Factors Affecting Cell Viability during the Enzymatic Dissociation of Human Endocrine Tumor Tissues
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Sampling
2.2. Histopathological Examination
2.3. Enzymatic Dissociation
2.4. Statistical Data Analysis
3. Results and Discussion
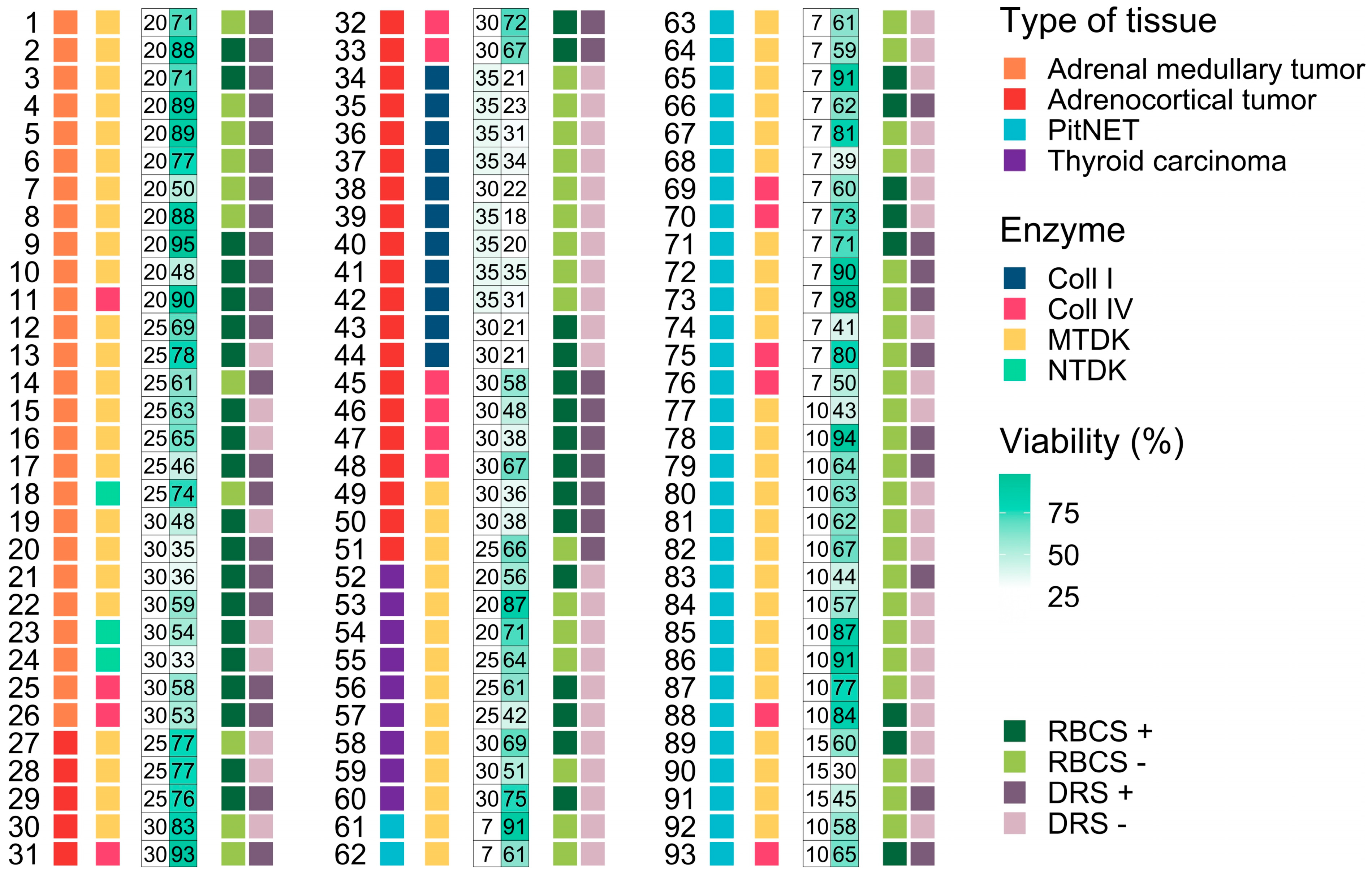
3.1. Influence of Morphological Features on the Dissociation Process and Cell Viability
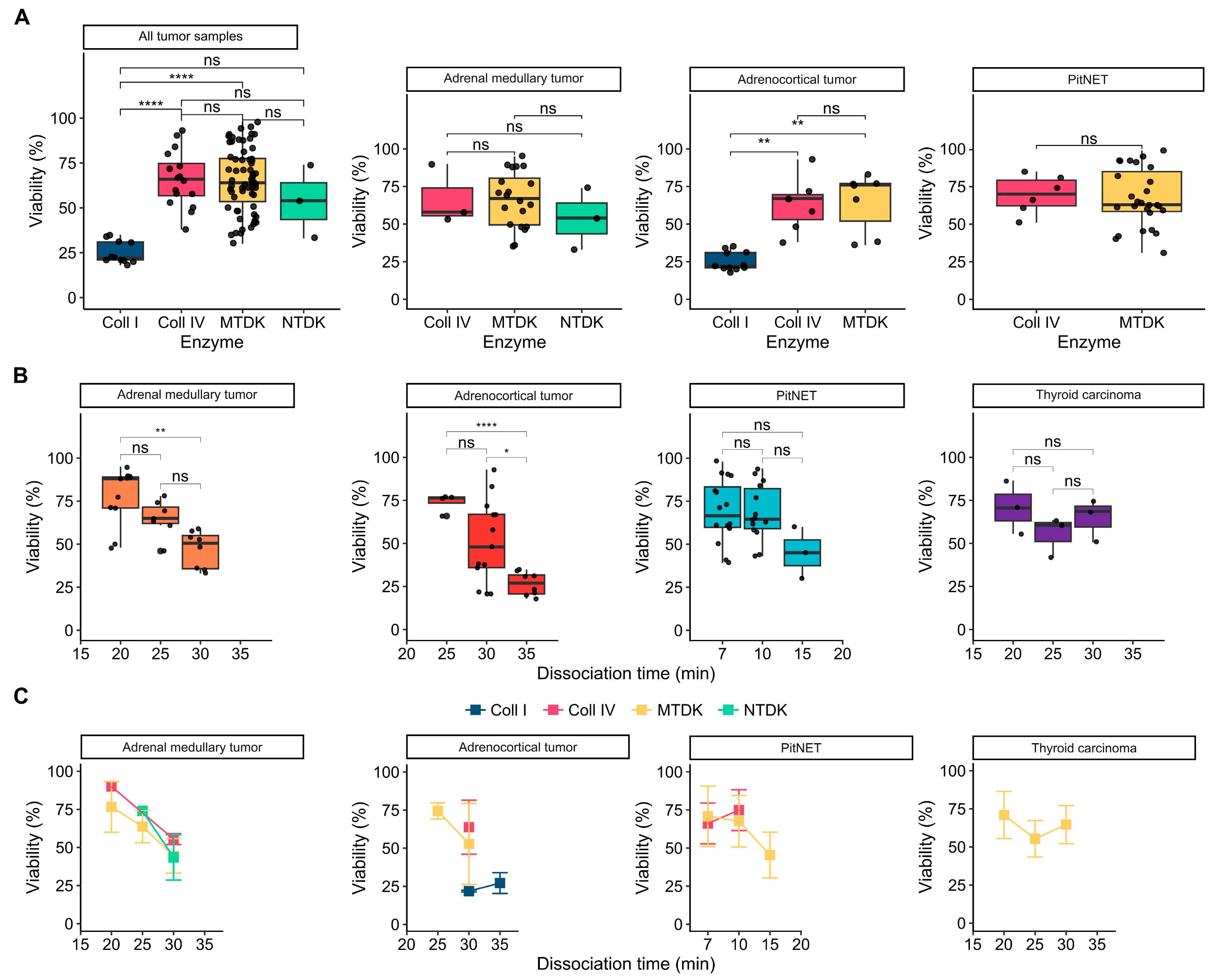
3.2. Influence of Enzymatic Activity on Cell Viability
3.3. Influence Dissociation Time on Cell Viability
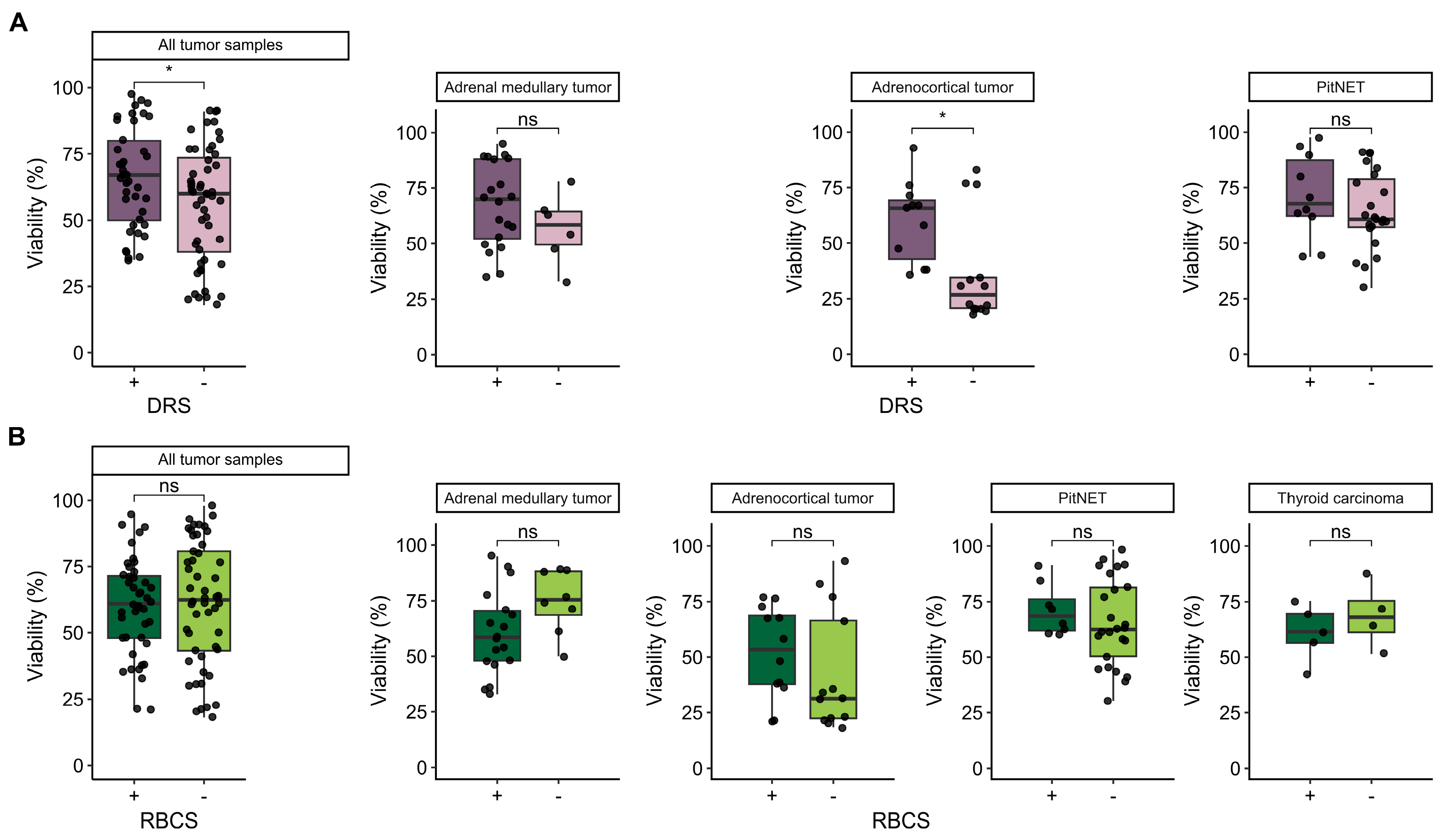
3.4. Influence of Additional Post-Dissociation Purification Procedures on Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Kuhn, J.R. Cell Isolation and Culture; WormBook: Pasadena, CA, USA, 2013; pp. 1–39. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.P.; Gimble, J.M.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Xenofree Enzymatic Products for the Isolation of Human Adipose-Derived Stromal/Stem Cells. Tissue Eng. Part C Methods 2013, 19, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Reichard, A.; Asosingh, K. Best Practices for Preparing a Single Cell Suspension from Solid Tissues for Flow Cytometry. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2019, 95, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The Extracellular Matrix in Development and Morphogenesis: A Dynamic View. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Okitsu, T.; Teramura, N.; Iijima, K.; Hayashida, O.; Teramae, H.; Hattori, S. Recombinant Collagenase from Grimontia Hollisae as a Tissue Dissociation Enzyme for Isolating Primary Cells. Sci. Rep. 2020, 10, 3927. [Google Scholar] [CrossRef]
