Simple Summary
Sexual reproduction in Cordyceps sensu lato is regulated by the mating-type locus, which has two alternate idiomorphs: MAT1-1 and MAT1-2. Ophiocordyceps xuefengensis, a heterothallic Cordyceps used as a traditional tonic by the Yao ethnic group in southern China, produces sterile fruiting bodies regardless of whether it is the MAT1-1 or MAT1-2 strain. However, the impact of mating types on the quality of these fruiting bodies is not well understood. In this study, we developed a loop-mediated isothermal amplification (LAMP) method that quickly and efficiently distinguishes between MAT1-1 and MAT1-2 strains of O. xuefengensis, with advantages including a short duration (1 h or less), visual detection, and the elimination of the need for a thermal cycler and gel electrophoresis. We also systematically compared biochemical contents of the fruiting bodies from MAT1-1 and MAT1-2 strains. Our findings show that the MAT1-1 strain of O. xuefengensis is a promising alternative for natural Cordyceps production, as it contains higher levels of adenosine and essential amino acids, as well as lower levels of toxic elements in the fruiting bodies compared to the MAT1-2 strain. This study provides a new approach to addressing issues related to unstable artificial cultivation, long cultivation periods, and high heavy metal content in Cordyceps.
Abstract
Many Cordyceps sensu lato species are used as traditional Chinese medicines. However, Cordyceps are entomopathogenic fungi in the family Clavicipitaceae of Ascomycota, and excessive harvesting severely disrupts natural habitat ecosystems. Artificial cultivation of Cordyceps fruiting bodies offers a viable strategy to protect the ecological environment and mitigate the depletion of wild resource. In this study, mononucleate hyphae were selected using DAPI fluorescence staining, the MAT1-1 and MAT1-2 strains of O. xuefengensis were successfully distinguished using loop-mediated isothermal amplification (LAMP). The chemical composition and bioactive components of fruiting bodies produced by these strains were compared. Results showed that the levels of adenosine, thymidine, adenine, guanosine, uridine, total amino acids, and total essential amino acids in the fruiting bodies of MAT1-1 strains were 1.31 mg/g, 0.15 mg/g, 0.26 mg/g, 2.40 mg/g, 2.34 mg/g, 270.3 mg/g, and 102.5 mg/g, respectively, which were significantly higher than those in the MAT1-2 sample. Contrastingly, the fruiting bodies of MAT1-2 strains contained higher levels of mannose and polysaccharides, at 11.7% and 12.2%, respectively. The levels of toxic elements such as Al, Pb, As, and Hg in the MAT1-1 fruiting bodies were 1.862 mg/kg, 0.0848 mg/kg, 0.534 mg/kg, and 0.0054 mg/kg, respectively, which were markedly lower than those in the MAT1-2 fruiting bodies.
1. Introduction
Many Cordyceps sensu lato species, such as Ophiocordyceps sinensis, Isaria cicadae, and Cordyceps militaris, are used as valuable traditional medicines or novel food in China, Japan, Korea, and other Eastern Asian countries [1,2,3,4]. Numerous studies have shown that Cordyceps contains a variety of bioactive constituents, including polysaccharides, adenosine, cordycepic acid, cordycepin, ergosterol, and minerals. Liu et al. [5] revealed that the polysaccharide from O. sinensis activated RAW264.7 macrophage cells and exhibited a stronger immunomodulatory effect through the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) signaling pathways. Le et al. [6] demonstrated that the polysaccharide PS-T80 from O. sobolifera exhibits promising antioxidant activity, with a scavenging rate of 93.85% for 1,1-Diphenyl-2-picrylhydrazyl (DPPH) at a concentration of 2.5 mg/mL. Adenosine, a major nucleoside in Cordyceps, can regulate macrophage phenotypic switching to reduce inflammatory responses. It also serves as a chemical marker for quantitatively monitoring the product quality of O. sinensis and C. militaris, as well as their substitutes in Asia [7,8]. Wang et al. [9] reported that cordycepic acid significantly inhibited the proliferation of human lung cancer A549 cells by regulating the Nrf-2/HO-1/NLRP3/NF-κB signaling pathway. Deng et al. [10] discovered that cordycepin lowered the expression of phagocytosis immune checkpoint cluster of differentiation 47 (CD47) in tumor cells, including the murine colon cancer cells CT26, human T-lymphoblastic (Jurkat) cells, and human colon cancer cell lines SW48, thereby promoting the phagocytosis of tumor cells by macrophages and enhancing anti-tumor immunity. Additionally, cordycepin exhibits excellent protective effects against inflammatory damage from acute lung injury (ALI), allergic asthma, liver fibrosis, rheumatoid arthritis, myocardial ischemia-reperfusion injury, and atopic dermatitis [11,12,13,14]. Ergosterols, a class of unique steroidal compounds specific to fungal cell membranes, belong to the group of organic compounds known as ergosterols and their derivatives. They consist of one cyclopentane ring, one cyclohexane ring, one cyclohexene ring, and one cyclobutane ring in their structure, and have been reported to exhibit antitrypanosomal, antimicrobial, antitumor, and various other activities [15,16,17].
Ophiocordyceps xuefengensis, the sister taxon of O. sinensis, parasitizes the larvae of Phassus nodus (Hepialidae) found in the living roots or trunks of Clerodendrum cyrtophyllum in the Xuefeng Mountains of Hunan Province in south China, and is well known as a folk tonic medicine by the local Yao ethnic group, also regarded as an ideal alternative for O. sinensis [18,19]. O. xuefengensis has been shown to be rich in various active chemical compounds, including nucleosides, nucleobases, cordycepic acid, amino acids, fatty acids, and ergosterol [20,21,22]. Qin et al. [23] demonstrated that the O. xuefengensis exhibited higher antioxidant capability than both C. militaris and O. sinensis. Jin et al. [24] indicated that the water extract of O. xuefengensis exerted antitumor activity against Michigan Cancer Foundation-7 (MCF-7), hepatocellular carcinoma G2 (HepG2), prostate cancer-3 (PC-3), A549, and Raji cell lines in vitro. Zheng et al. [25] demonstrated that the aqueous extract of O. xuefengensis can enhance antitumor activity against human HepG2 liver cancer by promoting the proliferation of dendritic cell-cytokine-induced killer (DC-CIK) cells.
With the rising market demand for this medicinal fungus, the prices of natural Cordyceps have soared dramatically over the past few decades, leading to excessive harvesting that has endangered the species and severely damaged the ecological systems of their natural habitats in China [2]. Artificial cultivation of Cordyceps fruiting bodies offers a viable strategy to safeguard the ecological environment and address the constraints of wild resource depletion. Sexual reproduction is crucial for the sustainable development of the Cordyceps industry, as mating-type loci play a decisive role in determining gender and sexual reproduction. In filamentous ascomycetes, sexual reproduction is governed by a single locus known as the mating type (MAT) locus. This locus has two highly divergent and nonhomologous variants, termed MAT1-1 and MAT1-2, which are referred to as idiomorphs rather than alleles. Heterothallic (self-sterile) fungal strains contain only one single idiomorph and require mating with a strain of the opposite mating type to achieve successful ascus formation [26]. In some heterothallic species of Cordyceps sensu lato in ascomycetes, such as C. militaris and O. xuefengensis, strains containing either the MAT1-1 or MAT1-2 idiomorph are self-sterile; however, they can still produce sterile fruiting bodies [26,27]. It remains unclear, however, whether the mating types of Cordyceps species influence the quality of these fruiting bodies.
