MAT1-1 and MAT1-2 Ophiocordyceps xuefengensis and Comparison of Their Chemical Composition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LAMP Reaction
2.3. Analysis of Nucleosides, Cordycepic Acid, and Amino Acid
2.4. Analysis of Elements
2.5. Statistical Analysis
3. Results
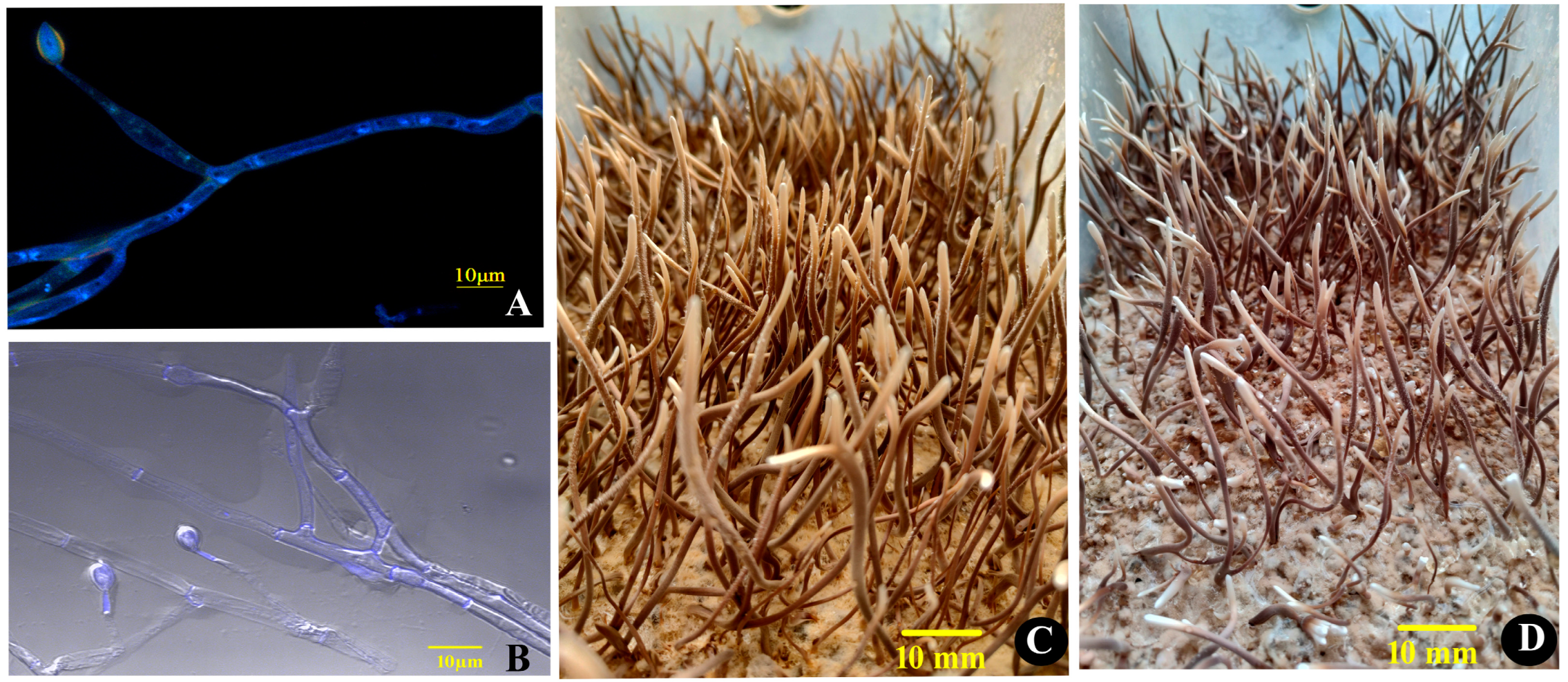
3.1. The Idiomorphs and the Fruiting Bodies
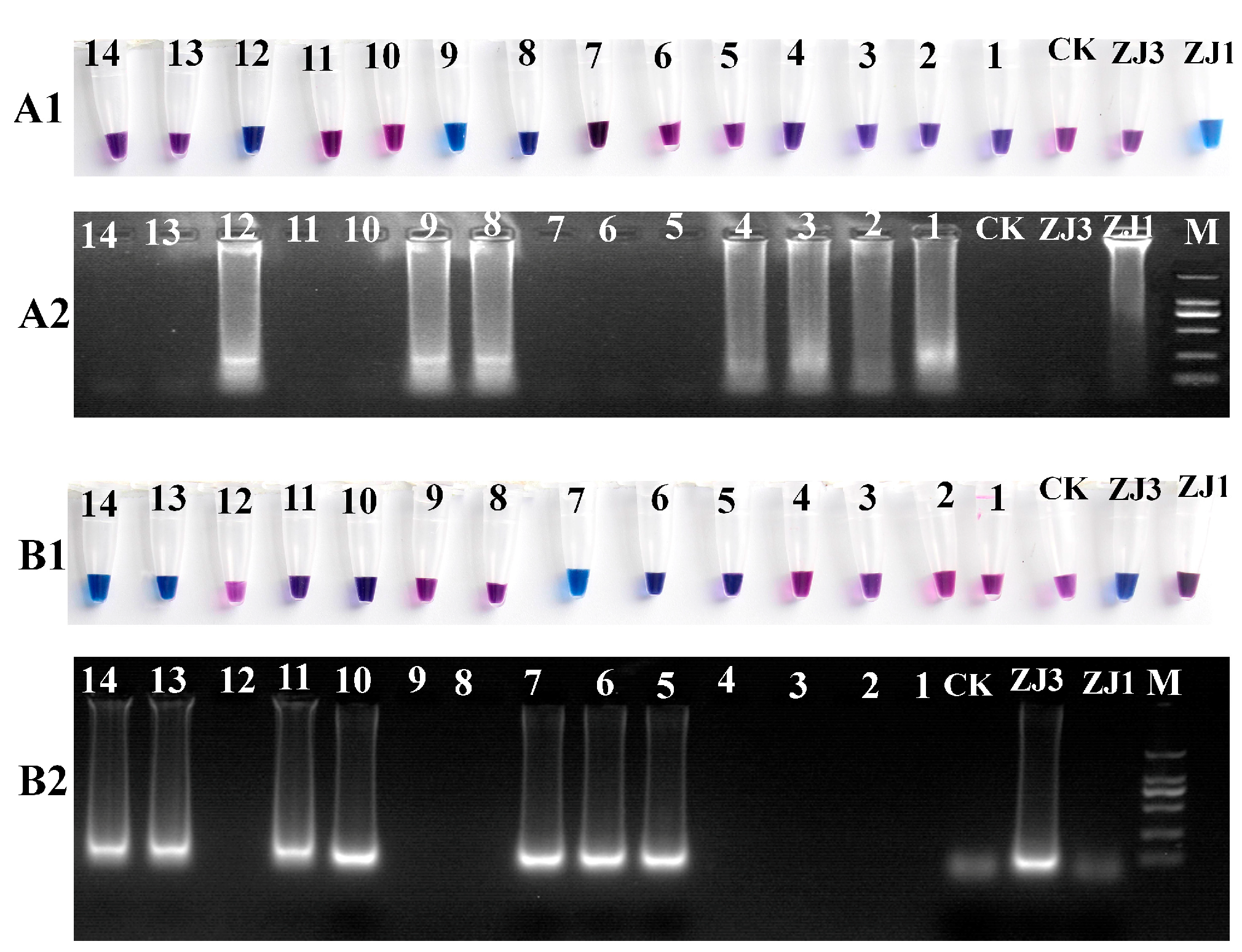
3.2. Specificity of LAMP
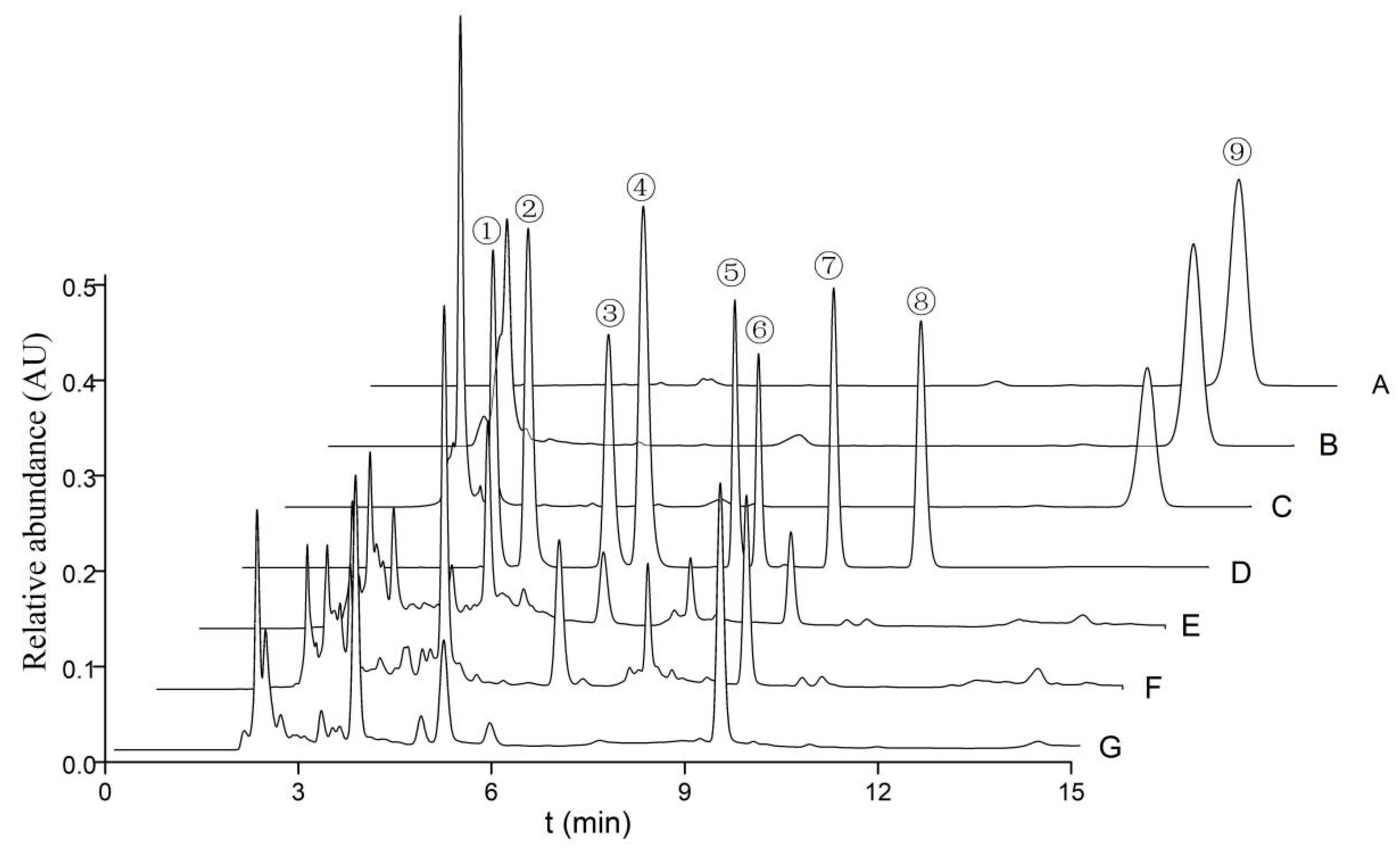
3.3. Carbohydrates, Nucleosides, and Ergosterol
3.4. Amino Acid Composition
3.5. Elements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, B.; Zhang, W.; Zhang, Y.; Liu, X. The medicinal fungus Cordyceps militaris: Research and development. Mycol. Prog. Progress. 2012, 11, 599–614. [Google Scholar] [CrossRef]
- Yue, K.; Ye, M.; Zhou, Z.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps sinensis: A review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Q.Z.; Li, L.D.J.; Zhou, X.W. Immunostimulatory effects of the intracellular polysaccharides isolated from liquid culture of Ophiocordyceps sinensis (Ascomycetes) on RAW264.7 cells via the MAPK and PI3K/Akt signaling pathways. J. Ethnopharmacol. 