- Shi, L.; Ermis, R.; Garcia, A.; Telgenhoff, D.; Aust, D. Degradation of Human Collagen Isoforms by Clostridium Collagenase and the Effects of Degradation Products on Cell Migration. Int. Wound J. 2010, 7, 87–95. [Google Scholar] [CrossRef]
- Balashova, A.; Pershin, V.; Zaborskaya, O.; Tkachenko, N.; Mironov, A.; Guryev, E.; Kurbatov, L.; Gainullin, M.; Mukhina, I. Enzymatic Digestion of Hyaluronan-Based Brain Extracellular Matrix in Vivo Can Induce Seizures in Neonatal Mice. Front. Neurosci. 2019, 13, 1033. [Google Scholar] [CrossRef]
- Corver, W.E.; Cornelisse, C.J.; Hermans, J.; Fleuren, G.J. Limited Loss of Nine Tumor-Associated Surface Antigenic Determinants after Tryptic Cell Dissociation. Cytometry 1995, 19, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Volovitz, I.; Shapira, N.; Ezer, H.; Gafni, A.; Lustgarten, M.; Alter, T.; Ben-Horin, I.; Barzilai, O.; Shahar, T.; Kanner, A.; et al. A Non-Aggressive, Highly Efficient, Enzymatic Method for Dissociation of Human Brain-Tumors and Brain-Tissues to Viable Single-Cells. BMC Neurosci. 2016, 17, 30. [Google Scholar] [CrossRef]
- Hussain, R.Z.; Miller-Little, W.A.; Doelger, R.; Cutter, G.R.; Loof, N.; Cravens, P.D.; Stüve, O. Defining Standard Enzymatic Dissociation Methods for Individual Brains and Spinal Cords in EAE. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e437. [Google Scholar] [CrossRef]
