MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution Data Acquisition and Processing
2.2. Environmental Parameters for Model Simulation
2.3. MaxEnt Model Description and Modeling
2.4. Division of Potentially Suitable Areas
3. Results
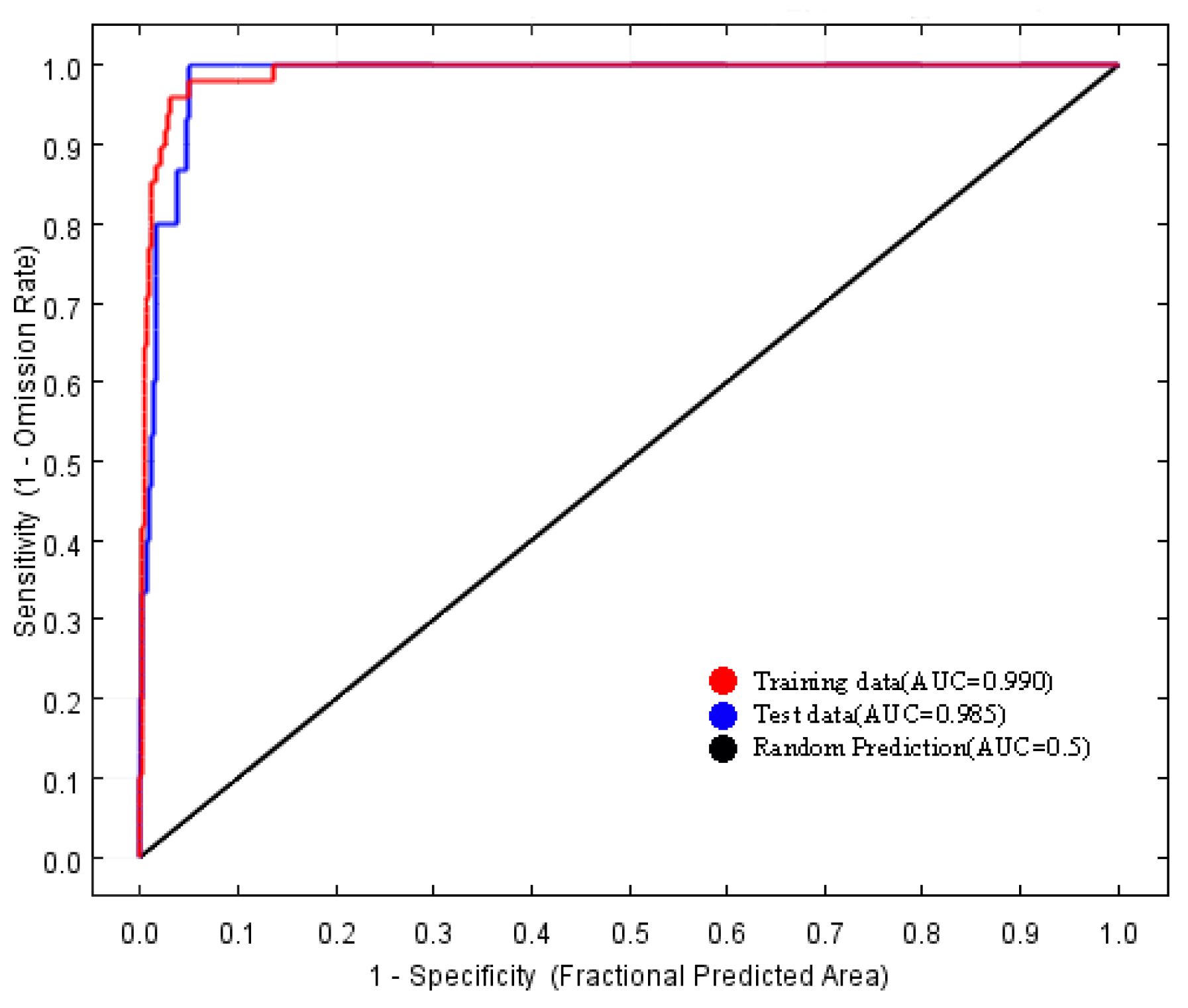
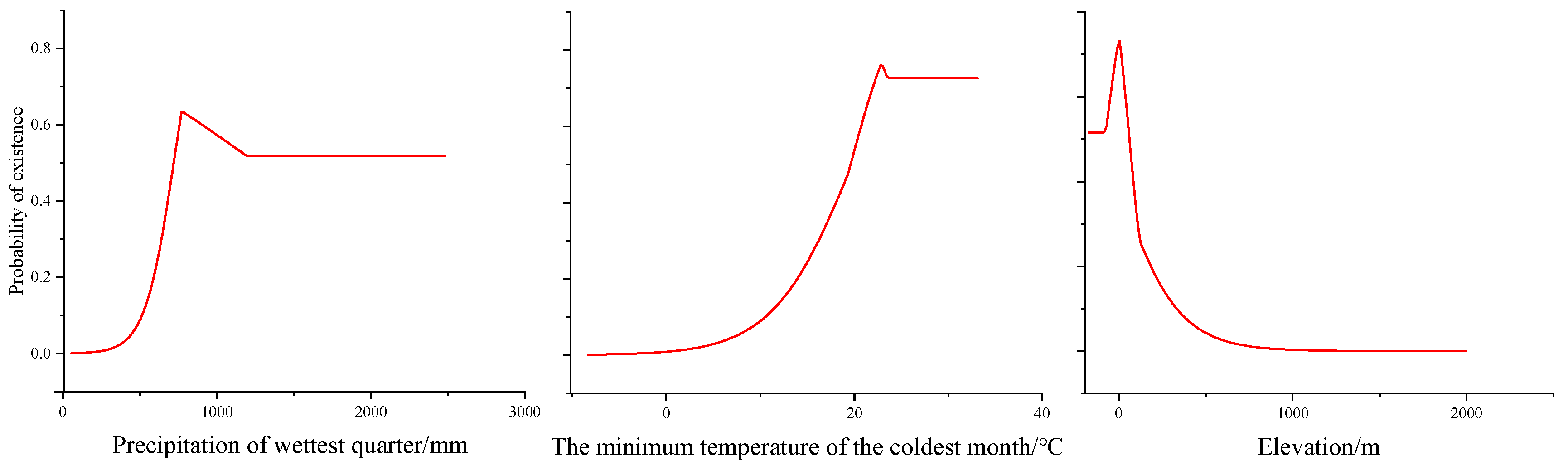
3.1. MaxEnt Model Evaluations and Environmental Characteristics of H. triflora
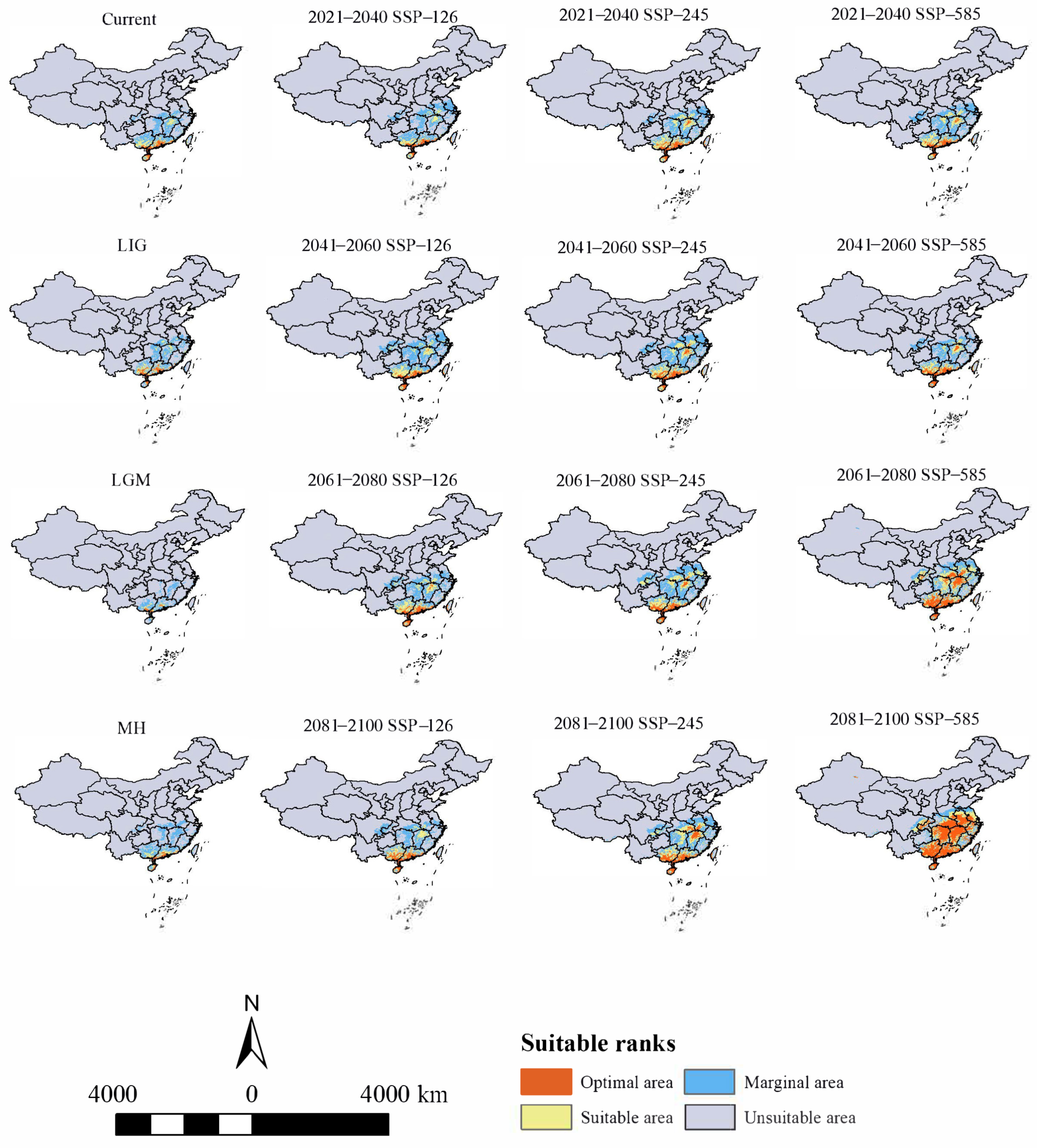
3.2. Historical Potential Distribution Area for H. triflora
3.3. Current Potential Distribution Area for H. triflora
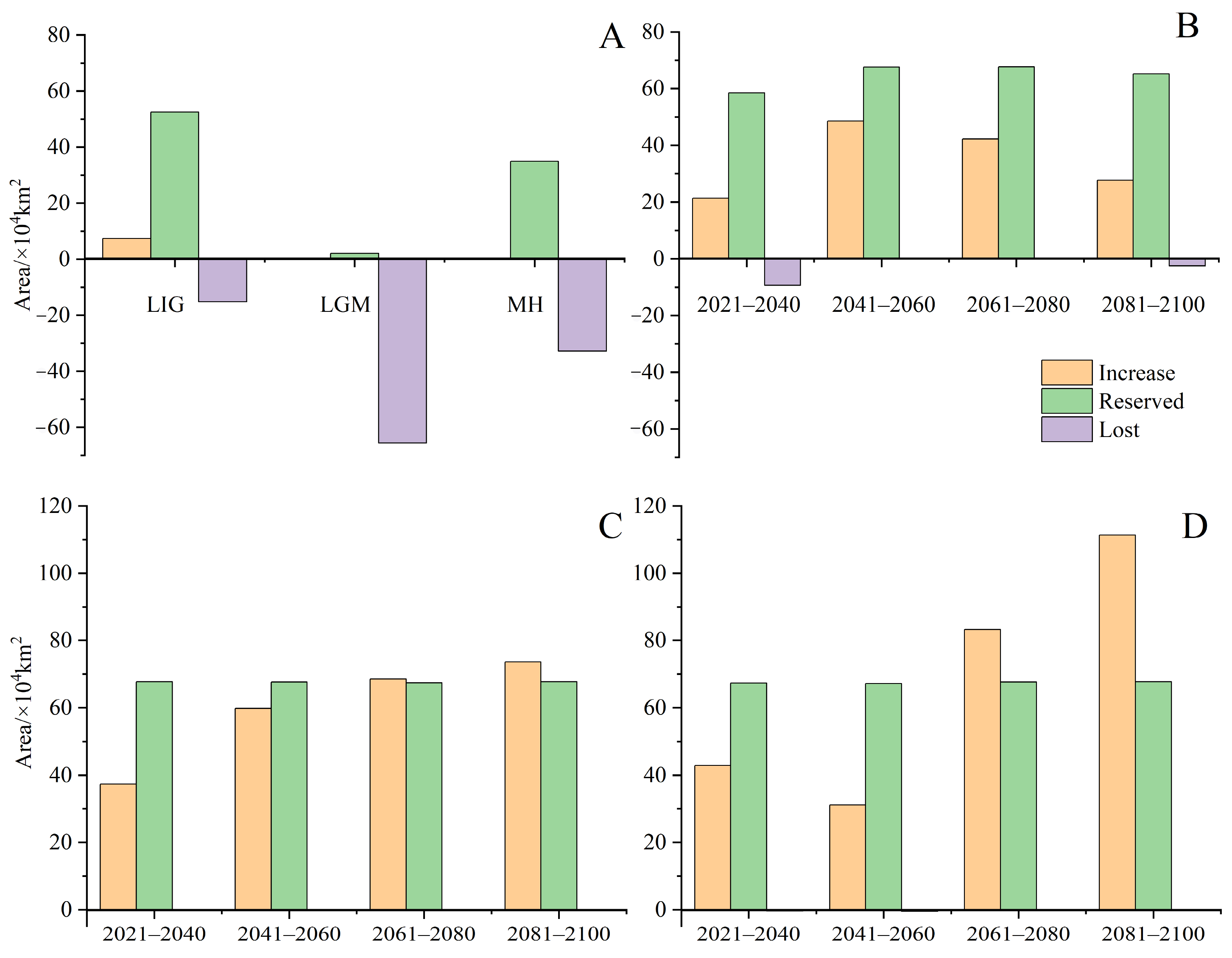
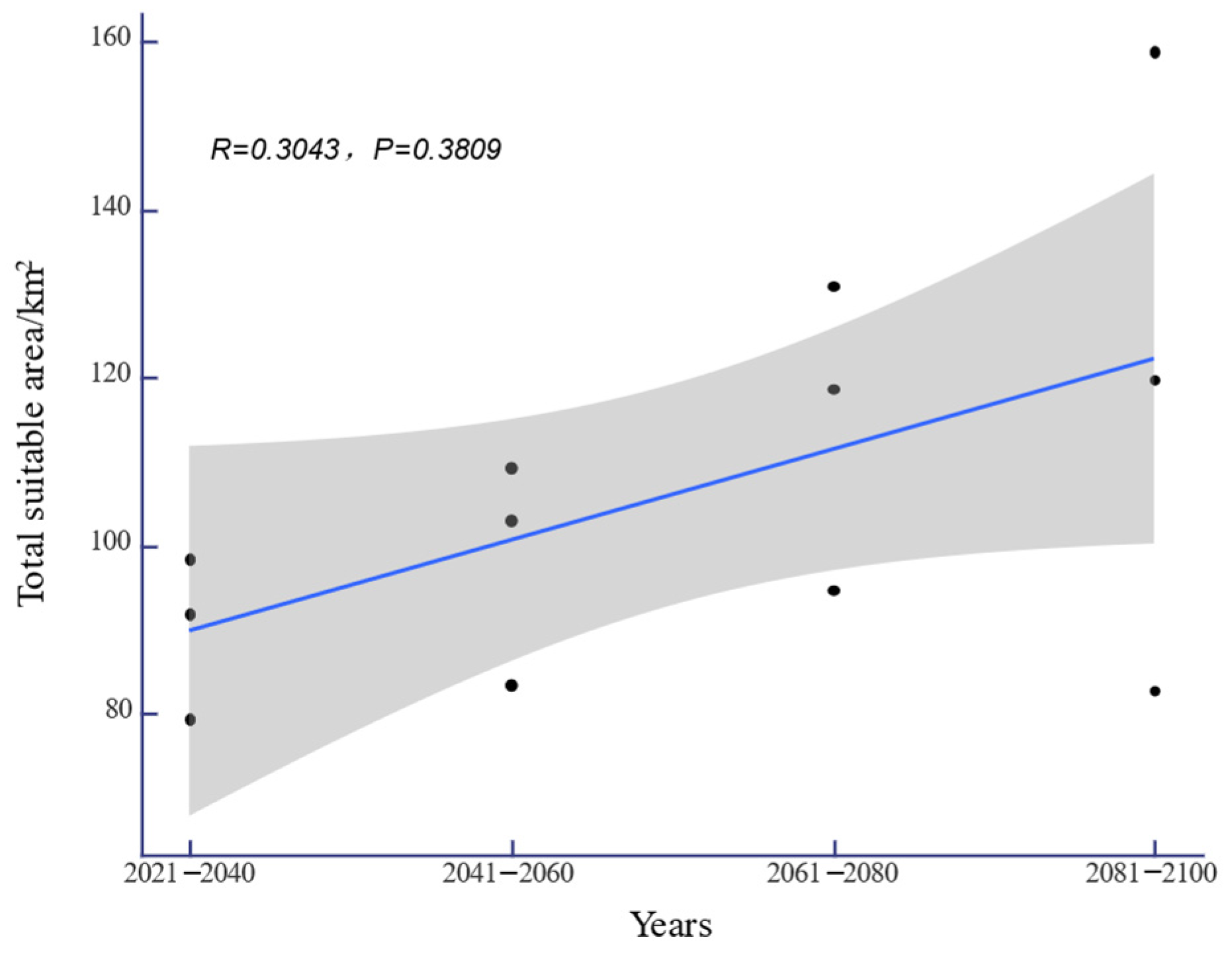
3.4. Changes in Future Potential Distribution Areas for H. triflora
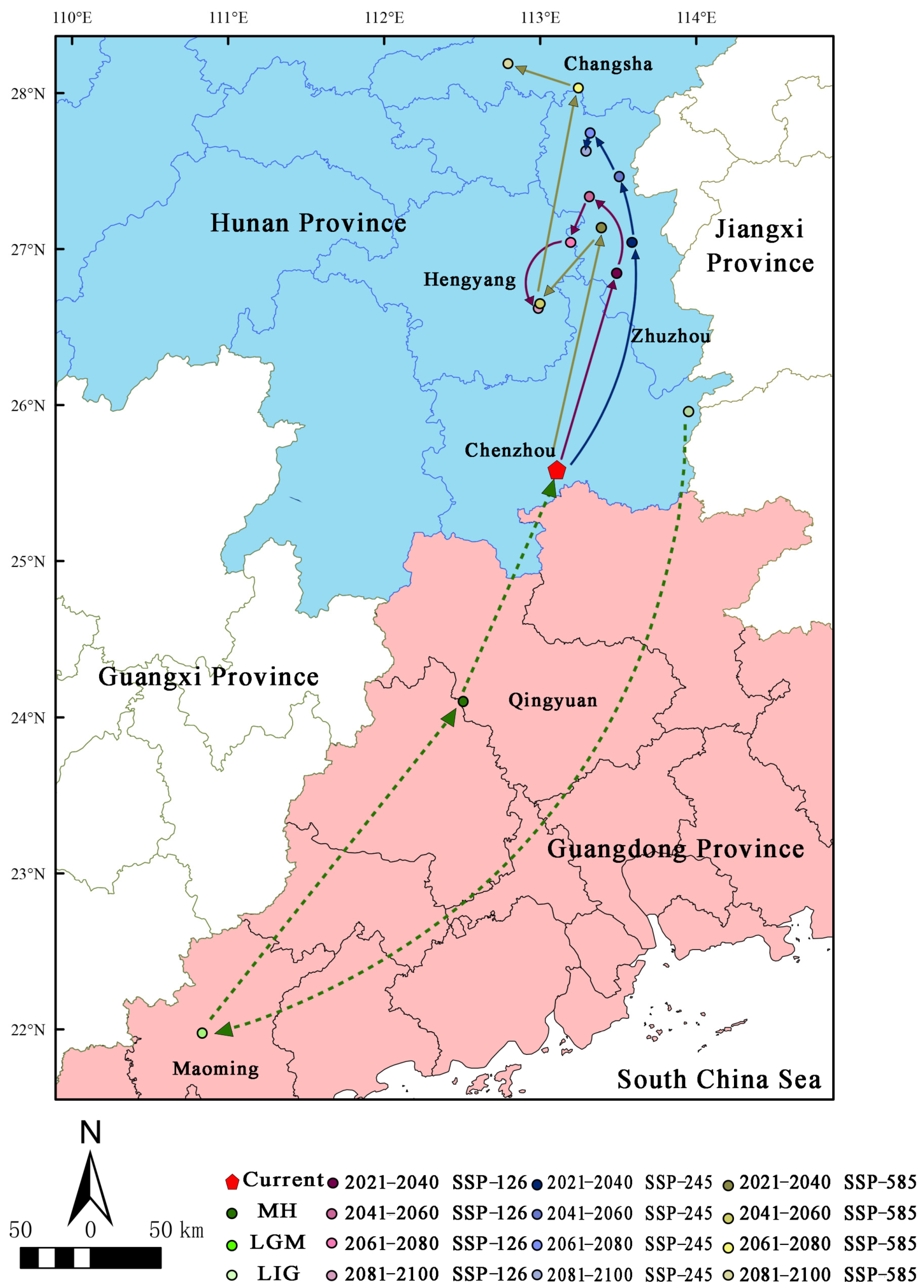