2021, 275, 114130. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Thi-Tran, T.V.; Tran, V.K.; Vu-Ho, X.A.; Tran, T.M.; Chau-Nguyen, D.G.; Chuong-Nguyen, T.H.; Varma, R.S.; Trinh, T.K.; Ho, T.T.; et al. Structural Characterization of Mannoglucan Isolated from Ophiocordyceps sobolifera and Its Antioxidant Activities. ACS Omega 2022, 7, 9397–9405. [Google Scholar] [CrossRef]
- Pharmacopoeia Committee of China. Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science and Technology Press: Beijing, China, 2020; p. 119.
- Singpoonga, N.; Rittiron, R.; Seang-On, B.; Chaiprasart, P.; Bantadjan, Y. Determination of adenosine and cordycepin concentrations in Cordyceps militaris fruiting bodies using near-infrared spectroscopy. ACS Omega 2020, 5, 27235–27244. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Li, W.; Shan, L. Cordyceps acid alleviates lung cancer in nude mice. J. Biochem. Mol. Toxicol. 2020, 35, 22670. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.; Fang, C.; Li, X.; Zhang, J.; Xi, Q.; Li, Y.; Zhang, R. Cordycepin enhances anti-tumor immunity in colon cancer by inhibiting phagocytosis immune checkpoint CD47 expression. Int. Immunopharmacol. 2022, 107, 108695. [Google Scholar] [CrossRef]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phytother. Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, X.; Zhang, G.; Bao, W.; Zhang, Y.; Zhang, M.; Zhou, X. Cordycepin inhibits airway remodeling in a rat model of chronic asthma. Biomed. Pharmacother. 2017, 88, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 110–114. [Google Scholar] [CrossRef]
- Ashraf, S.; Radhi, M.; Gowler, P.; Burston, J.J.; Gandhi, R.D.; Thorn, G.J.; de Moor, C.H. The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Sci. Rep. 2019, 9, 4696. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Takahashi, H.; Grudniewska, A.; Ban, S.; Umeyama, A.; Noji, M. Ergostane-Type Sterols From Several Cordyceps Strains. Nat. Prod. Commun. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Alexandre, T.R.; Lima, M.L.; Galuppo, M.K.; Mesquita, J.T.; do Nascimento, M.A.; Dos Santos, A.L.; Sartorelli, P.; Pimenta, D.C.; Tempone, A.G. Ergosterol isolated from the basidiomycete Pleurotus salmoneostramineus affects Trypanosoma cruzi plasma membrane and mitochondria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tao, Y.; Wang, Q.; Shen, L.; Yang, T.; Liu, Z.; Liu, C. Ergosterol Is the Active Compound of Cultured Mycelium Cordyceps sinensis on Antiliver Fibrosis. Evid. Based Complement. Alternat. Med. 2014, 2014, 537234. [Google Scholar] [CrossRef]
- Wen, T.C.; Zhu, R.C.; Kang, J.C.; Huang, M.H.; Tan, D.B.; Ariyawansha, H.; Hyde, K.D.; Liu, H. Ophiocordyceps xuefengensis sp. nov. from larvae of Phassus nodus (Hepialidae) in Hunan Province, southern China. Phytotaxa 2013, 123, 41–50. [Google Scholar] [CrossRef]
- Zhu, R.; Tan, D.; Qin, Y.; Liang, X.; Zhang, S.; Huang, H. Study on dynamic changes of adenosine in Ophiocordyceps xuefengensis from different harvest time. Mod. Chin. Med. 2015, 17, 1180–1183. Available online: https://www.zgxdzy.net/ch/reader/view_abstract.aspx?file_no=20151116&st=alljournals (accessed on 10 April 2024). (In Chinese).