The morphological differentiation between the MAT1-1 and MAT1-2 strain without quarantine status is difficult, thus rapid on-site identification is needed. An excellent approach is the loop-mediated isothermal DNA amplification (LAMP) assay, which has recently been shown to be an efficient and convenient technology for identifying viruses, bacteria, fungi, and other pathogens [28,29,30]. LAMP is extremely sensitive and highly specific because it uses two primer sets to effectively identify target DNA sequence sites [28]. Due to the DNA strand displacement activity of the Bst DNA polymerase, LAMP reactions are carried out at a constant temperature, eliminating the need for a thermal cycler compared to conventional polymerase chain reaction (PCR)-based assays. Moreover, LAMP has a distinct advantage over PCR-based technologies in its resilience against latent inhibitors in DNA extract, thereby eliminating the need for DNA purification [31]. Because of its simplicity, speed, and lack of requirement for a thermal cycler, LAMP can be conducted on-site using portable constant-temperature real-time detection equipment.
In this study, we developed a novel method for rapidly distinguishing between the MAT1-1 and MAT1-2 strains of O. xuefengensis using the LAMP reaction. This technique has several advantages, including the elimination of DNA purification steps, providing visual results within 1 h or less, and bypassing the need for a thermal cycler and gel electrophoresis. Furthermore, we conducted a thorough analysis comparing the contents of polysaccharides, adenosine, cordycepic acid, cordycepin, amino acids, fatty acid, and element components between the MAT1-1 and MAT1-2 fruiting bodies and investigated the effects of the mating-type genes on these components.
2. Materials and Methods
2.1. Materials
Mononucleate hyphae were selected for fruiting body cultivation using DAPI (4′,6-diamidino-2-phenylindole) staining: conidia from 3-week-old colonies of O. xuefengensis were cultured as reported by Tkaczuk and Majchrowska-Safaryan [32], and the hyphae were grown on a cover glass until reaching the appropriate growth stage. Methanol at 4 °C was then applied to the side of the cover glass containing the hyphae and left for 10–15 min. Subsequently, the hyphae were washed three times with phosphate-buffered saline (PBS) at pH 7.4, with each wash lasting 5 min and involving occasional gentle shaking. Next, 0.4% Triton X-100 was added to the cover glass containing the cultures and left for 5–10 min. The cultures were then washed with PBS 2–3 times, each wash lasting for 5 min. Following this, the cultures were treated with 1 μg/mL DAPI staining solution for 10 min, washed with PBS 2–3 times (each wash lasting 5 min), and then examined using laser confocal microscopy (Sunny Optical Technology Co., Ltd., Ningbo, China). Mononucleate hyphae of O. xuefengensis were used in culture to cultivate both liquid mycelium and fruiting bodies. The fungal strains numbered 1–14 were isolated from the fresh stromata of O. xuefengensis collected from the Xuefeng Mountains in China.
The strain ZJ3, genotype MAT1-2, is preserved in the China Center for Type Culture Collection (no. CCTCC M 2015777). The strain ZJ1 has the genotype MAT1-1 (GenBank no. MH176300). Strain no. 1–14, collected from Xuefeng Mountain at an altitude of over 400 m, with coordinates ranging from 110.40 to 110.69° E and 27.06 to 27.23° N, has ITS sequences with GenBank no. PQ159738–PQ159751, respectively. The fruiting bodies of O. xuefengensis were cultivated artificially at 19 °C in dark conditions on solid media as reported by Zou et al. [22]. The media comprised 40 g rice, 20 g oat, 10 g millet, 2 g pupa powder, 0.4 g CaCO3, and 0.4 g CaSO4·2H2O, along with a nutrient solution (120 mL) containing 200 g/L potato infusion, 10 g/L sugar, 5 g/L peptone, 1.18 g/L Na2HPO4, 1.13 g/L KH2PO4, 1.5 g/L MgSO4·7H2O, and 2 g/L KNO3 [22]. These components were mixed and poured into a 31.0 cm × 21.0 × 11.5 cm polyethylene cultivation pot. The state of fruiting bodies was captured using a Canon camera with an EF-S 55-250 mm lens (Canon, Zhuhai, China). The fruiting bodies of natural O. xuefengensis were derived from the larvae of Phassus nodus (Hepialidae) found in the living roots or trunks of Clerodendrum cyrtophyllum in the Xuefeng Mountains of Hunan Province, southern China. The MAT1-1 and MAT1-2 fruiting bodies were artificially cultivated on solid media in the laboratory, as detailed in our materials.
2.2. LAMP Reaction
The specific primer sets, shown in Table 1, were designed on the basis of conserved domain sequences of the MAT1-1-1 (MH176302.1) and the MAT1-2-1 (MH176301.1) of Ophiocordyceps xuefengensis, respectively, using Primer Explorer version 5 software (available at http://primerexplorer.jp/lampv5e/index.html, accessed on 20 August 2020) from the Eiken Chemical Company (http://primerexplorer.jp/lampv5e/index.html, accessed on 20 August 2020).

Table 1.
LAMP target genes and primers used for amplification.
A 100 mg fresh sample or a 50 mg dry sample of O. xuefengensis was placed in a mortar and mixed with three times its weight of sterile quartz sand. The mixture was thoroughly ground with a pestle, transferred into 300 μL of sterile double-distilled water in a 1.5 mL centrifuge tube, and incubated in a boiling water bath for 10 min. The supernatantcontaining DNA was then collected via centrifugation at 9800× g for 5 min, stored at −20 °C, and used within 48 h.
The LAMP reaction was performed in 20 µL mixtures containing 8 mM MgSO4, 1.4 mM dNTP mix, 1 × ThermoPol buffer (0.1% Triton X-100, 2 mM MgSO4, 20 mM Tris–HCl, 10 mM (NH4)2SO4, 10 mM KCl, pH 8.8), 1.25 µM of each FIP and BIP, 0.125 µM of each F3 and B3, 12 U Bst DNApolymerase (NEB, Ipswich, MA, USA), 1 µL DNA template, 120 µM hydroxynaphthol blue (HNB), 0.8 M betaine, and ddH2O to a final volume of 20 µL. The reaction was incubated at 61 °C for 1 h and then terminated by heating at 80 °C for 10 min. DNA amplification was assessed using two methods: 2% agarose gel electrophoresis and visual detection of the color changes in the LAMP mixture with HNB dye.
