Simple Summary
This study explores and develops eco-friendly alternatives for multidrug-resistant bacterial pathogens. Herein, the potential antibacterial activity of honeybee venom (BV) and Monascus purpureus red dye (RD) extracts was investigated. Both BV and RD extracts inhibited the bacterial growth of E. coli, S. aureus, and E. faecalis in a dose-dependent manner while being more effective against S. aureus (MIC = 3.18 and 6.315 µg·mL−1, respectively). It is worth mentioning that the highest concentration (200 μg·mL−1) of both extracts was more effective than several standard antibiotics, particularly RD, which produced a significant inhibition zone (~20 mm) against S. aureus. SEM-based microscopy showed that both extracts disrupt bacterial cell membranes, indicating their ability to penetrate and damage bacterial cell walls. Moreover, GC-MS-based analysis showed high diversity in BV’s chemical composition and bioactive compounds (42 metabolites) and RD (23 metabolites), including polycyclic systems, fatty acids, and esters. Collectively, these findings suggest that BV and RD have strong antibacterial properties and potential applications as alternatives to conventional antibiotics. However, supplementary investigations are required to better understand these extracts’ physio-biochemical and molecular mechanisms within treated bacterial cells.
Abstract
Antimicrobial resistance (AMR) poses a critical global health threat, driving the search for alternative treatments to conventional antibiotics. In this study, the antibacterial properties of honeybee venom (BV) and fungal Monascus purpureus red dye (RD) were evaluated against three multidrug-resistant bacterial pathogens. Extracts of BV and RD exhibited dose-dependent antibacterial activity against the three tested bacteria, with their strongest effectiveness against S. aureus (minimum inhibitory concentrations [MIC] = 3.18 and 6.315 μg·mL−1, respectively). Although the three bacterial strains were resistant to the antibiotic ampicillin-sulbactam (10/10 µg), both extracts exhibited superior antibacterial activity against the three bacterial strains compared to five standard antibiotics, especially RD extract, which produced the largest inhibition zone (20 ± 0.20 mm) against S. aureus. The larger inhibition zones against S. aureus suggest its high sensitivity, whereas E. coli and E. faecalis exhibited smaller inhibition zones, indicating less sensitivity to BV and RD extracts. Differences in the inhibition zones suggest the variations in antimicrobial activity between the two extracts and their strain-specific effectiveness. Scanning electron microscopy (SEM) revealed that BV and RD extracts disrupted the bacterial plasma membrane, suggesting that the bioactive compounds penetrate the bacterial cell wall and alter its integrity. Furthermore, GC–MS-based analysis revealed that the chemical composition of BV and RD extracts exhibited highly diverse structures, including complex polycyclic systems, porphyrins, steroids, and esters. For instance, 42 metabolites were identified in the BV extract, which mainly were organic and metal–organic compounds; however, only 23 molecules were identified in RD extract, which mainly were fatty acids and their derivatives. The diversity in the chemical compositions of both extracts highlights their potential applications in pharmaceuticals, materials, and biochemistry fields. Collectively, these findings indicate that honeybee venom and the red dye from M. purpureus have promising antibacterial properties and warrant further investigation as potential alternatives to conventional antibiotics. Further multi-ligand docking-based virtual screening studies are required to identify the most promising detected metabolite(s) within both BV and RD extracts.
1. Introduction
Antimicrobial resistance (AMR) is a significant challenge predicted by the World Health Organization (WHO) as a “global public health concern” [1]. It has serious global implications, leading to higher rates of illness and death from bacterial infections, and urgent measures are needed to combat this issue [2]. Over the last 30 years, the rate of new antibiotic approvals has declined, while antibiotic-resistant bacterial pathogens continue to emerge [3]. These pathogens are increasingly developing additional resistance mechanisms, leading to the rise of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) bacteria that resist all known antibiotics [4]. AMR poses a worldwide challenge, impacting the health of both humans and animals. While the primary cause of AMR is the overuse of antibiotics and antimicrobials, it is also fueled by poor sanitation, pollution, and other non-medical factors, with the natural environment significantly contributing to its spread [5,6]. The AMR phenomenon accounted for 1.27 million deaths in 2019 globally, and it is projected to cause over 10 million deaths by 2050. In 2020, the WHO warned that without changes in antibiotic usage, AMR could gradually lead to a pandemic [6]. In 2019, Egypt recorded 16,100 deaths directly attributable to AMR, along with an additional 56,600 deaths associated with it, ranking 58th in terms of age-standardized mortality rate per 100,000 populations among 204 countries [7].
In the context of antimicrobial resistance, the complex web of microbial interactions within ecosystems can be disrupted, exacerbating the spread of resistant pathogens [8]. Microorganisms, found throughout the biosphere, play vital roles in shaping their surroundings, with effects that can be beneficial, harmful, or subtle [9]. While many bacteria contribute positively to human health and other organisms, certain species are responsible for a wide array of diseases in humans and animals, causing illnesses that range from mild to life threatening. For instance, methicillin-resistant Staphylococcus aureus (MRSA) is a notable example of an AMR strain. Staphylococcus aureus is a noteworthy bacterial human pathogen in humans, accountable for a wide range of clinical conditions [10]. This Gram-positive coccal bacterium typically forms clusters [11]. S. aureus is one of the most prevalent bacterial pathogens in humans, causing various infections, including bacteremia, infective endocarditis, skin and soft tissue infections, gastroenteritis, meningitis, toxic shock syndrome, and urinary tract infections [12]. Infections with S. aureus occur frequently in both community and hospital settings, and treatment is complicated by the rise of multi-drug-resistant strains [13].
Likewise, AMR in Escherichia coli is a growing concern. E. coli has a complex relationship with its host [14]. While some strains harmlessly coexist in the intestines, aiding in digestion [15], others can cause serious systemic infections [16]. Genetic diversity among E. coli strains means that some possess virulence factors that enable them to cause diseases in various species. Pathogenic E. coli can produce toxins that disrupt the digestive system, resulting in symptoms like cramps, diarrhea, and vomiting [17]. Food contamination is a common route for these harmful strains, emphasizing the need for good food-handling practices to prevent E. coli infections [18].
Another example of AMR human pathogenic bacteria is the vancomycin-resistant Enterococcus faecalis (VRE). E. faecalis is a human pathobiont that can exhibit both commensal and pathogenic behaviors [19]. Its pathogenicity is often linked to host vulnerability, excessive intestinal growth, or the presence of medical devices [20]. Traditionally viewed as an extracellular pathogen, E. faecalis can adhere to and invade various mammalian cells, though not as effectively as typical intracellular pathogens [21]. The adhesion of E. faecalis to epithelial cells is likely mediated more by carbohydrate structures than by protein components [22]. E. faecalis is responsible for a significant portion of enterococcal infections and is mainly associated with urinary tract infections (UTIs), bloodstream infections, and endocarditis (inflammation of the heart valves) [19]. Some strains of E. faecalis are concerning due to their antibiotic resistance, complicating treatment options [23]. The cases of these three bacterial species underscore the alarming rise in antibiotic resistance and highlight the urgent need for improved hygiene and food-handling practices to prevent infections. Growing concerns over antibiotic overuse have led to increased interest in eco-friendly alternatives derived from biological sources [24]. Honeybee venom (BV) and Monascus fermentation products might be promising alternatives.