- Zhang, S.H.; Cai, P.; Chen, L.; Liang, X.J.; Qin, Y.; Zhu, R.C.; Huang, H.Y. Identification of chemical constituents in Ophiocordyceps xuefengensis by HPLC-Q-TOF-MS/MS. Chin. Tradit. Herb. Drugs 2015, 46, 817–821. (In Chinese) [Google Scholar] [CrossRef]
- Jin, J.; Zhong, C.; Qin, Y.; Cai, Y.; Zhen, L.; Shen, B.; Chen, L.; Wan, D.; Qin, Y.; Zhang, S. A new cordycepin-producing caterpillar fungus Ophiocordyceps xuefengensis with artificial infection to the host, cultivation of mycelia and stromata. FEMS Microbiol. Lett. 2017, 346, 181. [Google Scholar] [CrossRef]
- Zou, J.; Wu, L.; He, Z.M.; Zhang, P.; Chen, Z.H. Determination of the Main Nucleosides and Nucleobases in Natural and Cultured Ophiocordyceps xuefengensis. Molecules 2017, 22, 1530. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, L.; Jin, J.; Huang, J.; Li, J.; Zhou, Q.; Zhou, R.; Zhang, S. Screening and Identification of Antioxidants from Ophiocordyceps xuefengensis (Ascomycetes) by Using DPPH-HPLC-DAD-Q-TOF-MS/MS. Int. J. Med. Mushrooms 2018, 20, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kang, W.; Zhong, C.; Qin, Z.; Zhou, R.; Liu, H.; Xie, J.; Chen, L.; Qin, Y. The pharmacological properties of Ophiocordyceps xuefengensis revealed by transcriptome analysis. J. Ethnopharmacol. 2018, 219, 195–201. [Google Scholar] [CrossRef]
- Zheng, B.; Xie, F.Y.; Cai, G.H.; Zhu, R.C.; Ke, L.I.; Gao, S.Q. Effects of Ophiocordyceps xuefengensis on proliferation of DC-CIK cells and activity of killing HepG-2 cells by DC-CIK cells. Chin. J. Immunol. 2015, 12, 189–192. Available online: https://kns.cnki.net/knavi/journals/ZMXZ/detail?uniplatform=NZKPT (accessed on 10 April 2024). (In Chinese).
- Lu, Y.; Xia, Y.; Luo, F.; Dong, C.; Wang, C. Functional convergence and divergence of mating-type genes fulfilling in Cordyceps militaris. Fungal Genet. Biol. 2016, 88, 35–43. [Google Scholar] [CrossRef]
- Zou, J.; Zeng, T.T.; He, Z.M.; Zhang, P.; Chen, Z.H. Cloning and analysis of Ophiocordyceps xuefengensis mating type (MAT) loci. FEMS Microbiol. Lett. 2019, 366, 70. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, R. Sensitive and rapid detection of Zika virus by loop-mediated isothermal amplification. Virus Genes 2019, 55, 43–50. [Google Scholar] [CrossRef]
- He, Z.; Su, Y.; Li, S.; Long, P.; Zhang, P.; Chen, Z. Development and Evaluation of Isothermal Amplification Methods for Rapid Detection of Lethal Amanita Species. Front. Microbiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Li, G.R.; Huang, G.M.; Zhu, L.H.; Lv, D.; Cao, B.; Liao, F.; Luo, J.F. Loop-mediated isothermal amplification (LAMP) detection of Phytophthora hibernalis, P. syringae and P. cambivora. J. Plant Pathol. 2019, 101, 51–57. [Google Scholar] [CrossRef]