2.3. Analysis of Nucleosides, Cordycepic Acid, and Amino Acid
According to Zou et al. [22], nucleoside analogues and nucleobases were extracted and detected using reverse-phase HPLC with a Waters e2695 HPLC system equipped with a Diamonsil C18 (2) column (Diamonsil, 250 mm × 4.6 mm, 5 µm). The mobile phase was ultrapure water (A) and methanol (B). Elution conditions: 0–3 min at 12% B, 3–3.5 min gradient from 12% to 21% B, 3.5–8.5 min at 21% B, 8.5–9 min gradient from 21% to 35% B, and 9–15 min at 35% B. This was followed by a 40 min flush with 100% B and 30 min reconditioning at 12% B. The flow rate was 1 mL/min, the injection volume was 20 µL, and detection was performed at 260 nm and a temperature of 30 °C. The content of cordycepic acid (D-mannose) was determined via KIO4 reaction using a corresponding chemical standard [33]. Ergosterol in the fruiting bodies of O. xuefengensis was detected using a Waters e2695 HPLC system with a solvent system of methanol (98%) and water (2%) at a flow rate of 1 mL/min and a total analysis time of 15 min, as reported by Guo et al. [34]. Ergosterol separation was achieved on a C18 column (Diamonsil, 250 mm × 4.6 mm, 5 µm), and detection was performed at 283 nm UV.
The acid hydrolysis and alkaline hydrolysis of the materials were performed to quantitatively analyze amino acids [35]. The hydrolyzed amino acid samples were filtered through a 0.45 µm membrane. Automated pre-column derivatization of the filtrate was achieved using o-phthalaldehyde (OPA) and 2-mercaptoethanol for primary amino acids, followed by 9-fluorenylmethyl chloroformate (FMOC-Cl) for secondary amino acids. Separation was performed using a pre-packed Hypersil ODS C18 column (4.6 × 250 mm, particle size 5 µm; Dalian Elite Analytical Instruments Co., Dalian, China), and determination was carried out with a photometric detector at 338 nm (262 nm for hypro and proline) using the HP-1100 automatic amino acid analyzer (Agilent, Santa Clara, CA, USA). The levels of amino acids were quantitatively analyzed using a calibration curve constructed with known concentrations of a standard (A9781 from Sigma–Aldrich, Burlington, MA, USA, 0.5 µmol/mL) [36].
2.4. Analysis of Elements
The materials, with 10 g of each per sample, were digested separately with a 1:4 (v/v) mixture of HClO4 and HNO3, as described by Wang et al. [35]. Elements were detected using atomic absorption spectroscopy (Optima 5300DV, PerkinElmer, Waltham, MA, USA), except for Se, Pb, and Hg, which were analyzed with atomic fluorescence spectrometry (AFS-230E, Haiguang Instruments, Beijing, China). Element standard solutions were used to calibrate the analyzer and to calculate the content of elements in the samples.
2.5. Statistical Analysis
All experiments were conducted in triplicate, and statistical significance was determined using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Results are presented as mean ± standard deviation (SD). Significant differences among groups were assessed using Duncan’s multiple range test, with significance indicated at the 0.05 level by different lowercase letters [37].
3. Results
3.1. The Idiomorphs and the Fruiting Bodies
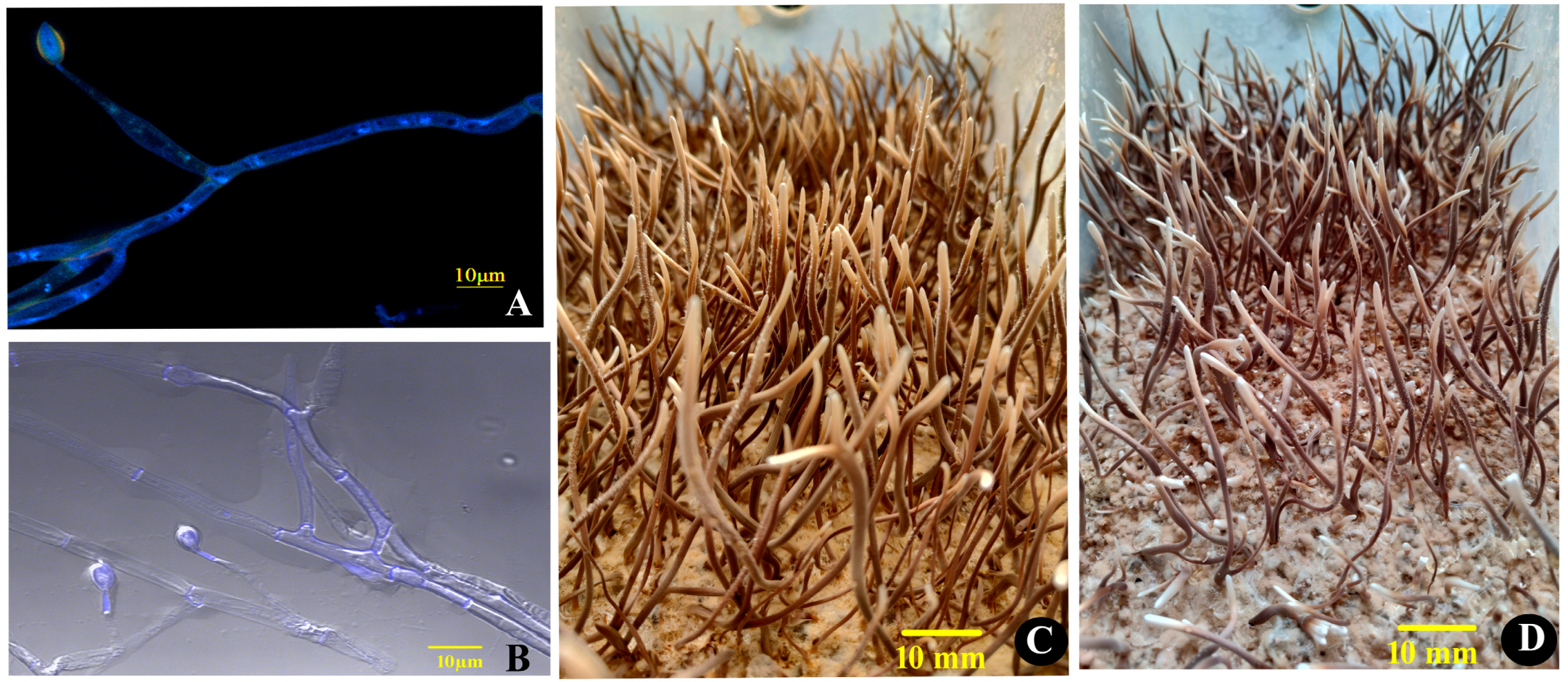
O. xuefengensis has so far been discovered exclusively in Dongkou County and Wugang City within Hunan, China, at elevations exceeding 600 m in the Xuefeng Mountain region. During cultivation, the presence of two mating types of mycelium simultaneously results in sparse and low-yielding fruiting bodies in solid culture. In this experiment, mononucleate hyphae were selected using DAPI fluorescence staining. On 1% PDA medium, the colonies of O. xuefengensis exhibited circular, powdery, and dense morphology, with branched, septate hyphae measuring 2 to 5 µm in diameter. Conidiophores were solitary, producing bottle-shaped structures tapering to a narrow neck, and bearing solitary conidia that were elliptical or elongated, slightly pointed at the apex, measuring (8 to 12) µm × (5 to 7) µm (see Figure 1A,B). The stromata, inoculated onto fruiting bodies culture medium, were cylindrical, with diameters ranging from 1 to 6 mm and lengths of up to 130 mm (Figure 1C,D). The growing tip was beige, and as growth progressed, the outer wall of the stroma gradually changed to yellow-brown while the interior remained beige, displaying a hard texture consistent with natural O. xuefengensis stromata.