Honeybee venom (BV) is a promising therapeutic candidate, known for its health benefits across various organisms. Traditionally used as a natural pain reliever and anti-inflammatory agent, honeybee venom contains over 40 bioactive substances, including peptides, enzymes, and other compounds that contribute to its medicinal properties [25]. Key components such as melittin, apamin, and adolapin are believed to play significant roles in their anti-inflammatory, antibacterial, and pain-relieving effects [26]. Additionally, some compounds, like apamin and phospholipase A2, show potential for treating immune disorders through their immune-regulating effects [27]. BV’s therapeutic advantages extend to veterinary medicine, benefiting both livestock and companion animals [28]. Similarly, Monascus pigments, derived from species such as Monascus purpureus, are popular as natural food colorants in East Asian countries, where they enhance both color and sensory quality [29]. Due to increasing interest in natural products, Monascus purpureus has been widely studied for its bioactive compounds, including flavonoids, phenols, tannins, and various secondary metabolites. Research suggests that fermenting rice with Monascus purpureus may offer additional health benefits, such as cholesterol regulation, diabetes management, cardiovascular support, and possibly even cancer prevention [30,31].
Antimicrobial resistance (AMR) is a serious global health challenge, yet sustainable alternatives to conventional antibiotics remain poorly studied. Although both honeybee venom (BV) and Monascus purpureus red dye (RD) have been recognized for their medicinal and antimicrobial properties, their comparative effectiveness against multidrug-resistant (MDR) human pathogenic bacteria is poorly studied. Moreover, the exact cellular mechanisms behind their activity, particularly their effects on bacterial membranes and the role of specific metabolites, remain largely unexplored. This study hypothesizes that BV and RD extracts exhibit differential antibacterial activity against common MDR human bacterial pathogens such as E. coli, S. aureus, and E. faecalis. Moreover, we aimed to evaluate their antibacterial activity compared to traditional antibiotics, investigate their mode of action using SEM imaging, characterize their chemical composition through GC–MS, and correlate dominant metabolites with potential antimicrobial activity. By investigating the antimicrobial mechanisms of these two natural products, this research aims to provide insights that could inform the development of alternative antimicrobial strategies to combat the growing challenge of antibiotic resistance.
2. Materials and Methods
2.1. Source and Extraction of Honeybee Venom
The honeybee venom (BV) used in this study was sourced from honeybee (Apis mellifera) colonies maintained at the apiary of Tanta University’s Faculty of Agriculture. Venom was collected using a semi-automatic extraction device consisting of stainless-steel electrodes connected to a battery and pulse generator, along with a glass collection slide. To extract the venom, the bees were stimulated with controlled electrical impulses, which prompted them to sting the glass collection surface. The venom secreted by the bees was deposited on the glass slide, and the volatile components were allowed to evaporate, leaving behind a white residue of crude honeybee venom. This sediment was carefully scraped off the glass slide and stored at 4 °C before further use. For the experiments, 1 mg of the collected honeybee venom was dissolved in 1 mL of dimethyl sulfoxide (DMSO) to prepare a 1000 μg·mL−1 stock solution. This stock solution was used to prepare the desired concentrations of honeybee venom for the antimicrobial assays.
2.2. Source and Extraction of Red Dye
The Monascus purpureus strain ATCC 16436 used in this study was obtained from the Microbiological Resources Center (MIRCEN) in Cairo, Egypt, which is part of the Egyptian National Culture Collection (ENCC). This specific strain is known for its ability to produce red pigments. For activating and short-term preservation of the fungal strain, potato dextrose agar (PDA) was used. To prepare the modified minimal medium, as described in previous studies [32,33], 4 mL of the medium was mixed with 3.0 g of rice grains per 100 mL. The composition of the modified medium included 0.5 g each of NH4SO4, NH4NO3, KNO3, and peptone, along with 0.2 g of KH2PO4 and K2HPO4, 0.2 g of MgSO4, 0.001 mM ZnSO4, and 0.002 mM MnSO4. This modified medium was the primary medium used for the biosynthesis of Monascus pigments, with the final pH adjusted to 4.5. A fungal plug was transferred to the PDA plates and then incubated at 30 °C for 7 days. Subsequently, the inoculated plates were used to prepare a 2 × 104 spores·mL−1 fungal spore suspension using sterile water.
For the extraction of Monascus pigments, a slightly modified method from previous studies was employed [34]. After the 7-day incubation period, 20 mL of 96% ethanol was added to the fermented rice substrate, and the mixture was agitated at 180 rpm for 2 h. The mixture was then filtered through Whatman filter paper No. 1. The filtrate was centrifuged at 10,000 rpm for 10 min, and the resulting supernatant containing the Monascus red pigments was collected and stored at 4 °C for further use. The alcohol trace was eliminated by volatilization under vacuum at room temperature. To prepare the extract for antibacterial activity testing, 1 mg of the concentrated Monascus red pigment was dissolved in 1 mL of DMSO to obtain a 1000 μg·mL−1 stock solution.
2.3. Chemical Profiling of the Honeybee Venom and Red Dye Extracts
The chemical compositions of the honeybee venom and Monascus red dye extracts were analyzed using gas chromatography–tandem mass spectrometry (GC–MS/MS). The GC–MS/MS analysis was conducted with the following parameters: column oven temperature initially set to 50 °C, then increased by 5 °C/min to 250 °C and held for 2 min, further increased to a final temperature of 300 °C at a rate of 30 °C/min and maintained for another 2 min; injector and MS transfer line temperatures of 270 °C and 260 °C, respectively; helium carrier gas at a constant flow rate of 1 mL per minute; 4-min solvent delay; and 1 μL aliquot of the diluted samples injected automatically in split mode using an autosampler AS1300 (Thermo Fisher Scientific Inc., Rodano-Milan, Italy). An MS detector (Perkin Elmer, Waltham, MA, USA) was set to electron ionization (EI) at 70 eV, scanning 50–650 m/z in full scan mode with 200 °C as the ion source temperature. GC–MS chromatograms of honeybee venom and Monascus red dye extracts were analyzed, and detected peaks were identified by comparing their mass spectra with library entries of NIST-14 and Wiley, 9th edition.
2.4. Antibacterial Activity
2.4.1. Bacterial Strains
The antibacterial properties of BV and RD extracts were evaluated using three reference bacterial strains, Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, and Enterococcus faecalis ATCC 25923, obtained from the Global Bioresource Center (ATCC, Manassas, VA, USA). These strains were selected, as they represent standard reference organisms widely used in antimicrobial susceptibility testing and serve as essential baseline controls for comparing novel antimicrobial agents [35]. E. coli was chosen as a representative Gram-negative organism, while S. aureus and E. faecalis represent Gram-positive bacteria. These well-characterized strains provide a foundational framework for understanding the antimicrobial mechanisms of BV and RD, establishing a methodological baseline for future studies with resistant strains such as MRSA.