- Blaser, S.; Diem, H.; von Felten, A.; Gueuning, M.; Andreou, M.; Boonham, N.; Tomlinson, J.; Müller, P.; Utzinger, J.; Frey, B.; et al. A Loop-mediated isothermal amplification (LAMP) assay for rapid identification of Bemisia tabaci. J. Vis. Exp. 2018, 29, 58502. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Majchrowska-Safaryan, A. Temperature Requirements for the Colony Growth and Conidial Germination of Selected Isolates of Entomopathogenic Fungi of the Cordyceps and Paecilomyces Genera. Agriculture 2023, 13, 1989. [Google Scholar] [CrossRef]
- West, C.D.; Rapoport, S. Modification of colorimetric method for determination of mannitol and sorbitol in plasma and urine. Proc. Soc. Exp. Biol. Med. 1949, 70, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, X.; Wang, N.; Mo, P.; Shen, J.; Liu, M.; Zhang, H.; Wang, P.; Zhang, Z. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol. 2023, 237, 930–943. [Google Scholar] [CrossRef]
- Wang, J.; Kan, L.; Nie, S.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT-Food Sci. Technol. 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Li, C.; Yang, H.; Li, Y.; Cheng, L.; Zhang, M.; Zhang, L.; Wang, W. Novel bioconversions of municipal effluent and CO2; into protein riched Chlorella vulgaris biomass. Bioresour. Technol. 2013, 132, 171–177. [Google Scholar] [CrossRef]
- Lawrence, R.A. A pocket calculator program for Duncan’s New Multiple Range Test and analysis of variance. Comput. Biol. Med. 1984, 14, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bushley, K.E.; Li, Y.; Wang, W.J.; Wang, X.L.; Jiao, L.; Spatafora, J.W.; Yao, Y.J. Isolation of the MAT1-1 mating typeidiomorph and evidence for selfing in the Chinese medicinal fungus Ophiocordyceps sinensis. Fungal Biol. 2013, 117, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Li, P.; Dong, T.T.X.; Tsim, K.W.K. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine 2001, 8, 207–212. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Hu, C.; Feng, Z.; Chen, W.; Shi, H. Comparison of Ergosterol and Vitamin D2 in Mushrooms Agaricus bisporus and Cordyceps militaris Using Ultraviolet Irradiation Directly on Dry Powder or in Ethanol Suspension. ACS Omega 2021, 6, 29506–29515. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Lai, C.M.; Gong, Y.X.; Kan, K.K.; Dong, T.T.; Tsim, K.W.; Wang, Y.T. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2004, 1036, 239–243. [Google Scholar] [CrossRef]