Figure 1.
Ophiocordyceps xuefengensis. Micrographs of MAT1-1 hyphae (A) and MAT1-2 hyphae (B); macrographs of MAT1-1 fruiting bodies (C) and MAT1-2 fruiting bodies (D). Scale bars: (A,B) = 10 µm; (C,D) = 10 mm.
3.2. Specificity of LAMP
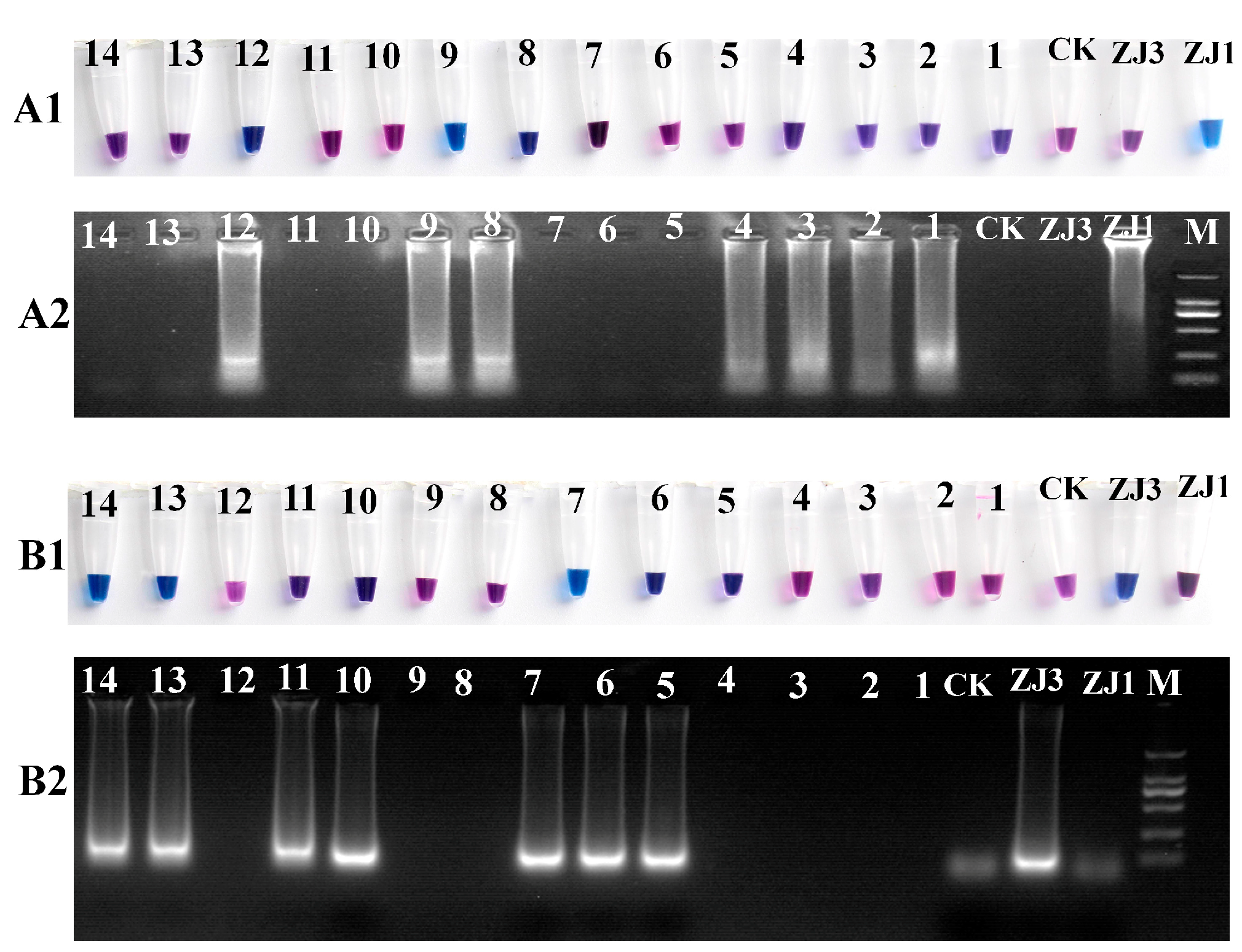
Because it is difficult to distinguish between the MAT1-1 and MAT1-2 strains based on the morphology, a real-time molecular identification assay using LAMP technology was designed. The results of the LAMP reaction were visualized using HNB dye staining, and the reaction products were analyzed via 2% agarose gel electrophoresis (Figure 2). The positive reaction mixtures stained with HNB appeared sky-blue, and the gel electrophoresis revealed a characteristic DNA ladder of different sizes, displaying a reproducible ladder-like pattern. In contrast, the negative reactions remained violet, and DNA laddering were undetectable after electrophoresis. We further tested a range of isolated O. xuefengensis strains using this assay. A total of 14 strains of O. xuefengensis with unknown mating types were evaluated to assess the practicality of LAMP detection. The assay clearly differentiated all 14 O. xuefengensis isolates, as shown in Figure 2(A1,B1). The specific primers for MAT1-1 and MAT1-2 effectively recognized and distinguished the mating types of O. xuefengensis. The genotypes of strains numbered 1–4, 8, 9, and 12 are MAT1-1, while strains numbered 5–7, 10, 11, 13, and 14 are MAT1-2. Overall, both the chromogenic reaction and agarose gel electrophoresis were consistent.
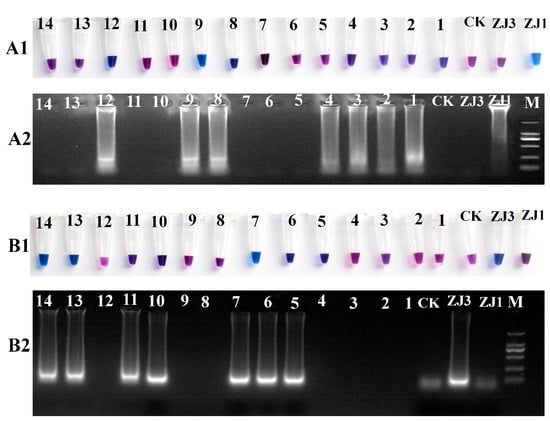
Figure 2.
LAMP assay reactions of O. xuefengensis. (A1,B1) visual inspection of LAMP products; (A2,B2) gel images showing LAMP products; (A1,A2) specific primers for MAT1-1; (B1,B2) specific primers for MAT1-2; M: DL2000; ZJ1: MAT1-1 strain; ZJ3: MAT1-2 strain; CK: negative control (no DNA); 1–14, 14 strains with unknown mating types.