2.4.2. Determination of Minimum Inhibitory Concentration (MIC) and the Half-Maximal Inhibitory Concentration (IC50)
The minimum inhibitory concentration (MIC) and the half-maximal inhibitory concentration (IC50) were determined using the Clinical and Laboratory Standards Institute (CLSI) method [36] with 96-well microplates. Briefly, a bacterial inoculum of 1.5 × 108 CFU·mL−1 was introduced into each well. Different concentrations of the BV and RD extracts were prepared in DMSO (200, 100, 50, 25, 12.5, 6.25, 3.125, 1.562, 0.781, and 0.390 μg·mL−1) and tested against the three bacterial strains in Mueller–Hinton broth (Oxoid, Basingstoke, Hampshire, UK). For each test, three wells containing bacterial suspension with solvent (DMSO), but without the extract served as growth controls, while three additional wells without bacterial inoculum acted as background controls. The optical density (O.D.) was measured at 620 nm using a microtiter plate reader (Thermo Scientific, Norristown, PA, USA). MIC (concentration that inhibited over 95% of bacterial growth) was calculated using a modified Gompertz function [37] using Equation (1) as follows:
where B is a slope parameter and M is the log concentration of the inflexion point.
MIC = 10(M+[1/B])
On the other hand, the half-maximal inhibitory concentration (IC50) was calculated via probit regression analysis.
2.4.3. Comparison of the Antibacterial Activity of the Extracts and Traditional Antibiotics
The comparison was carried out using the disc diffusion method according to the protocol established by Surendra et al. [38]. Initially, the three bacterial strains (E. coli, S. aureus, and E. faecalis) were cultured in 1.5 mL of brain heart infusion broth (Oxoid, Basingstoke, Hampshire, UK), and then incubated at 37 °C for 24 h to prepare the pure bacterial cultures. Subsequently, the newly prepared bacterial cultures were streaked onto brain heart infusion agar medium and then incubated at 37 °C for an additional 24 h. A 0.5 McFarland standard (1.5 × 105 CFU·mL−1) of each bacterial strain was prepared by transferring a proper amount of the three tested bacterial strains into 10 mL of sterile saline. Then, bacterial cultures were evenly streaked on Mueller–Hinton agar (MH, Oxoid, Basingstoke, Hampshire, UK) plates using a sterile cotton swab.
Cellulose discs (6.3 mm diameter) were infused with the BV and RD extracts at the highest concentration used in the study (200 μg·mL−1) and placed on the agar surface. The tests were performed in triplicate. After incubating for 24 h at 37 °C, the plates were examined, and the inhibition zones were measured to calculate the average inhibition zone. Additionally, antibiotic discs, including ciprofloxacin (CIP; 5 µg), azithromycin (AZM; 15 µg), streptomycin (S; 10 µg), ampicillin-sulbactam (A/S; 10/10 µg), and clarithromycin (CLR; 15 µg), were added to the plates. After 24-h incubation at 37 °C, the inhibition zones were measured, and the results were reported as the mean inhibition zone in millimeters. The interpretation of antibiotic susceptibility was based on the Clinical and Laboratory Standards Institute (CLSI) guidelines, allowing direct comparison between the extracts’ inhibition zones and standard antibiotic performance under identical experimental conditions.
2.4.4. Cellular Structure of the Tested Bacterial Strains
To examine malformations in bacterial cellular structures, the tested bacterial strains were cultured in nutrient broth (NB) media (Merck Millipore, Darmstadt, Germany) with BV and RD extracts (200 μg·mL−1) and incubated at 37 °C for 12 h, alongside a non-treated control. After incubation, formalin–glutaraldehyde fixative (4F1G) in 0.1 M phosphate buffer (pH 7.4) was used to fix the bacteria after centrifugation. In the same buffer, the specimens were subsequently fixed once again using 1% osmium tetroxide. Following fixation, the specimens were dehydrated through a series of acetone concentrations. Then, they were coated with gold–palladium using a Polaron E500 sputter coater (Polaron Equipment Ltd., Hertfordshire, England). The bacterial specimens were analyzed using a scanning electron microscope (Model JSM 35C, JEOL Ltd., Akishima, Tokyo, Japan).
