination and microbiology of methane formation in tropical anoxic lake sediments. Biogeosciences 2011, 8, 795–814. [Google Scholar] [CrossRef]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef]
- Mach, V.; Blaser, M.B.; Claus, P.; Chaudhary, P.P.; Rulík, M. Methane production potentials, pathways, and communities of methanogens in vertical sediment profiles of river Sitka. Front. Microbiol. 2015, 6, 506. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, X.; Zhao, T.; Chen, F.; Xu, W.; Duan, X.; Wang, X.; Ouyang, Z. Spatial–temporal variations of methane emissions from the Ertan hydroelectric reservoir in southwest China. Hydrol. Process. 2011, 25, 1391–1396. [Google Scholar] [CrossRef]
- Shi, W.; Chen, Q.; Yi, Q.; Yu, J.; Ji, Y.; Hu, L.; Chen, Y. Carbon emission from cascade reservoirs: Spatial heterogeneity and mechanisms. Environ. Sci. Technol. 2017, 51, 12175–12181. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.J.; Delwiche, K.; Long, L.H.; Liu, J.; Liu, D.F.; Wang, C.F.; Bodmer, P.; Lorke, A. Spatial and temporal variability of methane emissions from cascading reservoirs in the Upper Mekong River. Water Res. 2020, 186, 116319. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Q.; Wang, C.; Pang, G.; Yang, L. Spatial distribution of vegetation coverage and lts affecting factors in the upper reaches of the Yellow River. Arid. Zone Res. 2019, 36, 546–555. [Google Scholar] [CrossRef]
- Li, G.; Gao, X.; Xiao, N. Spatial and temporal changes of ecosystem quality based on key indicators in Yellow River basin from 2000 to 2018. Res. Environ. Sci. 2021, 34, 2945–2953. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Jiao, L.; Zhang, L.; Nan, W.; Dong, Z. Evaluation index system of comprehensive benefits of ecological Evaluation index system of comprehensive benefits of ecologica. Acta Ecol. Sin. 2022, 42, 7565–7576. [Google Scholar] [CrossRef]
- State Environmental Protection Administration. Methods for Water and Wastewater Monitoring and Analysis; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Luo, F.; Fu, Y.; Li, S.; Nong, Y.; Feng, Q.; Qin, Y. Analysis of bacterial community in sediments Longjiang River and lts response to environmental changes. Environ. Sci. Technol. 2023, 46, 34–44. [Google Scholar] [CrossRef]
- Cui, H.; Su, X.; Wei, S.; Zhu, Y.; Lu, Z.; Wang, Y.; Li, Y.; Liu, H.; Zhang, S.; Pang, S. Comparative analyses of methanogenic and methanotrophic communities between two different water regimes in controlled wetlands on the Qinghai-Tibetan Plateau, China. Curr. Microbiol. 2018, 75, 484–491. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Liu, X.; Yao, P.; Ge, T.; Zhang, X. Spatiotemporal dynamics of the archaeal community in coastal sediments: Assembly process and co-occurrence relationship. ISME J. 2020, 14, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dang, C.; Zhong, S.; Wang, J.; Zheng, T.; Ni, J. Community characteristics of dominant archaea before and after the Danjiangkou Dam. Acta Sci. Nat. Univ. Pekin. 2020, 56, 509–517. [Google Scholar] [CrossRef]
- Peng, X.; Qin, Y.; Shu, Y.; Li, Y.; Zhang, X. Effects of functional methane community in sediments of Wanzhou section of the Three Gorges Reservoir on methane emissions in summer. Chin. J. Environ. Eng. 2022, 16, 1028–1038. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Hou, W.; Feng, K.; Li, F.; Hai, W.; Zhang, Y.; Sun, Y.; Deng, Y. Temperature and microbial interactions drive the deterministic assembly processes in sediments of hot springs. Sci. Total Environ. 2021, 772, 145465. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zheng, X.; Zhang, H.; Xiao, F.; Gu, H.; Zhang, K.; He, Z.; Liu, X.; Yan, Q. Bacterial community responses to tourism development in the Xixi National Wetland Park, China. Sci. Total Environ. 2020, 720, 137570. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Y.; Zhen, M.; Mi, T.; Li, J.; Liu, C. Methanogenic pathways and methanogen communities in the sediments from Bohai Sea. Mar. Geol. Quat. Geol. 2022, 42, 50–61. [Google Scholar] [CrossRef]
- Youngblut, N.D.; Dell'Aringa, M.; Whitaker, R.J. Differentiation between sediment and hypolimnion methanogen communities in humic lakes. Environ. Microbiol. 2014, 16, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, L.; Chen, L.; Liu, Y.; Dong, X. Methanogen communities and predominant methanogenic pathways in three saline-alkaline lakes on the Tibetan Plateau. Acta Microbiol. Sin. 2020, 60, 161–171. [Google Scholar] [CrossRef]
- Wang, D.; Huang, Y.; Yang, H. Seasonal differences of lake bacterial community structures and their driving mechanisms in the northeastern of the Qinghai-Tibet Plateau. J. Lake Sci. 2023, 35, 267–282. [Google Scholar] [CrossRef]