3.3. Carbohydrates, Nucleosides, and Ergosterol
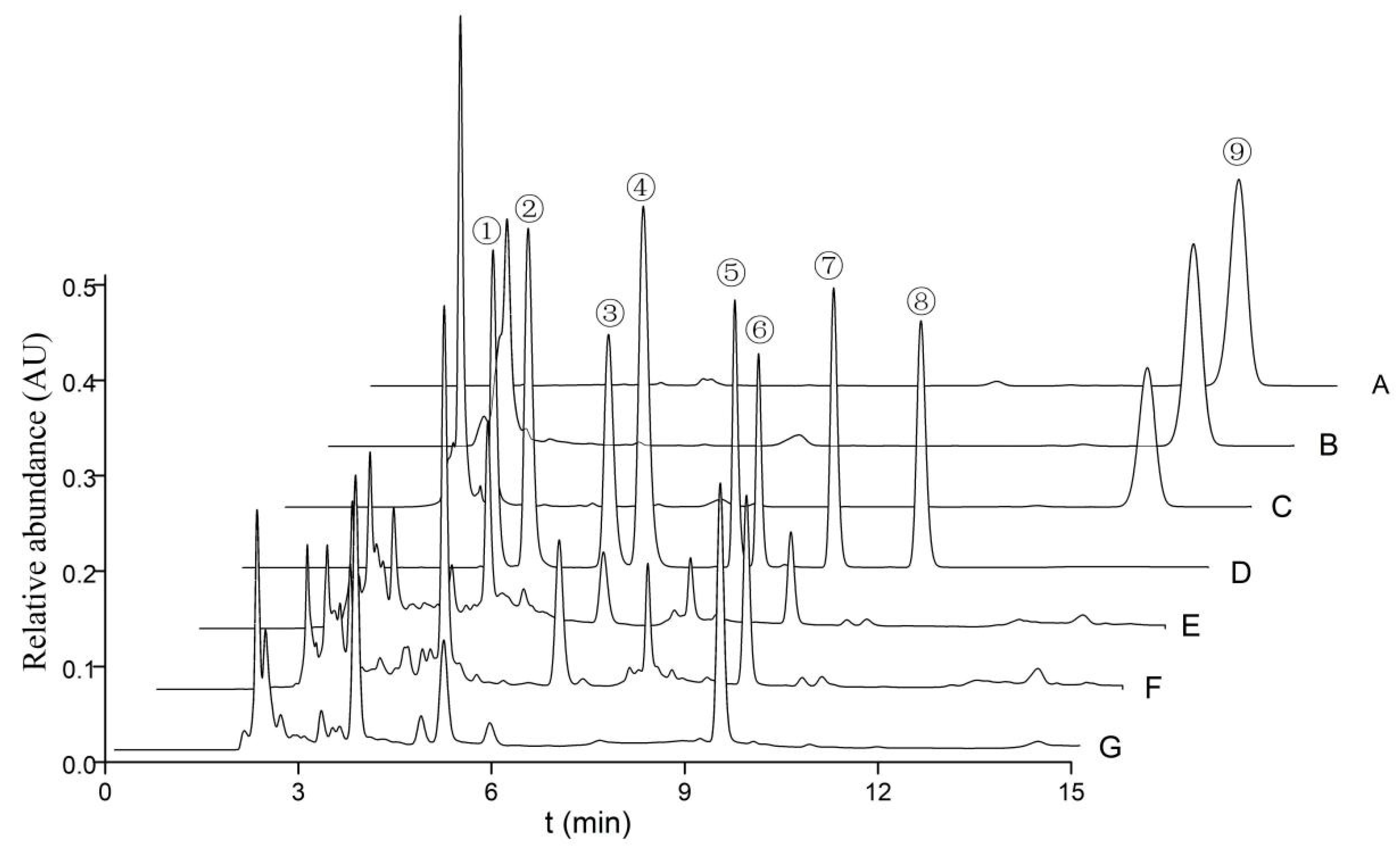
The quantities of cordycepic acid, total polysaccharides, eight nucleosides, and ergosterol in the various fruiting bodies of O. xuefengensis were illustrated in Figure 3 and detailed in Table 2, with results presented as mean ± standard deviation. It is evident from Table 2 that there were statistically significant differences in cordycepic acid, with the MAT1-2 fruiting bodies showing a slight predominance. The cordycepic acid contents in three samples were 6.7%, 11.7%, and 7.8%, respectively. No statistically significant difference in polysaccharide content was found between the MAT1-1 fruiting bodies and the MAT1-2 fruiting bodies. The concentration of adenosine in MAT1-1 fruiting bodies was 1305 ± 56 mg/kg, which was obviously higher than MAT1-2 fruiting bodies (649 ± 14 mg/kg) and natural fruiting bodies (762 ± 56 mg/kg). For thymidine, guanosine, adenine, inosine, uracil, and uridine, the following observations were made: thymidine was detected only in the cultured fruiting bodies, while inosine was found exclusively in the natural fruiting bodies. The highest concentrations of adenine, guanosine, and uridine were found in MAT1-1 fruiting bodies, which contained 256.5 ± 8.2 mg/kg, 2395 ± 40 mg/kg, and 2336 ± 40 mg/kg, respectively. The uracil content in the MAT1-2 fruiting bodies was 147.4 ± 6.4 mg/kg, which was higher compared to that in MAT1-2 fruiting bodies and natural fruiting bodies. Except for uracil, the MAT1-1 fruiting bodies had significantly higher amounts of adenosine, thymidine, guanosine, adenine, and uridine compared to the MAT1-2 fruiting bodies. Thus, the MAT1-1 fruiting bodies of O. xuefengensis may be particularly favorable for further studies on pharmacological activity of this species and may have potential applications in the development of health and functional medical products.
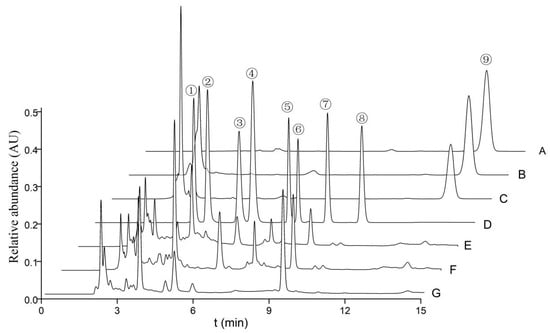
Figure 3.
HPLC profile of nucleosides, nucleobases, and ergosterols from O. xuefengensis. A–C: Ergosterol (A. standard sample; B. MAT1-2 fruiting bodies; C. MAT1-1 fruiting bodies); D–G: nucleosides and nucleobases (D. standard sample; E. MAT1-2 fruiting bodies; F. MAT1-1 fruiting bodies; G. natural fruiting bodies). 1. uracil; 2. uridine; 3. inosine; 4. guanosine; 5. adenine; 6. thymidine; 7. adenosine; 8. cordycepin; and 9. ergosterol.

Table 2.