2.5. Statistical Analysis
The completely randomized design, unless otherwise stated, was used as the standard experimental design throughout this study. All experiments were conducted in triplicate, with three biological replicates for each treatment, and all tests were performed in triplicate. One-way ANOVA (Analysis of Variance) was used to test the statistically significant differences between treatments, followed by Tukey’s HSD for post hoc pairwise comparisons (p ≤ 0.05). The MIC was calculated using a modified Gompertz function [37], whereas the IC50 was calculated using probit regression analysis with 95% confidence intervals. The overall model fit of the probit regression model was stated using the chi-square (χ2), p-value, and coefficient of determination (R2) of Cox & Snell and Nagelkerke. To minimize variability across replicates, all experiments were conducted under controlled temperature conditions (±0.1 °C), using single batches of reagents, and following a standardized protocol with precise timing of sample preparation steps; each analysis was performed by the same researcher to eliminate inter-operator variation.
3. Results
3.1. Honeybee Venom (BV) Extract Exhibited Notable Antibacterial Activity Against MDR Human Pathogenic Bacteria
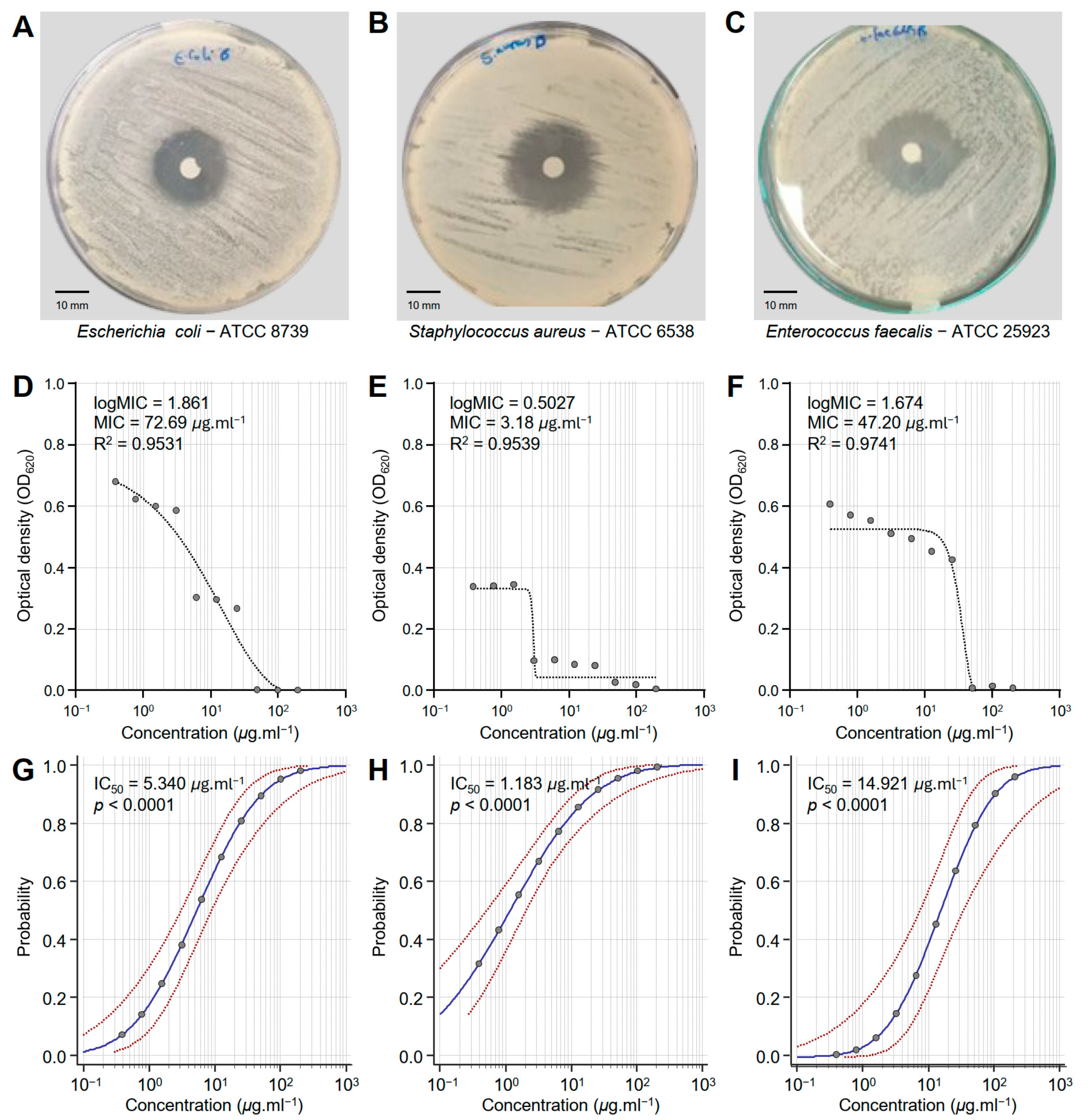
Honeybee venom (BV) extract exhibited significant antibacterial activity against E. coli—ATCC 8739 (Figure 1A), S. aureus—ATCC 6538 (Figure 1B), and E. faecalis—ATCC 25923 (Figure 1C). Although BV extract showed putative antibacterial activity against E. coli (logMIC = 1.861, MIC = 72.69 μg·mL−1, and R2 = 0.9531) (Figure 1D), it demonstrated its strongest effectiveness against S. aureus (logMIC = 0.5027, MIC = 3.18 μg·mL−1, and R2 = 0.9539) (Figure 1E). Moreover, BV extract displayed comparable antibacterial activity against E. faecalis (logMIC = 1.674, MIC = 47.20 μg·mL−1, and R2 = 0.9741) (Figure 1F). It is worth mentioning that the probit regression (dose–response analysis) of honeybee venom (BV) extract against the three bacterial strains (E. coli, S. aureus, and E. faecalis) (Figure 1G, Figure 1H and Figure 1I, respectively) exhibited strong overall high chi-square (χ2) values and significant p-values (p < 0.0001) (Table 1). S. aureus is the most sensitive bacterial strain (IC50 = 1.183 μg·mL−1), followed by E. coli (IC50 = 5.340 μg·mL−1), and E. faecalis as the least sensitive (IC50 = 14.921 μg·mL−1). These findings highlight the potential of BV extract as a promising antibacterial alternative across different pathogens, as well as its potential for targeted applications.
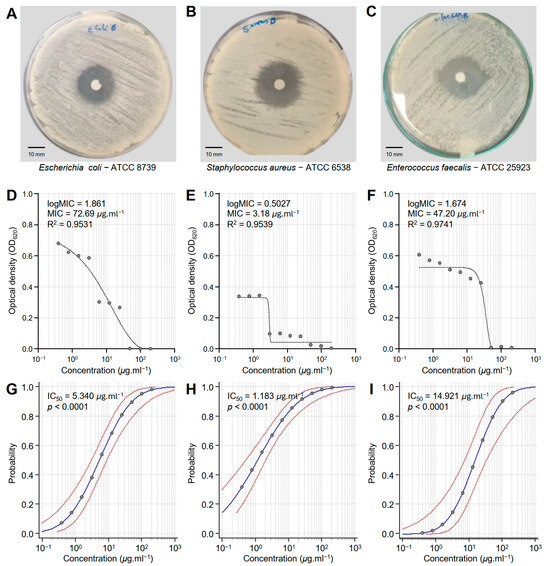
Figure 1.
In vitro antibacterial activity of honeybee venom (BV) extracts against three multidrug-resistant human pathogenic bacteria. (A–C) Disc diffusion method of the BV extract (200 μg·mL−1) against Escherichia coli—ATCC 8739, Staphylococcus aureus—ATCC 6538, and Enterococcus faecalis—ATCC 25923, respectively. (D–F) Susceptibility analysis and minimum inhibitory concentrations (MICs) of BV extract against E. coli, S. aureus, and E. faecalis, respectively. Different concentrations of the BV extract were prepared in DMSO (200, 100, 50, 25, 12.5, 6.25, 3.125, 1.562, 0.781, and 0.390 μg·mL−1). (G–I) Probit regression (dose–response analysis) of BV extract against E. coli, S. aureus, and E. faecalis, respectively. Gray dots present the means of three replicates of each concentration. Blue solid lines represent the probit regression lines, whereas red dashed lines edge represent the 95% confidence intervals for the estimated regression. Probit-associated half-maximal inhibitory concentrations (IC50; μg·L−1), 95% confidence intervals, and overall model fit are listed in Table 1. For BV extract, the IC50 against E. coli ATCC8739 was 5.340 μg·mL−1 (95% CI: 3.080–8.929 μg·mL−1), against S. aureus ATCC 6538 it was 1.183 μg·mL−1 (95% CI: 0.524–2.069 μg·mL−1), and against E. faecalis ATCC 25923 it was 14.921 μg·mL−1 (95% CI: 7.181–32.692 μg·mL−1).

Table 1.
The half-maximal inhibitory concentration (IC50; μg·L−1) results of honeybee venom (BV) and Monascus red dye (RD) extracts against multidrug-resistant human pathogenic bacteria.