- Lyautey, E.; Billard, E.; Tissot, N.; Jacquet, S.; Domaizon, I. Seasonal dynamics of abundance, structure, and diversity of methanogens and methanotrophs in lake sediments. Microb. Ecol. 2021, 82, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Chen, Z.; Chen, S.; Shen, L. Progress in research on the adaptability of microorganisms to extremely cold environments. Acta Microbiol. Sin. 2022, 62, 2150–2164. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Mechanism of bacterial adaptation to low temperature. J. Biosci. 2006, 31, 157–165. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Yu, G.; Huang, K.; Chen, F.; Hao, H.; Zhao, C. Eco-environmental problems with the development on Large-scale reservoirs in Xinyang City—Using Nanwan Reservoir as an Example. J. Hydroecol. 2009, 30, 142–146. [Google Scholar]
- Wang, J.; Yuan, J.J.; Liu, D.Y.; Xiang, J.; Ding, W.X.; Jiang, X.J. Research progresses on methanogenesis pathway and methanogens in coastal wetlands. Ying Yong Sheng Tai Xue Bao 2016, 27, 993–1101. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, K.; Pedersen, M.W.; Wang, F.; Chen, Y.; Zeng, C.; Liu, Y. Community assembly processes underlying the temporal dynamics of glacial stream and lake bacterial communities. Sci. Total Environ. 2021, 761, 143178. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liu, Y.; Miao, L.; Wang, F.; Chu, L.; Wang, J.; Liu, Z. Prokaryotic community structure driven by salinity and ionic concentrations in plateau lakes of the Tibetan Plateau. Appl. Environ. Microbiol. 2016, 82, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wegner, C.E.; Bei, Q.; Liu, P.; Liesack, W. Metatranscriptomics reveals a differential temperature effect on the structural and functional organization of the anaerobic food web in rice field soil. Microbiome 2018, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Ou, Y. A research overview of methanogens. Environ. Sci. Technol. 2014, 35, 2025–2030. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zhou, J.; Xie, X.; Yong, X.; Yan, Z.; Ge, Y.; Zheng, T. Effect of temperature on methanogens metabolie pathway and structures of predominant bacteria. CIESC J. 2014, 65, 1602–1606. [Google Scholar] [CrossRef]
- Tang, Q.; Xue, X.; Wang, H.; Xing, P. New knowledge of methanogens and methanotrophs in lake ecosystems. J. Lake Sci. 2018, 30, 597–610. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Lu, Y. Microbial mechanisms of methane production and oxidation in terrestrial ecosystems. Acta Ecol. Sin. 2015, 35, 6592–6603. [Google Scholar] [CrossRef]
- Großkopf, R.; Janssen Peter, H.; Liesack, W. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl. Environ. Microbiol. 1998, 64, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Franzmann, P.D.; Liu, Y.; Balkwill, D.L.; Aldrich, H.C.; Conway De Macario, E.; Boone, D.R. Methanogenium frigidum sp. nov., a psychrophilic, H2-Using methanogen from ace lake, Antarctica. Int. J. Syst. Evol. Microbiol. 1997, 47, 1068–1072. [Google Scholar] [CrossRef]
- Li, S.; Zang, K.; Song, L. Review on methanogens and methanotrophs metabolised by methane in wetland. Mar. Environ. Sci. 2020, 39, 488–496. [Google Scholar] [CrossRef]
- Ma, T.; Cheng, L.; Zheng, Z.; Qin, Q.; Dai, R.; Zhang, H. Effects of pH on methanogenesis and methanogenic community in the cultures amended with acetate. Acta Microbiol. Sin. 2014, 54, 1453–1461. [Google Scholar] [CrossRef]
- Nie, M.; Yang, Y.; Li, Z. Research progress in the mechanisms of maintaining intracellular and extracellular pH homeostasis in microorganisms. Acta Microbiol. Sin. 2024, 64, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Liu, Q.; Han, Y. Research progress on enhancement of methane production through direct interspecific electron transfer by conductive materials. Acta Microbiol. Sin. 2021, 61, 1507–1524. [Google Scholar] [CrossRef]
- Garcia, J.L.; Patel, B.K.; Ollivier, B. Taxonomic, phylogenetic, and ecological diversity of methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Mao, X.; Wu, Y.; Xia, L.; Yu, H.; Tang, W.; Qi, Y.; Zhang, Z.; Xiao, F.; Ji, H. Methanogenic Community Characteristics and Its Influencing Factors in Reservoir Sediments on the Northeastern Qinghai Plateau. Biology 2024, 13, 615. https://doi.org/10.3390/biology13080615
Liu Z, Mao X, Wu Y, Xia L, Yu H, Tang W, Qi Y, Zhang Z, Xiao F, Ji H. Methanogenic Community Characteristics and Its Influencing Factors in Reservoir Sediments on the Northeastern Qinghai Plateau. Biology. 2024; 13(8):615. https://doi.org/10.3390/biology13080615
Chicago/Turabian StyleLiu, Zebi, Xufeng Mao, Yi Wu, Liang Xia, Hongyan Yu, Wenjia Tang, Yanhong Qi, Ziping Zhang, Feng Xiao, and Haichuan Ji. 2024. "Methanogenic Community Characteristics and Its Influencing Factors in Reservoir Sediments on the Northeastern Qinghai Plateau" Biology 13, no. 8: 615. https://doi.org/10.3390/biology13080615
APA StyleLiu, Z., Mao, X., Wu, Y., Xia, L., Yu, H., Tang, W., Qi, Y., Zhang, Z., Xiao, F., & Ji, H. (2024). Methanogenic Community Characteristics and Its Influencing Factors in Reservoir Sediments on the Northeastern Qinghai Plateau. Biology, 13(8), 615. https://doi.org/10.3390/biology13080615