Contents of cordycepic acid, 8 nucleosides, and ergosterol in the fruiting bodies of MAT1-1 and MAT1-2 of O. xuefengensis.
Ergosterol, a type of mycosterol abundantly present in the cell membranes of fungi, performs a multitude of functions similar to those of cholesterol in animal cells. This study revealed that the ergosterol content in the MAT1-2 fruiting bodies of O. xuefengensis was higher, at 7116 ± 151 mg/kg, compared to 4660 ± 88 mg/kg in the MAT1-1 fruiting bodies and 5308 ± 10 mg/kg in the natural fruiting bodies.
3.4. Amino Acid Composition
The amino acid compositions of different fruiting bodies of O. xuefengensis are given in Table 3. Significant discrepancies in amino acid components among the three samples were observed. The contents of total amino acids and total essential amino acids in the MAT1-1 fruiting bodies were 270.3 ± 2.9 mg/g and 102.5 ± 1.5 mg/g, respectively, which were significantly higher than those in the MAT1-2 fruiting bodies. The abundance of individual amino acids in the MAT1-1 sample was much higher than in the MAT1-2 sample, except for three amino acids: histidine, proline, and arginine. Aspartic acid was the principal amino acid in all three samples of O. xuefengensis. The four principal amino acids and their amounts in the MAT1-1 fruiting bodies were as follows: glutamic acid (38.0 ± 1.7 mg/g), aspartic acid (31.4 ± 1.4 mg/g), leucine (26.3 ± 0.4 mg/g), and arginine (25.4 ± 2.0 mg/g). However, their levels in the MAT1-2 fruiting bodies and the natural fruiting bodies differed: glutamic acid (21.2 ± 1.0 mg/g and 15.2 ± 1.0 mg/g), aspartic acid (27.8 ± 1.2 mg/g and 20.0 ± 1.2 mg/g), leucine (11.1 ± 0.3 mg/g and 19.0 ± 0.9 mg/g), and arginine (25.5 ± 0.6 mg/g and 15.3 ± 0.6 mg/g), respectively. In the natural fruiting bodies, the total amino acid (TAA) content was 211.55 mg/g, which was notably lower than that in the MAT1-1 fruiting bodies. There were no notable differences in the content of total essential amino acids (TEAs) between the MAT1-1 fruiting bodies and natural fruiting bodies. These data indicate that the cultured MAT1-1 fruiting bodies have a better amino acid composition than the natural fruiting bodies and may be considered as a potential substitute for natural O. xuefengensis.

Table 3.
Amino acid composition of the fruiting bodies of O. xuefengensis.
3.5. Elements
According to their relationship to human health, elements can be categorized into three groups: essential trace elements, possibly essential elements, and potentially toxic elements. Essential trace elements include iron (Fe), zinc (Zn), copper (Cu), cobalt (Co), selenium (Se), strontium (Sr), molybdenum (Mo), and chromium (Cr), etc. Possibly essential elements include manganese (Mn), nickel (Ni), vanadium (V), and barium (Ba), etc. Potentially toxic elements include arsenic (As), lead (Pb), mercury (Hg), cadmium (Cd), aluminum (Al), and tin (Sn), etc. The average concentrations of these elements, expressed in milligram per kilograms of dry weight in samples, are presented in Table 4.

Table 4.
Elemental composition of natural and cultured fruiting bodies of O. xuefengensis (mg/kg).
The concentrations of two essential elements, Cu and Zn, in the cultured MAT1-2 fruiting bodies were noticeably higher than those in MAT1-1 and natural fruiting bodies. Selenium (Se) is an important essential microelement known for its antioxidant properties and its role in thyroid hormone metabolism, immune responses, and reproduction in humans and animals. Among the different samples, the Se content in MAT1-1 fruiting bodies was the highest, reaching 0.43 ± 0.02 mg/kg. Regarding possibly essential elements, the concentration of Mn in MAT1-1 fruiting bodies (9.52 ± 0.46 mg/kg) was slightly higher than that in the MAT1-2 fruiting bodies (6.33 ± 0.31 mg/kg). Among potential toxicity elements, the MAT1-1 fruiting bodies had lower levels of Pb compared to the other two samples, and no significant differences in As levels were observed among the three samples. Overall, MAT1-1 fruiting bodies exhibited lower levels of toxic elements.
4. Discussion
In heterothallic ascomycetes, a single-spore isolate contains either the MAT1-1 or the MAT1-2 idiomorph [38]. Currently, the MAT1-1 and MAT1-2 strains have identical ITS sequences and can only be distinguished via PCR amplification of mating type genes. In our research, we designed a LAMP assay to rapidly differentiate between the two idiomorphs of O. xuefengensis. This is also the first time LAMP technology has been employed to distinguish between the two idiomorphs of Cordyceps sensu lato. Compared to PCR, LAMP displayed several advantages, including a short duration (1 h or less), visual detection, and the elimination of the need of a thermal cycler and gel electrophoresis. This technology has proven effective for precise species differentiation. For instance, Blaser et al. [31] distinguished Bemisia tabaci from other invasive pests using LAMP, based on the mitochondrial COI gene. He et al. [29] developed a LAMP assay to distinguish lethal Amanita species from the other Amanita species, using the section Phalloideae as the out-group. Li et al. [30] optimized the LAMP detection procedure for Phytophthora hibernalis, P. cambivora, and P. syringae by designing specific primers based on the sequences of internal transcribed spacers (ITS), enolase (Enol), and ras-like protein Ypt1 genes. Zhao and Feng [28] developed a LAMP assay for detecting the Zika virus using the envelope protein (EP) coding region and the NS5 protein coding region as target sequences.
Comparative research was conducted to assess the chemical composition of the fruiting bodies of two mating-type idiomorphs of cultured O. xuefengensis and natural O. xuefengensis. The cordycepic acid levels in the three fruiting bodies were found to be comparable to those reported for O. sinensis by Wang et al. [35], ranging from approximately 6.7% to 11.7% and 4.8% to 11.5%, respectively. The polysaccharide content in the fruiting bodies of O. xuefengensis (10.33–12.2%) was slightly higher than the values (4.2–10.43%) reported for natural and cultured O. sinensis by Li et al. [39] and Wang et al. [35]. The ergosterol content in the three fruiting bodies of O. xuefengensis (ranging from 4.66 mg/g to 7.11 mg/g) was markedly higher than the value reported for cultivated mushrooms of C. militaris (3.461 mg/g) by Hu et al. [40] as well as the range observed in wild O. sinensis (0.91 mg/g to 4.19 mg/g) by Li et al. [41]. The amounts of five nucleosides (adenosine, thymidine, adenine, guanosine, and uridine) as well as the total essential amino acids and total amino acids in the MAT1-1 fruiting bodies of O. xuefengensis were significantly higher than those in the MAT1-2 fruiting bodies of O. xuefengensis and in natural O. sinensis [35,42,43,44]. Cordycepin was first identified in C. militaris in 1964 and subsequently attracted widespread attention due to its antioxidant activity and anticancer activity [45]. However, recent studies have raised controversy over whether O. sinensis and O. xuefengensis contained cordycepin [21,22,24]. In our test, cordycepin was undetectable in both MAT1-1 and MAT1-2 O. xuefengensis.