3.2. Monascus Red Dye (RD) Extract Exhibited Strong Antibacterial Activity Against MDR Human Pathogenic Bacteria
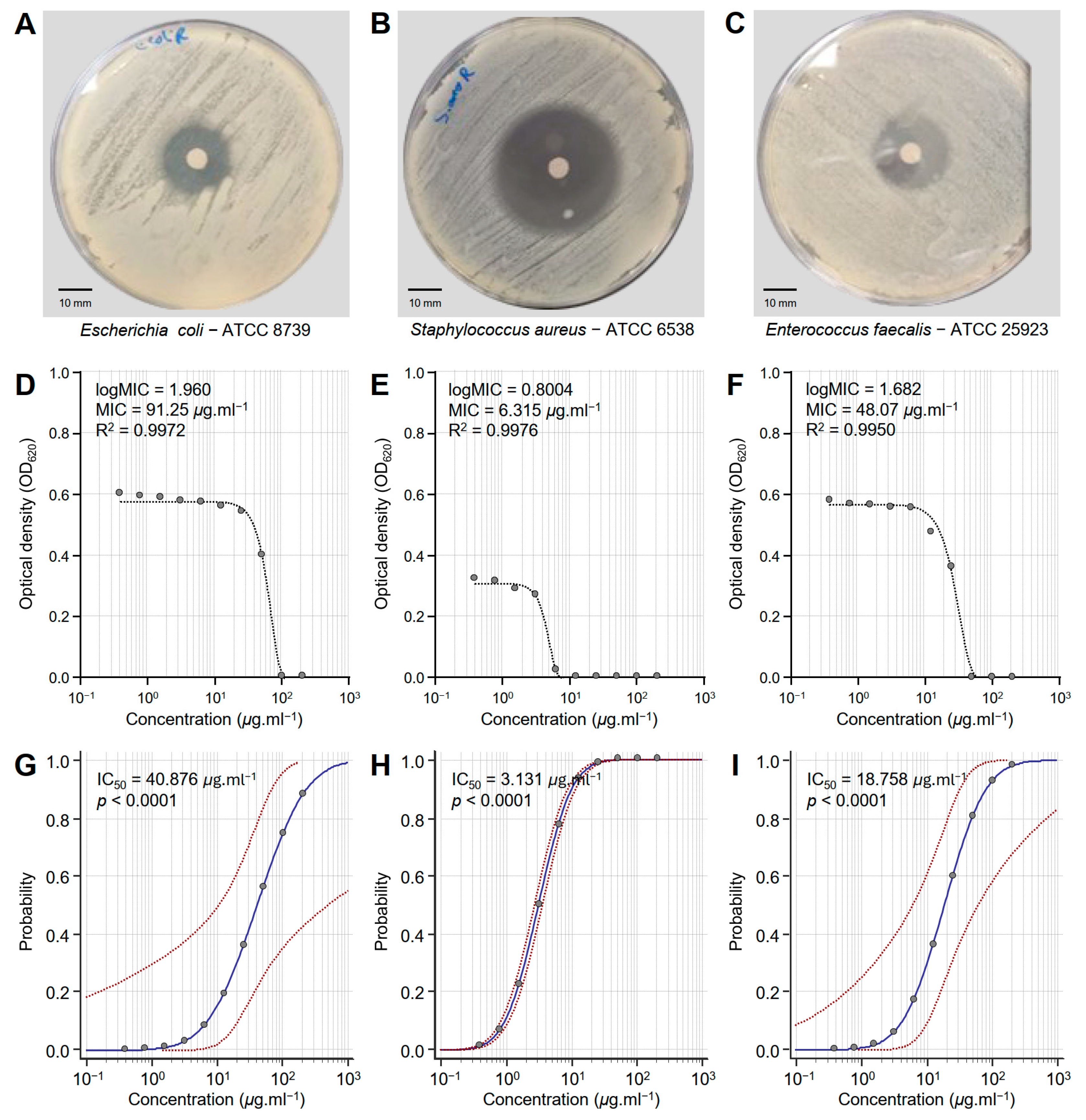
The susceptibility of three multidrug-resistant human pathogenic bacteria to different concentrations of the Monascus red dye (RD) extract was tested in vitro (Figure 2). RD extract showed strong antibacterial activity against E. coli—ATCC 8739 (Figure 2A), S. aureus—ATCC 6538 (Figure 2B), and E. faecalis—ATCC 25923 (Figure 2C). However, S. aureus was more susceptible to RD extracts than the other bacterial strains.
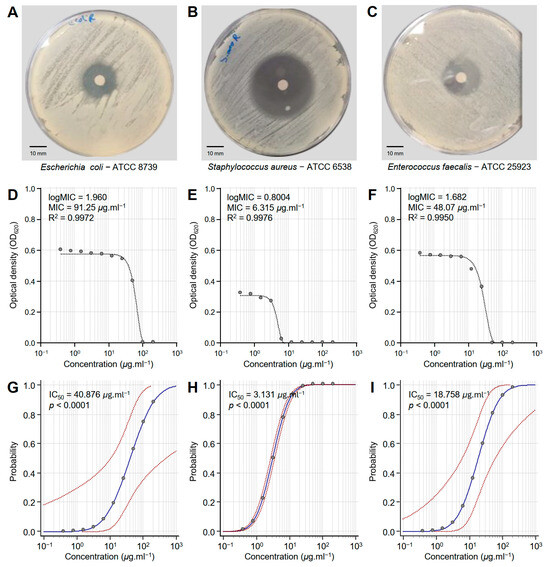
Figure 2.
In vitro antibacterial activity of Monascus red dye (RD) extracts against three multidrug-resistant human pathogenic bacteria. (A–C) Disc diffusion method of the RD extract (200 μg·mL−1) against Escherichia coli—ATCC 8739, Staphylococcus aureus—ATCC 6538, and Enterococcus faecalis—ATCC 25923, respectively. (D–F) Susceptibility analysis and minimum inhibitory concentrations (MICs) of RD extract against E. coli, S. aureus, and E. faecalis, respectively. Different concentrations of the RD extract were prepared in DMSO (200, 100, 50, 25, 12.5, 6.25, 3.125, 1.562, 0.781, and 0.390 μg·mL−1). (G–I) Probit regression (dose–response analysis) of RD extract against E. coli, S. aureus, and E. faecalis, respectively. Gray dots present the means of three replicates of each concentration. Blue solid lines represent the probit regression lines, whereas red dashed lines edge represent the 95% confidence intervals for the estimated regression. Probit-associated half-maximal inhibitory concentrations (IC50; μg·L−1), 95% confidence intervals, and overall model fit are listed in Table 1. For RD extract, the IC50 against E. coli ATCC8739 was 40.876 μg·mL−1 (95% CI: 10.632–491.037 μg·mL−1), against S. aureus ATCC 6538 it was 3.131 μg·mL−1 (95% CI: 2.729–3.592 μg·mL−1), and against E. faecalis ATCC 25923 it was 18.758 μg·mL−1 (95% CI: 6.201–61.248 μg·mL−1).
Susceptibility analysis showed that the minimum inhibitory concentration (MIC) of RD extract that was required to inhibit E. coli (logMIC = 1.960, MIC = 91.25 μg·mL−1, and R2 = 0.9972) was relatively high compared to other bacterial strains (Figure 2D). However, the dose–response curve of S. aureus demonstrated the highest sensitivity with an MIC of 6.315 μg·mL−1 (logMIC = 0.8004 and R2 = 0.9976) (Figure 2E). On the other hand, E. faecalis exhibited intermediate sensitivity to RD extracts with an MIC of 48.07 μg·mL−1 (logMIC = 1.682 and R2 = 0.9950) (Figure 2F). It is worth mentioning that all models were fitted with high R2 values (more than 0.99), highlighting the strong fit of the model and confirming the reliability of the predicted MIC.
Moreover, probit analysis showed the probability of RD extract to inhibit E. coli (Figure 2G), S. aureus (Figure 2H), and E. faecalis (Figure 2I). Briefly, E. coli showed a steep response curve, indicating that higher concentrations are required for effective inhibition (IC50 = 40.876, p < 0.0001), suggesting reduced sensitivity (Table 1 and Figure 2G). On the other hand, S. aureus exhibited a sharper transition and a more centered curve at lower concentrations (IC50 = 3.131, p < 0.0001), reflecting higher sensitivity (Table 1 and Figure 2H). Similarly, E. faecalis exhibited an intermediate sensitivity to RD extract, with the response curve shifted slightly towards higher concentrations (IC50 = 18.758, p < 0.0001) compared to S. aureus but relatively lower than E. coli (Table 1 and Figure 2I).