- Jager, L.D.; Canda, C.-M.A.; Hall, C.A.; Heilingoetter, C.L.; Huynh, J.; Kwok, S.S.; Kwon, J.H.; Richie, J.R.; Jensen, M.B. Effect of Enzymatic and Mechanical Methods of Dissociation on Neural Progenitor Cells Derived from Induced Pluripotent Stem Cells. Adv. Med. Sci. 2016, 61, 78–84. [Google Scholar] [CrossRef]
- Van den Brink, S.C.; Sage, F.; Vértesy, Á.; Spanjaard, B.; Peterson-Maduro, J.; Baron, C.S.; Robin, C.; van Oudenaarden, A. Single-Cell Sequencing Reveals Dissociation-Induced Gene Expression in Tissue Subpopulations. Nat. Methods 2017, 14, 935–936. [Google Scholar] [CrossRef]
- Miller, R.G.; Phillips, R.A. Separation of Cells by Velocity Sedimentation. J. Cell. Physiol. 1969, 73, 191–201. [Google Scholar] [CrossRef]
- Singh, S.; Ponnappan, N.; Verma, A.; Mittal, A. Osmotic Tolerance of Avian Erythrocytes to Complete Hemolysis in Solute Free Water. Sci. Rep. 2019, 9, 7976. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Mete, O.; Erickson, L.; Juhlin, C.; Krijger, R.; Sasano, H.; Volante, M.; Papotti, M. Overview of the 2022 WHO Classification of Adrenal Cortical Tumors. Endocr. Pathol. 2022, 33, 1–42. [Google Scholar] [CrossRef]
- Mortini, P.; Albano, L.; Barzaghi, L.R.; Losa, M. Pituitary Surgery. La Presse Médicale 2021, 50, 104079. [Google Scholar] [CrossRef]
- Sun, B. The Mechanics of Fibrillar Collagen Extracellular Matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef]
- Lebrun, L.; Salmon, I. Pathology and New Insights in Thyroid Neoplasms in the 2022 WHO Classification. Curr. Opin. Oncol. 2024, 36, 13–21. [Google Scholar] [CrossRef]