- Fan, H.; Li, S.P.; Xiang, J.J.; Lai, C.M.; Yang, F.Q.; Gao, J.L.; Wang, Y.T. Qualitative and quantitative determnation of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography–electrospray ionization tandem mass spectrometry HPLC–ESI–MS/MS). Anal. Chim. Acta 2006, 567, 218–228. [Google Scholar] [CrossRef]
- Xie, J.W.; Huang, L.F.; Hu, W.; He, Y.B.; Wong, K.P. Analysis of the main nucleosides in Cordyceps sinensis by LC/ESI-MS. Molecules 2010, 15, 305–314. [Google Scholar] [CrossRef]
- Zong, S.Y.; Han, H.; Wang, B.; Li, N.; Dong, T.T.; Zhang, T.; Tsim, K.W. Fast simultaneous determination of 13 nucleosides and nucleobases in Cordyceps sinensis by UHPLC-ESI-MS/MS. Molecules 2015, 20, 21816–21825. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002, 3, 415–424. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer Name | Length (bp) | Sequence(5′-3′) |
|---|---|---|---|
| MAT1-1-1 | F3-M1 | 24 | GTGGCTGACAATAAAACTGTAGGT |
| FIP-M1 | 46 | CCCACTTGTTTCTGAACGGGTCTTtaatTTCCCGACGTGCAGCAAA | |
| BIP-M1 | 46 | TGGCAAAGACAAAGTTTCTCTGGCtataGCAGCGGGCTCGATGAT | |
| B3-M1 | 17 | CCCAGCGCGTTCAGGTA | |
| MAT1-2-1 | F3-M2 | 20 | CAACATGAACCCCAATCCTC |
| FIP-M2 | 45 | AATCCCCCTCCAGGCAAAGAACtaatACGAGGCAATCTGGAAAGG | |
| BIP-M2 | 50 | TCATGTGAGTCTGATGTTAACCGCAtataTGACTCCTGAACATGCTCCCT | |
| B3-M2 | 16 | CCCGGTCGGGTCCATT |
| Analyte | The Fruiting Bodies of MAT1-1 | The Fruiting Bodies of MAT1-2 | Natural Fruiting Bodies |
|---|---|---|---|
| Nucleosides (mg/kg) | |||
| Adenine | 256.5 ± 8.2 a | 105.0 ± 9.2 b | 83.7 ± 8.9 c |
| Adenosine | 1305 ± 56 a | 649 ± 14 c | 762 ± 56 b |
| Cordycepin | nd | nd | nd |
| Guanosine | 2395 ± 40 a | 1017 ± 26 b | 514 ± 18 c |
| Inosine | nd | nd | 178 ± 1 |
| Thymidine | 148.4 ± 6.6 | 83.1 ± 2.1 | nd |
| Uridine | 2336 ± 40 a | 1400 ± 35 b | 975 ± 42 c |
| Uracil | 101.7 ± 7.3 b | 147.4 ± 6.4 a | 69.1 ± 2.7 c |
| Carbohydrates (%) | |||
| Cordycepic acid (Mannose) | 6.7 ± 0.2 c | 11.7 ± 0.7 a | 7.8 ± 0.5 b |
| Polysaccharides | 11.8 ± 0.6 a | 12.2 ± 0.8 a | 10.0 ± 0.6 b |
| Ergosterol (mg/kg) | 4660 ± 88 c | 7116 ± 151 a | 5308 ± 10 b |
| Amino Acid (mg/g) | MAT1-1 | MAT1-2 | Natural Fruiting Bodies |
|---|---|---|---|
| Ile * | 14.4 ± 0.2 a | 6.8 ± 0.3 c | 13.0 ± 1.0 b |
| Leu * | 26.3 ± 0.4 a | 11.1 ± 0.3 c | 19.0 ± 0.5 b |
| Lys * | 11.7 ± 0.2 b | 10.2 ± 0.4 c | 15.6 ± 0.8 a |
| Met * | 3.7 ± 0.2 b | 2.7 ± 0.2 c | 9.4 ± 0.6 a |
| Phe * | 12.3 ± 0.8 b | 9.2 ± 0.8 c | 19.8 ± 0.9 a |
| Thr * | 10.8 ± 0.7 a | 7.9 ± 0.4 b | 7.7 ± 0.3 b |
| Trp * | 11.9 ± 1.1 a | 2.6 ± 0.3 b | 3.1 ± 0.2 b |
| Val * | 11.4 ± 0.7 b | 10.5 ± 0.6 b | 17.2 ± 0.4 a |