3.3. Antibacterial Activity of the BV and RD Extracts Against Traditional Antibiotics
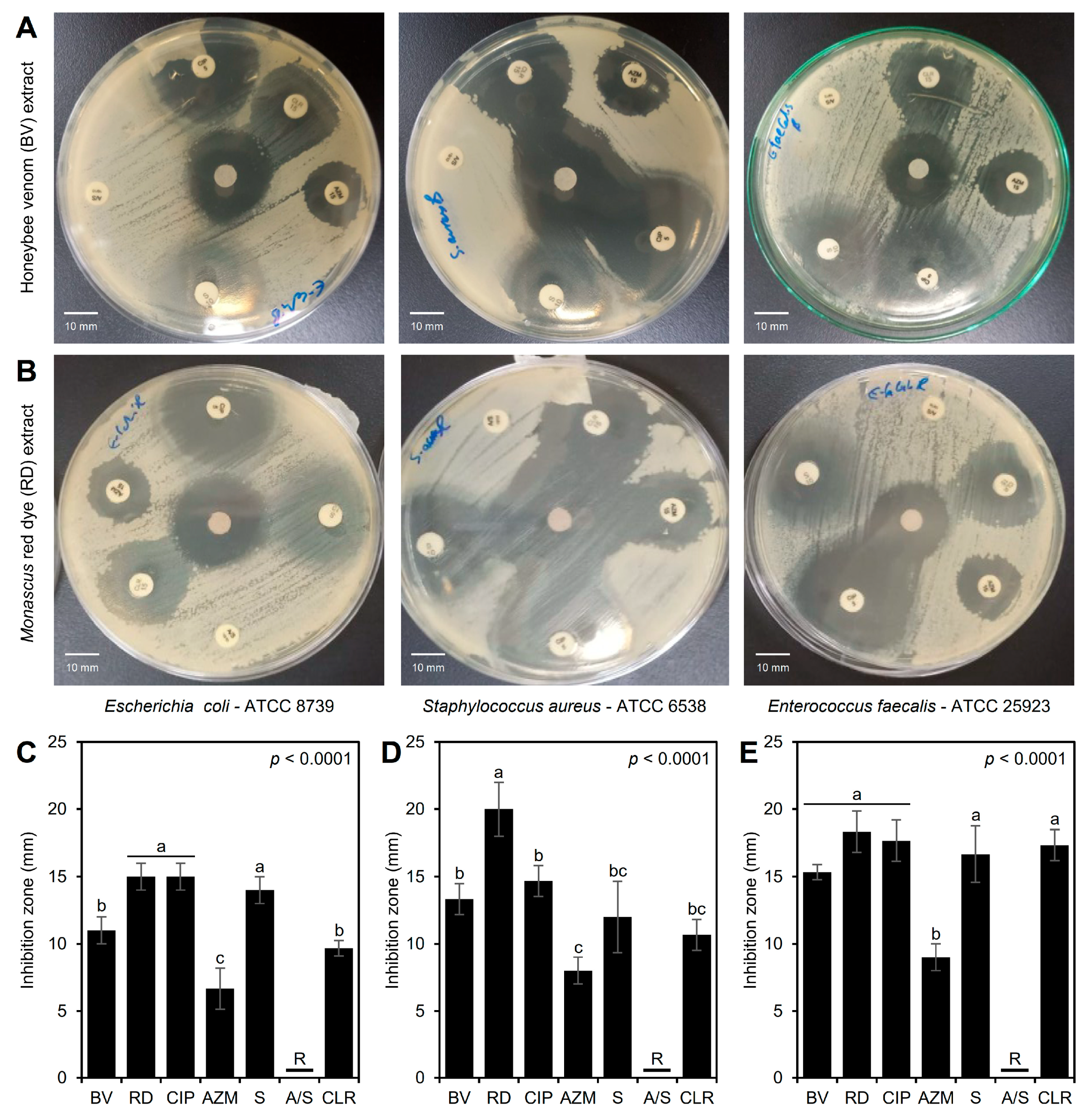
Although the three tested bacterial strains were resistant to ampicillin-sulbactam (A/S; 10/10 µg) and compared to conventional antibiotics, both BV (Figure 3A) and RD (Figure 3B) extracts exhibited significant antibacterial activity against the three tested bacterial strains. Briefly, BV and RD extracts exhibited noticeable inhibition zones across all three bacterial strains, with the largest inhibition zones observed for S. aureus, suggesting it is the most sensitive strain, whereas both E. coli and E. faecalis exhibited smaller inhibition zones, indicating less sensitivity (Figure 3A,B).
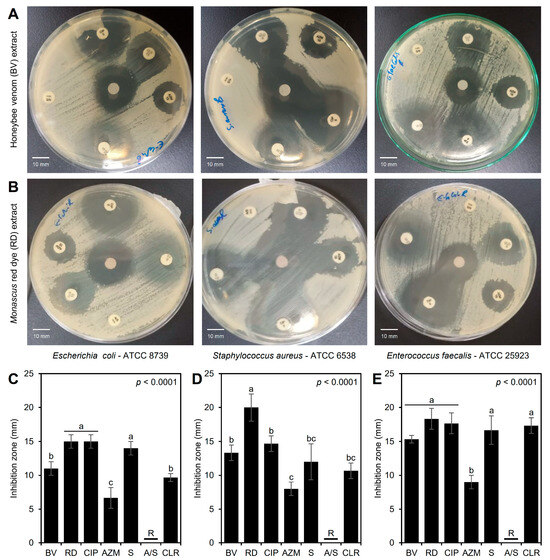
Figure 3.
In vitro antibacterial activity of honeybee venom (BV) and Monascus red dye (RD) extracts against multidrug-resistant human pathogenic bacteria in comparison with traditional antibiotics. (A,B) Disc diffusion method of the BV and RD extract (200 μg·mL−1) compared with the traditional antibiotics against Escherichia coli—ATCC 8739, Staphylococcus aureus—ATCC 6538, and Enterococcus faecalis—ATCC 25923, respectively, at 24 h post-incubation (hpi) at 37 °C. (C–E) Inhibition zones (mm) of BV and RD extract (200 μg·mL−1) compared with traditional antibiotics against E. coli, S. aureus, and E. faecalis, respectively. Bars and whiskers represent the means and standard deviations (Means ± SDs) of three biological replicates. Different letters signify statistically significant differences between treatments using Tukey’s HSD (p < 0.05). CIP: ciprofloxacin (5 µg), AZM: azithromycin (15 µg), S: streptomycin (10 µg), A/S: ampicillin-sulbactam (10/10 µg), and CLR: clarithromycin (15 µg).
In agreement with these visual observations, RD extract (200 μg·mL−1) showed the highest inhibition zones against E. coli (15.0 ± 1.0 mm) when directly compared with standard antibiotics, and was comparable to the most effective traditional antibiotic, ciprofloxacin (5 µg), with no significant differences between them, and followed by BV extract (11.0 ± 1.0 mm) (Figure 3C). On the other hand, RD extract proved to be more effective against S. aureus (inhibition zone = 20.0 ± 2.0 mm) than BV extract (13.3 ± 1.2 mm) and all other antibiotics tested (ranged from 8.0 ± 1.0 to 14.7 ± 1.2 mm) (Figure 3D). Additionally, both extracts (BV and RD) showed wide inhibition zones against E. faecalis (15.3 ± 0.6 and 18.3 ± 1.5 mm, respectively) similar to those caused by traditional antibiotics, and even were better than azithromycin (AZM; 15 µg) (p < 0.0001) (Figure 3E). These differences in the inhibition zones suggest the variability in antimicrobial efficacy between the two extracts and their strain-specific effectiveness.