- Ferreira, L.; Gimba, E.; Vinagre, J.; Sobrinho-Simões, M.; Soares, P. Molecular Aspects of Thyroid Calcification. Int. J. Mol. Sci. 2020, 21, 7718. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, A.V.; Tarasov, P.A.; Semianov, K.A.; Nekrasov, V.M.; Hoekstra, A.G.; Maltsev, V.P. Erythrocyte Lysis in Isotonic Solution of Ammonium Chloride: Theoretical Modeling and Experimental Verification. J. Theor. Biol. 2008, 251, 93–107. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakova, A.; Utkina, M.; Valyaeva, A.; Pachuashvili, N.; Bondarenko, E.; Urusova, L.; Popov, S.; Mokrysheva, N. Factors Affecting Cell Viability during the Enzymatic Dissociation of Human Endocrine Tumor Tissues. Biology 2024, 13, 665. https://doi.org/10.3390/biology13090665
Shcherbakova A, Utkina M, Valyaeva A, Pachuashvili N, Bondarenko E, Urusova L, Popov S, Mokrysheva N. Factors Affecting Cell Viability during the Enzymatic Dissociation of Human Endocrine Tumor Tissues. Biology. 2024; 13(9):665. https://doi.org/10.3390/biology13090665
Chicago/Turabian StyleShcherbakova, Anastasia, Marina Utkina, Anna Valyaeva, Nano Pachuashvili, Ekaterina Bondarenko, Liliya Urusova, Sergey Popov, and Natalya Mokrysheva. 2024. "Factors Affecting Cell Viability during the Enzymatic Dissociation of Human Endocrine Tumor Tissues" Biology 13, no. 9: 665. https://doi.org/10.3390/biology13090665
APA StyleShcherbakova, A., Utkina, M., Valyaeva, A., Pachuashvili, N., Bondarenko, E., Urusova, L., Popov, S., & Mokrysheva, N. (2024). Factors Affecting Cell Viability during the Enzymatic Dissociation of Human Endocrine Tumor Tissues. Biology, 13(9), 665. https://doi.org/10.3390/biology13090665