| Ala | 10.5 ± 0.6 a | 8.8 ± 0.3 b | 11.0 ± 1.0 a |
| Arg | 25.4 ± 2.0 a | 25.5 ± 0.6 a | 15.3 ± 0.6 b |
| Asp | 31.4 ± 1.4 a | 27.7 ± 1.2 b | 20.0 ± 1.2 c |
| Cys | 4.7 ± 0.3 a | 1.8 ± 0.2 c | 3.9 ± 0.3 b |
| Glu | 38.0 ± 1.7 a | 21.2 ± 1.1 b | 15.2 ± 0.9 c |
| Gly | 12.7 ± 0.5 a | 8.0 ± 0.4 b | 8.6 ± 0.4 b |
| His | 12.3 ± 1.3 b | 14.6 ± 0.4 a | 8.6 ± 0.5 c |
| Pro | 10.4 ± 0.6 b | 13.0 ± 0.9 a | 11.9 ± 0.4 a |
| Ser | 11.7 ± 0.3 a | 6.1 ± 0.3 b | 6.8 ± 0.5 b |
| Tyr | 10.7 ± 0.9 a | 3.9 ± 0.1 c | 5.4 ± 0.4 b |
| Total amino acids (TAAs) | 270.3 ± 2.9 a | 191.5 ± 2.8 c | 211.5 ± 1.7 b |
| Total essential amino acids (TEAs) | 102.5 ± 1.5 a | 60.8 ± 2.0 b | 104.9 ± 1.0 a |
| TEA/TAA (%) | 37.9 | 31.7 | 49.6 |
| Minerals | MAT1-1 | MAT1-2 | Natural Fruiting Bodies |
|---|---|---|---|
| Ca | 1222 ± 77 b | 1200 ± 61 b | 4900 ± 139 a |
| Mg | 887.33 ± 34.85 c | 1300 ± 45.18 a | 1000 ± 19.08 b |
| K | 10763 ± 1292 b | 14967 ± 1320 a | 7800 ± 300 c |
| Na | 203.33 ± 14.98 b | 147 ± 9.85 b | 6000 ± 148.39 a |
| Fe | 10.77 ± 0.61 b | 9.85 ± 0.62 c | 59.34 ± 2.23 a |
| Zn | 71.00 ± 4.58 b | 142.8 ± 8.0 a | 43.86 ± 2.88 c |
| Cu | 12.12 ± 1.65 b | 16.75 ± 2.25 a | 6.84 ± 0.24 c |
| Co | nd | nd | nd |
| Se | 0.43 ± 0.02 a | 0.14 ± 0.01 c | 0.35 ± 0.01 b |
| Sr | 0.78 ± 0.02 | nd | nd |
| Mo | nd | nd | nd |
| Cr | 0.76 ± 0.04 c | 1.87 ± 0.03 a | 0.85 ± 0.05 b |
| Mn | 9.52 ± 0.46 b | 6.33 ± 0.31 c | 23.99 ± 1.32 a |
| Ni | nd | nd | nd |
| V | nd | nd | nd |
| Ba | nd | nd | nd |
| As | 0.53 ± 0.04 a | 0.54 ± 0.02 a | 0.53 ± 0.03 a |
| Pb | nd | 2.84 ± 0.07 | 0.40 ± 0.02 |
| Hg | nd | nd | nd |
| Cd | nd | nd | nd |
| Sn | nd | 1.23 ± 0.06 | 0.26 ± 0.01 |
| Al | nd | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Zhang, Y.; Luo, Y.; Fu, M.; Sun, B.; Liu, S. MAT1-1 and MAT1-2 Ophiocordyceps xuefengensis and Comparison of Their Chemical Composition. Biology 2024, 13, 686. https://doi.org/10.3390/biology13090686
Zou J, Zhang Y, Luo Y, Fu M, Sun B, Liu S. MAT1-1 and MAT1-2 Ophiocordyceps xuefengensis and Comparison of Their Chemical Composition. Biology. 2024; 13(9):686. https://doi.org/10.3390/biology13090686
Chicago/Turabian StyleZou, Juan, Yating Zhang, Yan Luo, Miaohua Fu, Beilin Sun, and Shenggui Liu. 2024. "MAT1-1 and MAT1-2 Ophiocordyceps xuefengensis and Comparison of Their Chemical Composition" Biology 13, no. 9: 686. https://doi.org/10.3390/biology13090686
APA StyleZou, J., Zhang, Y., Luo, Y., Fu, M., Sun, B., & Liu, S. (2024). MAT1-1 and MAT1-2 Ophiocordyceps xuefengensis and Comparison of Their Chemical Composition. Biology, 13(9), 686. https://doi.org/10.3390/biology13090686