3.4. Effects of BV and RD Extracts on Cellular Morphology of Tested Strains
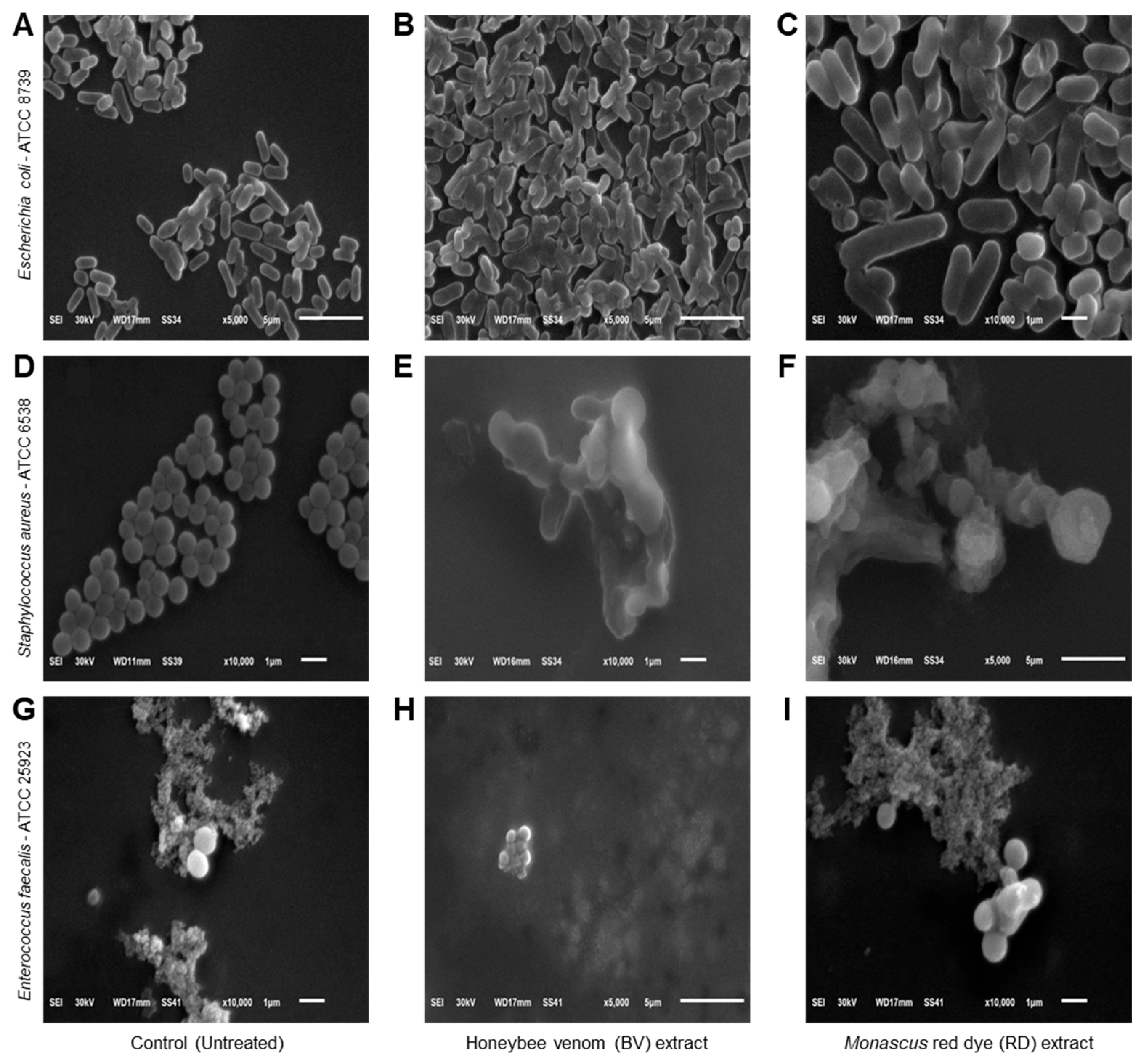
Scanning electron microscopy (SEM) was used to examine the morphological changes in bacterial cells exposed to BV and RD extracts at 200 μg·mL−1 compared to untreated controls. Control E. coli cells displayed typical rod-shaped morphology with intact, smooth surfaces (Figure 4A). In contrast, extract-treated E. coli cells showed severe structural damage, characterized by wrinkled and shriveled surfaces with visible perforations and indentations (Figure 4B,C). Some cells appeared hollow with depleted cellular contents. Control S. aureus exhibited characteristic spherical cells in grape-like clusters with uniform, smooth surfaces (Figure 4D). Following exposure to BV (Figure 4E) and RD (Figure 4F) extracts, most cells showed significant morphological alterations including distortion, enlargement, and surface irregularities with visible holes and indentations. A small subset of cells maintained their original morphology with intact surfaces. Untreated E. faecalis cells displayed their typical ovoid shape with smooth surfaces, predominantly appearing in diplococcal arrangements (Figure 4G). Treatment with the extracts induced substantial morphological changes, resulting in cell shrinkage and surface damage characterized by multiple small perforations and indentations (Figure 4H,I).
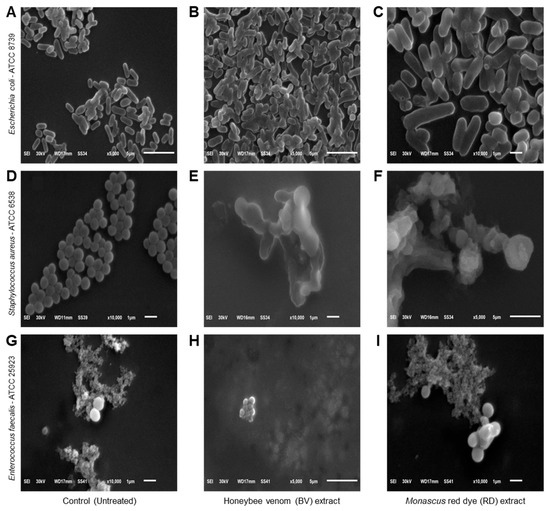
Figure 4.
Effects of honeybee venom (BV) and Monascus red dye (RD) extracts on cellular morphology of three multidrug-resistant human pathogenic bacteria. (A–C) Scanning electron microscopy (SEM) micrographs illustrating the cytomorphology of E. coli before the treatment (control), after treatment with the BV extract, and after treatment with the RD extract, respectively. (D–F) SEM-based micrographs of S. aureus before the treatment (control), after treatment with the BV extract, and after treatment with the RD extract, respectively. (G–I) SEM-based micrographs of E. faecalis before the treatment (control), after treatment with the BV extract, and after treatment with the RD extract, respectively. The micrographs are presented at varying magnification levels, denoted by the scale bars. Images (A,B,F,H) were acquired at 5000× magnification, while micrographs (C–E,G,I) were captured at higher magnification (10,000×).