Wang et al. [34] found that the content of toxicity elements in O. sinensis was somewhat above the standard, particularly for Al, Pb, As, and Hg. Remarkably, the concentrations of toxic elements (Al, Pb, and Hg) in the MAT1-1 fruiting bodies of O. xuefengensis were undetectable. These data suggest that the MAT1-1 fruiting bodies of O. xuefengensis could be a potential substitute for natural O. sinensis. Excessive levels of toxic elements in medicinal and edible fungi have long been a serious problem, becoming a main factor limiting the development of their production. Therefore, selecting specific mating type strains may offer a new solution.
5. Conclusions
In the present study, the specific designed nested primer sets allowed for rapid and sensitive differentiation of the two MAT idiomorphs of O. xuefengensis using the LAMP reaction, providing a new technique for distinguishing different genotypes of the same species. The contents of active constituents in MAT1-1 O. xuefengensis were significantly higher than those in MAT1-2. More importantly, the contents of heavy metals in the MAT1-1 fruiting bodies were within safe limits. Altogether, the findings of this study suggest that MAT1-1 O. xuefengensis might be a potential substitute for natural O. sinensis. Currently, the exploitation and utilization of these species are severely restricted due to the relatively fragile ecological environment of their habitats, limited wild resources, excessive heavy metal content, and high cultivation difficulty. The artificial cultivation of O. sinensis fruiting bodies offers a sustainable alternative to harvesting wild specimens, thereby supporting ecological conservation. This approach addresses the scarcity of natural resources and marks a significant advancement toward industrial-scale production of Cordyceps. Based on the practical needs for the safe production and green development of Cordyceps medicinal materials, our research offers new solutions to the challenges of difficult, lengthy, and unstable artificial cultivation of Cordyceps fruiting bodies, as well as the issue of excessive heavy metal content.
Author Contributions
Methodology, J.Z. and M.F.; investigation, S.L., Y.L. and J.Z.; data curation, J.Z. and Y.L.; writing—original draft preparation, J.Z. and Y.Z.; writing—review and editing, S.L. and Y.L.; validation, Y.L., M.F. and B.S.; formal analysis, Y.Z.; project administration, Y.Z.; resources, M.F.; supervision, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Foundation of Hunan Double First-rate Discipline Construction Projects of Bioengineering, Hunan Provincial Natural Science Foundation of China (No. 2023JJ30483) and the Science and Technology Plan Project of Huaihua city (No. 2021R3127).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We are grateful to the editors and anonymous reviewers for their valuable comments and reviews.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shrestha, B.; Zhang, W.; Zhang, Y.; Liu, X. The medicinal fungus Cordyceps militaris: Research and development. Mycol. Prog. Progress. 2012, 11, 599–614. [Google Scholar] [CrossRef]
- Yue, K.; Ye, M.; Zhou, Z.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps sinensis: A review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.Z.; Li, L.D.J.; Zhou, X.W. Immunostimulatory effects of the intracellular polysaccharides isolated from liquid culture of Ophiocordyceps sinensis (Ascomycetes) on RAW264.7 cells via the MAPK and PI3K/Akt signaling pathways. J. Ethnopharmacol. 2021, 275, 114130. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Thi-Tran, T.V.; Tran, V.K.; Vu-Ho, X.A.; Tran, T.M.; Chau-Nguyen, D.G.; Chuong-Nguyen, T.H.; Varma, R.S.; Trinh, T.K.; Ho, T.T.; et al. Structural Characterization of Mannoglucan Isolated from Ophiocordyceps sobolifera and Its Antioxidant Activities. ACS Omega 2022, 7, 9397–9405. [Google Scholar] [CrossRef]
- Pharmacopoeia Committee of China. Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science and Technology Press: Beijing, China, 2020; p. 119.
- Singpoonga, N.; Rittiron, R.; Seang-On, B.; Chaiprasart, P.; Bantadjan, Y. Determination of adenosine and cordycepin concentrations in Cordyceps militaris fruiting bodies using near-infrared spectroscopy. ACS Omega 2020, 5, 27235–27244. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Li, W.; Shan, L. Cordyceps acid alleviates lung cancer in nude mice. J. Biochem. Mol. Toxicol. 2020, 35, 22670. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.; Fang, C.; Li, X.; Zhang, J.; Xi, Q.; Li, Y.; Zhang, R. Cordycepin enhances anti-tumor immunity in colon cancer by inhibiting phagocytosis immune checkpoint CD47 expression. Int. Immunopharmacol. 2022, 107, 108695. [Google Scholar] [CrossRef]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phytother. Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, X.; Zhang, G.; Bao, W.; Zhang, Y.; Zhang, M.; Zhou, X. Cordycepin inhibits airway remodeling in a rat model of chronic asthma. Biomed. Pharmacother. 2017, 88, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 110–114. [Google Scholar] [CrossRef]
- Ashraf, S.; Radhi, M.; Gowler, P.; Burston, J.J.; Gandhi, R.D.; Thorn, G.J.; de Moor, C.H. The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Sci. Rep. 2019, 9, 4696. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Takahashi, H.; Grudniewska, A.; Ban, S.; Umeyama, A.; Noji, M. Ergostane-Type Sterols From Several Cordyceps Strains. Nat. Prod. Commun. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Alexandre, T.R.; Lima, M.L.; Galuppo, M.K.; Mesquita, J.T.; do Nascimento, M.A.; Dos Santos, A.L.; Sartorelli, P.; Pimenta, D.C.; Tempone, A.G. Ergosterol isolated from the basidiomycete Pleurotus salmoneostramineus affects Trypanosoma cruzi plasma membrane and mitochondria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tao, Y.; Wang, Q.; Shen, L.; Yang, T.; Liu, Z.; Liu, C. Ergosterol Is the Active Compound of Cultured Mycelium Cordyceps sinensis on Antiliver Fibrosis. Evid. Based Complement. Alternat. Med. 2014, 2014, 537234. [Google Scholar] [CrossRef]
- Wen, T.C.; Zhu, R.C.; Kang, J.C.; Huang, M.H.; Tan, D.B.; Ariyawansha, H.; Hyde, K.D.; Liu, H. Ophiocordyceps xuefengensis sp. nov. from larvae of Phassus nodus (Hepialidae) in Hunan Province, southern China. Phytotaxa 2013, 123, 41–50. [Google Scholar] [CrossRef]
- Zhu, R.; Tan, D.; Qin, Y.; Liang, X.; Zhang, S.; Huang, H. Study on dynamic changes of adenosine in Ophiocordyceps xuefengensis from different harvest time. Mod. Chin. Med. 2015, 17, 1180–1183. Available online: https://www.zgxdzy.net/ch/reader/view_abstract.aspx?file_no=20151116&st=alljournals (accessed on 10 April 2024). (In Chinese).