3.5. Migration of the Distribution Centroid in the Potential Distribution Area under Different Periods
4. Discussion
4.1. Model Accuracy Assessment
4.2. Environmental Factors Limit the Distribution Area of H. triflora
4.3. Changes in Potential Distribution Area under Historic, Current, and Future Periods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, P.T.; Caldeira, K. Greater future global warming inferred from Earth’s recent energy budget. Nature 2017, 552, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.S.; Broadman, E. Revisiting the Holocene global temperature conundrum. Nature 2023, 614, 425–435. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. Bmc Ecol. 2018, 18, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Uszko, W.; Huss, M.; Gardmark, A. Smaller species but larger stages: Warming effects on inter- and intraspecific community size structure. Ecology 2022, 103, e3699. [Google Scholar] [CrossRef] [PubMed]
- Ntilde, P.; Uelas, J.; Boada, M.I. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Chang. Biol. 2003, 9, 131–140. [Google Scholar]
- Shi, H.; Zhou, Q.; Xie, F.L.; He, N.J.; He, R.; Zhang, K.R.; Zhang, Q.F.; Dang, H.S. Disparity in elevational shifts of upper species limits in response to recent climate warming in the Qinling Mountains, North-central China. Sci. Total Environ. 2020, 706, 135718. [Google Scholar] [CrossRef]
- Rewicz, A.; Myśliwy, M.; Rewicz, T.; Adamowski, W.; Kolanowska, M. Contradictory effect of climate change on American and European populations of Impatiens capensis Meerb.—Is this herb a global threat? Sci. Total Environ. 2022, 850, 157959. [Google Scholar] [CrossRef]