- Zhang, S.H.; Cai, P.; Chen, L.; Liang, X.J.; Qin, Y.; Zhu, R.C.; Huang, H.Y. Identification of chemical constituents in Ophiocordyceps xuefengensis by HPLC-Q-TOF-MS/MS. Chin. Tradit. Herb. Drugs 2015, 46, 817–821. (In Chinese) [Google Scholar] [CrossRef]
- Jin, J.; Zhong, C.; Qin, Y.; Cai, Y.; Zhen, L.; Shen, B.; Chen, L.; Wan, D.; Qin, Y.; Zhang, S. A new cordycepin-producing caterpillar fungus Ophiocordyceps xuefengensis with artificial infection to the host, cultivation of mycelia and stromata. FEMS Microbiol. Lett. 2017, 346, 181. [Google Scholar] [CrossRef]
- Zou, J.; Wu, L.; He, Z.M.; Zhang, P.; Chen, Z.H. Determination of the Main Nucleosides and Nucleobases in Natural and Cultured Ophiocordyceps xuefengensis. Molecules 2017, 22, 1530. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, L.; Jin, J.; Huang, J.; Li, J.; Zhou, Q.; Zhou, R.; Zhang, S. Screening and Identification of Antioxidants from Ophiocordyceps xuefengensis (Ascomycetes) by Using DPPH-HPLC-DAD-Q-TOF-MS/MS. Int. J. Med. Mushrooms 2018, 20, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kang, W.; Zhong, C.; Qin, Z.; Zhou, R.; Liu, H.; Xie, J.; Chen, L.; Qin, Y. The pharmacological properties of Ophiocordyceps xuefengensis revealed by transcriptome analysis. J. Ethnopharmacol. 2018, 219, 195–201. [Google Scholar] [CrossRef]
- Zheng, B.; Xie, F.Y.; Cai, G.H.; Zhu, R.C.; Ke, L.I.; Gao, S.Q. Effects of Ophiocordyceps xuefengensis on proliferation of DC-CIK cells and activity of killing HepG-2 cells by DC-CIK cells. Chin. J. Immunol. 2015, 12, 189–192. Available online: https://kns.cnki.net/knavi/journals/ZMXZ/detail?uniplatform=NZKPT (accessed on 10 April 2024). (In Chinese).
- Lu, Y.; Xia, Y.; Luo, F.; Dong, C.; Wang, C. Functional convergence and divergence of mating-type genes fulfilling in Cordyceps militaris. Fungal Genet. Biol. 2016, 88, 35–43. [Google Scholar] [CrossRef]
- Zou, J.; Zeng, T.T.; He, Z.M.; Zhang, P.; Chen, Z.H. Cloning and analysis of Ophiocordyceps xuefengensis mating type (MAT) loci. FEMS Microbiol. Lett. 2019, 366, 70. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, R. Sensitive and rapid detection of Zika virus by loop-mediated isothermal amplification. Virus Genes 2019, 55, 43–50. [Google Scholar] [CrossRef]
- He, Z.; Su, Y.; Li, S.; Long, P.; Zhang, P.; Chen, Z. Development and Evaluation of Isothermal Amplification Methods for Rapid Detection of Lethal Amanita Species. Front. Microbiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Li, G.R.; Huang, G.M.; Zhu, L.H.; Lv, D.; Cao, B.; Liao, F.; Luo, J.F. Loop-mediated isothermal amplification (LAMP) detection of Phytophthora hibernalis, P. syringae and P. cambivora. J. Plant Pathol. 2019, 101, 51–57. [Google Scholar] [CrossRef]
- Blaser, S.; Diem, H.; von Felten, A.; Gueuning, M.; Andreou, M.; Boonham, N.; Tomlinson, J.; Müller, P.; Utzinger, J.; Frey, B.; et al. A Loop-mediated isothermal amplification (LAMP) assay for rapid identification of Bemisia tabaci. J. Vis. Exp. 2018, 29, 58502. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Majchrowska-Safaryan, A. Temperature Requirements for the Colony Growth and Conidial Germination of Selected Isolates of Entomopathogenic Fungi of the Cordyceps and Paecilomyces Genera. Agriculture 2023, 13, 1989. [Google Scholar] [CrossRef]
- West, C.D.; Rapoport, S. Modification of colorimetric method for determination of mannitol and sorbitol in plasma and urine. Proc. Soc. Exp. Biol. Med. 1949, 70, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, X.; Wang, N.; Mo, P.; Shen, J.; Liu, M.; Zhang, H.; Wang, P.; Zhang, Z. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol. 2023, 237, 930–943. [Google Scholar] [CrossRef]
- Wang, J.; Kan, L.; Nie, S.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT-Food Sci. Technol. 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Li, C.; Yang, H.; Li, Y.; Cheng, L.; Zhang, M.; Zhang, L.; Wang, W. Novel bioconversions of municipal effluent and CO2; into protein riched Chlorella vulgaris biomass. Bioresour. Technol. 2013, 132, 171–177. [Google Scholar] [CrossRef]
- Lawrence, R.A. A pocket calculator program for Duncan’s New Multiple Range Test and analysis of variance. Comput. Biol. Med. 1984, 14, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bushley, K.E.; Li, Y.; Wang, W.J.; Wang, X.L.; Jiao, L.; Spatafora, J.W.; Yao, Y.J. Isolation of the MAT1-1 mating typeidiomorph and evidence for selfing in the Chinese medicinal fungus Ophiocordyceps sinensis. Fungal Biol. 2013, 117, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Li, P.; Dong, T.T.X.; Tsim, K.W.K. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine 2001, 8, 207–212. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Hu, C.; Feng, Z.; Chen, W.; Shi, H. Comparison of Ergosterol and Vitamin D2 in Mushrooms Agaricus bisporus and Cordyceps militaris Using Ultraviolet Irradiation Directly on Dry Powder or in Ethanol Suspension. ACS Omega 2021, 6, 29506–29515. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Lai, C.M.; Gong, Y.X.; Kan, K.K.; Dong, T.T.; Tsim, K.W.; Wang, Y.T. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2004, 1036, 239–243. [Google Scholar] [CrossRef]
- Fan, H.; Li, S.P.; Xiang, J.J.; Lai, C.M.; Yang, F.Q.; Gao, J.L.; Wang, Y.T. Qualitative and quantitative determnation of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography–electrospray ionization tandem mass spectrometry HPLC–ESI–MS/MS). Anal. Chim. Acta 2006, 567, 218–228. [Google Scholar] [CrossRef]
- Xie, J.W.; Huang, L.F.; Hu, W.; He, Y.B.; Wong, K.P. Analysis of the main nucleosides in Cordyceps sinensis by LC/ESI-MS. Molecules 2010, 15, 305–314. [Google Scholar] [CrossRef]
- Zong, S.Y.; Han, H.; Wang, B.; Li, N.; Dong, T.T.; Zhang, T.; Tsim, K.W. Fast simultaneous determination of 13 nucleosides and nucleobases in Cordyceps sinensis by UHPLC-ESI-MS/MS. Molecules 2015, 20, 21816–21825. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002, 3, 415–424. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).