- Shao, X.; Liu, X.; Cai, J.; Cai, Y.Z.; Cui, B.S. Identifying priority areas of Four Major Chinese carps’ species in the Pearl River basin based on the MaxEnt model. Waters Ecol. Environ. 2023, 5, 18–23. [Google Scholar] [CrossRef]
- Stockman, A.K.; Beamer, D.A.; Bond, J.E. An evaluation of a GARP model as an approach to predicting the spatial distribution of non-vagile invertebrate species. Divers. Distrib. 2010, 12, 81–89. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Zhang, D.; Yu, W.W.; Chen, B. Mapping the potential of mangrove forest restoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Murase, H.; Nagashima, H.; Yonezaki, S.; Matsukura, R.; Kitakado, T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: A case study in Sendai Bay, Japan. ICES J. Mar. Sci. 2009, 66, 1417–1424. [Google Scholar] [CrossRef]
- Mahanand, S.; Behera, M.D.; Roy, P.S. Rapid assessment of plant diversity using MODIS biophysical proxies. J. Environ. Manag. 2022, 311, 114778. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 201803456. [Google Scholar] [CrossRef]
- Ngarega, B.K.; Nzei, J.M.; Saina, J.K.; Halmy, M.W.A.; Chen, J.M.; Li, Z.Z. Mapping the habitat suitability of Ottelia species in Africa. Plant Divers. 2022, 44, 468–480. [Google Scholar] [CrossRef]
- Jane, E.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. S 2009, 40, 677–697. [Google Scholar]
- Costa, G.C.; Nogueira, C.; Machado, R.B.; Colli, G.R. Sampling bias and the use of ecological niche modeling in conservation planning: A field evaluation in a biodiversity hotspot. Biodivers. Conserv. 2010, 19, 883–899. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of The People’s Republic of China. The China Biodiversity Red List-Higher Plants Volume. Available online: https://www.plantplus.cn/rep/protlist/4?key=Hydrocera%20triflora (accessed on 18 January 2024).
- Ministry of Ecology and Environment of The People’s Republic of China. Announcement on the Release of The China Biodiversity Red List—Vertebrate Volume (2020) and The China Biodiversity Red List-Higher Plants Volume (2020). Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202305/t20230522_1030745.html (accessed on 18 January 2024).
- Chen, Y.L. Flora of China (Volume 47-2); Science Press: Beijing, China, 2002. [Google Scholar]
- Li, Z.Z.; Saina, J.; Gichira, A.; Kyalo, C.; Wang, Q.F.; Chen, J.M. Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera triflora and Impatiens pinfanensis. Int. J. Mol. Sci. 2018, 19, 319. [Google Scholar] [CrossRef]
- Ramadevi, D.; Narayana, L.L. Morphology of the flower and fruit of Hydrocera triflora Wight and Arn. emend Venkat. and Dutt—An elucidation. Proc. Plant Sci. 1990, 100, 43–49. [Google Scholar]
- Puri, R.; Choudhary, A.K.; Barman, P.; Mishra, G.; Geeta, R. Two unusual conjugated fatty acids, parinaric acid and α-eleostearic acid, are present in several Impatiens species, but not in congener Hydrocera triflora. Physiol. Mol. Biol. Plants 2022, 28, 1109–1118. [Google Scholar] [CrossRef]
- Wang, J.; Lv, D.; Huang, S.; Qi, H.S.; Ren, J.F.; Pan, M. Resource status and investigation of Hydrocera triflora of endangered aquatic plants in Hainan Province. Chin. Hortic. Abstr. 2017, 32, 67–91. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, Y.; Zhang, H.; Ying, B. Phylogeographic structure of Syntrichia caninervis Mitt, a xerophytic moss, highlights the expanded during glacial period. J. Plant Ecol. 2023, 16, rta057. [Google Scholar] [CrossRef]
- Wang, M.; Guan, Q. Prediction of potential suitable areas for Broussonetia papyrifera in China using the MaxEnt model and CIMP6 data. J. Plant Ecol. 2023, 16, rtad006. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhu, B.; Cheng, X.F.; Kong, R. MaxEnt Modeling Based on CMIP6 Models to Project Potential Suitable Zones for Cunninghamia lanceolata in China. Forests 2021, 12, 752. [Google Scholar] [CrossRef]
- Meng, Y.; Ma, J.M.; Wang, Y.Q.; Mo, Y.H. Prediction of distribution area of Loropetalum chinense based on Maxent model. Acta Ecol. Sin. 2020, 40, 8287–8296. [Google Scholar]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.F. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Monica, P.; Havel, J.E.; Vander Zanden, M.J. Using maximum entropy to predict the potential distribution of an invasive freshwater snail. Freshw. Biol. 2016, 61, 457–471. [Google Scholar]
- Taylor, I.E.P.; Frey, J.K. Predicting the distribution of a rare chipmunk (Neotamias quadrivittatus oscuraensis): Comparing MaxEnt and occupancy models. J. Mammal. 2020, 101, 1035–1048. [Google Scholar] [CrossRef]
- Xie, C.; Li, M.; Chen, L.; Jim, C.Y. Climate-driven changes to the spatial–temporal pattern of endangered tree Toona ciliata Roem. in China. Theor. Appl. Climatol. 2024, 155, 2071–2085. [Google Scholar] [CrossRef]
- Sarma, K.; Kumar, A.; Krishna, M.; Medhi, M.; Tripathi, O.P. Predicting Suitable Habitats for the Vulnerable Eastern Hoolock Gibbon, Hoolock leuconedys, in India Using the MaxEnt Model. Folia. Primatol. 2015, 86, 387–397. [Google Scholar] [CrossRef]
- Su, Q.T.; Zou, Z.R.; Du, Z.X.; Zhou, B. Distribution pattern of suitable area and early warning of invasive species Mirabilis japala under climate change scenarios in China. Chin. J. Ecol. 2024, 43, 833–841. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distribution. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Guardia, M.S.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.; Silander, J.J. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Fang, J.; Lechowicz, M.J. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J. Biogeogr. 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.P.; Zhang, Q.; Zhang, D.F.; Xie, C.X.; Liu, X. Prediction for the potential distribution area of Codonopsis pilosula at global scale based on Maxent model. Chine J. Appl. Ecol. 2017, 28, 992–1000. [Google Scholar]
- Wang, K.; Sun, J.; Cheng, G. Effect of Altitude and Latitude on Surface Air Temperature across the Qinghai-Tibet Plateau. J. Mt. Sci. 2011, 8, 808–816. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, S.; Yang, L.; Qi, X.J. Prediction of potential distribution of Cypripedium macranthos under climate change scenarios in China. Acta Ecol. Sin. 2024, 44, 209–223. [Google Scholar]
- Chen, D.; Kang, H.; Liu, C. An Overview on the Potential Quaternary Glacial Refugia of Plants in China Mainland. Bull. Bot. Res. 2011, 31, 623–632. [Google Scholar]
- Wang, Y.; Wang, J.; Wang, F.; Liu, Y.F.; Tan, C.C.; Yuan, Y.C.; Nie, W.; Liu, J.F.; Chang, E.M.; Jia, Z.R. Simulation of Suitable Distribution Areas of Picea koraiensis in China Since the Last Interglacial and under Future Climate Scenarios. Sci. Silvae Sin. 2023, 59, 1–12. [Google Scholar]
- Cohmap, M. Climatic Changes of the Last 18,000 Years: Observations and Model Simulations. Science 1998, 241, 1043–1052. [Google Scholar] [CrossRef]
- Birks, J.; Battarbee, R.; Mackay, A.; Oldfield, F. Global Change in the Holocene; Routledge: London, UK, 2014. [Google Scholar]
- Hou, G.; Fang, X. Characteristics of Holocene Temperature Change in China. Prog. Geogr. 2011, 30, 1075–1080. [Google Scholar]
- Boubli, J.P.; Ribas, C.; Alfaro, J.W.L.; Alfaro, M.E.; Silva, M.N.F.D.; Pinho, G.M.; Farias, I.P. Spatial and temporal patterns of diversification on the Amazon: A test of the riverine hypothesis for all diurnal primates of Rio Negro and Rio Branco in Brazil. Mol. Phylogenet Evol. 2015, 82, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ling, S.; Chen, W.; Ren, M.X.; Tang, L. Effects of geographic isolation caused by Changhua River on gentic diversity of Hainan-endemic Metapetrocosmea peltata (Gesneriaceae). Guihaia 2020, 40, 1505–1513. [Google Scholar]
- Zhang, L.; Chen, X.; Xin, X. Short commentary on CMIP6 Scenario Model Intercomparison Project (ScenarioMIP). Clim. Chang. Res. 2019, 15, 519–525. [Google Scholar]

| Variable | Description | Percent Contribution/% |
|---|---|---|
| bio02 | Mean diurnal range | 3.9 |
| bio04 | Variation in temperature seasonality | 0.9 |
| bio06 | Minimum temperature of the coldest month | 24.3 |
| Bio10 | Mean temperature of warmest quarter | 4.5 |
| Bio11 | Mean temperature of coldest quarter | 3.9 |
| Bio14 | Precipitation of the driest month | 0.6 |
| Bio15 | Variation in precipitation seasonality | 3.4 |
| Bio16 | Precipitation of wettest quarter | 36.1 |
| Bio19 | Precipitation of coldest quarter | 3.2 |
| Elev | Elevation position of species | 20.0 |
| Slope | Slope position of species | 1.5 |
| Aspect | Aspect position of species | 1.0 |
| Climate Scenario | Year | Optimal Area | Suitable Area | Marginal Area | Unsuitable Area |
|---|---|---|---|---|---|
| LIG | 7.43 | 13.79 | 39.92 | 902.71 | |
| LGM | 1.60 | 4.33 | 21.10 | 936.82 | |
| MH | 4.77 | 9.81 | 41.30 | 907.97 | |
| Current | 8.45 | 16.11 | 43.25 | 896.04 | |
| SSP-126 | 2021–2040 | 8.83 | 15.36 | 54.89 | 884.82 |
| SSP-126 | 2041–2060 | 10.81 | 23.86 | 68.04 | 861.14 |
| SSP-126 | 2061–2080 | 13.49 | 24.34 | 56.64 | 869.38 |
| SSP-126 | 2081–2100 | 13.46 | 19.97 | 49.03 | 881.39 |
| SSP-245 | 2021–2040 | 13.93 | 25.64 | 52.02 | 872.26 |
| SSP-245 | 2041–2060 | 15.56 | 28.11 | 65.36 | 854.82 |
| SSP-245 | 2061–2080 | 15.86 | 34.56 | 67.95 | 845.47 |
| SSP-245 | 2081–2100 | 22.72 | 35.27 | 61.51 | 844.35 |
| SSP-585 | 2021–2040 | 12.34 | 25.28 | 60.46 | 865.76 |
| SSP-585 | 2041–2060 | 14.90 | 20.32 | 47.92 | 880.71 |
| SSP-585 | 2061–2080 | 34.05 | 44.35 | 52.29 | 833.15 |
| SSP-585 | 2081–2100 | 75.94 | 45.97 | 36.72 | 805.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Du, Z.; Luo, Y.; Zhou, B.; Xiao, Y.; Zou, Z. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios. Biology 2024, 13, 745. https://doi.org/10.3390/biology13090745
Su Q, Du Z, Luo Y, Zhou B, Xiao Y, Zou Z. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios. Biology. 2024; 13(9):745. https://doi.org/10.3390/biology13090745
Chicago/Turabian StyleSu, Qitao, Zhixuan Du, Yi Luo, Bing Zhou, Yi’an Xiao, and Zhengrong Zou. 2024. "MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios" Biology 13, no. 9: 745. https://doi.org/10.3390/biology13090745
APA StyleSu, Q., Du, Z., Luo, Y., Zhou, B., Xiao, Y., & Zou, Z. (2024). MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios. Biology, 13(9), 745. https://doi.org/10.3390/biology13090745