3.5. Chemical Characterizations of the BV and RD Extracts
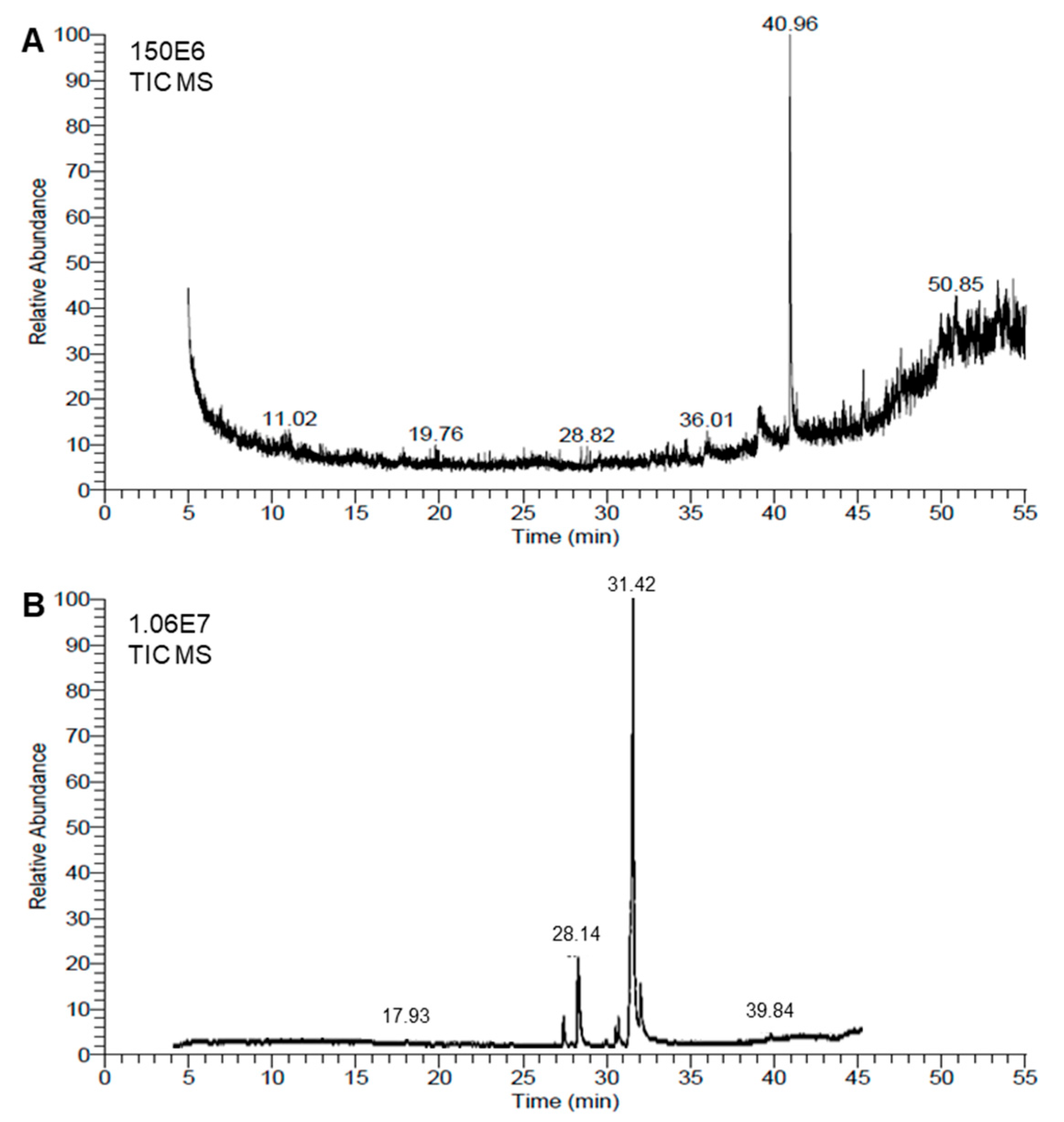
Chemical analyses of the honeybee venom (BV) extract and Monascus red dye (RD) extract were conducted using the GC–MS method. The resulting chromatograms are presented in Figure 5A,B, respectively. The GC–MS analysis showed that the BV extract was richer than the RD extract, since about 42 metabolites were identified in the BV extract (Table 2) compared with only 23 molecules in the red dye extract (Table 3).
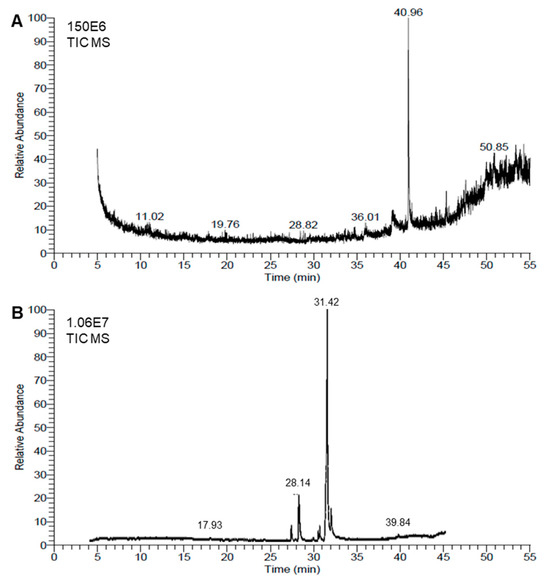
Figure 5.
Chemical analyses of honeybee venom (BV) and Monascus red dye (RD) extracts using gas chromatography–mass spectrometry (GC–MS) running in the full-scan mode. (A,B) Representative chromatograms of BV dimethyl sulfoxide (DMSO)-based extract and RD extract, respectively.

Table 2.
Retention times (min), peak area percentages (%), molecular formulas, and molecular masses of detected metabolites in the extract of honeybee venom (BV) using gas chromatography–mass spectrometry (GC–MS) running in full-scan mode.

Table 3.
Retention times (min), peak area percentages (%), molecular formulas, and molecular masses of detected metabolites in Monascus red dye (RD) extract using gas chromatography–mass spectrometry (GC–MS) running in full-scan mode.
The chemical composition of BV extract exhibited highly diverse structures, including complex polycyclic systems, porphyrins, steroids, and esters. The most abundant compound, 9-Octadecen-1-o (RT = 40.96), accounts for over a quarter of the total peak areas (28.35%), followed by Aralionine (RT = 49.94, 5.16%). Notable compounds include Dotriacontane (RT = 44.56, 1.55%), a long-chain hydrocarbon, and 1,2-Benzenedicarboxylic acid, di-isooctyl ester (RT = 45.34 and 4.45%), a widely used plasticizer. Other significant entries feature bioactive molecules like Astaxanthin (RT = 48.23, 1.6%) and structural components like Flavone 4′-oh,5-oh,7-di-o-glucoside (RT = 47.34, 3.43%) (Figure 5A and Table 2). This diversity highlights a mix of organic and metal–organic compounds with applications across pharmaceuticals, materials, and biochemistry fields.
On the other hand, among the 23 metabolites identified in the extract of RD, fatty acids and their derivatives were dominant, with oleic acid being the most abundant (RT = 31.42, 62.48%). Additionally, high peak-area percentages were noticed for n-hexadecanoic acid (RT = 28.14 and 12.76%), Octadecanoic acid (RT = 31.87 and 10.38%), and methyl esters such as Hexadecanoic acid, methyl ester (RT = 27.26 and 2.76%) (Table 2). Other notable compounds include cis-5,8,11,14,17-Eicosapentaenoic acid (0.87%), an omega-3 fatty acid, and 1,25-Dihydroxyvitamin D3 (0.3%), a bioactive vitamin derivative. Collectively, this highlights their applications in antimicrobial, biochemistry, and pharmaceutical fields (Figure 5B and Table 3).
4. Discussion
Antimicrobial resistance (AMR) represents a critical global health challenge, characterized by microorganisms’ ability to survive exposure to antimicrobial agents, particularly antibiotics. While AMR occurs naturally, its acceleration has been driven by inappropriate antibiotic use [1]. The magnitude of this crisis is reflected in recent statistics: AMR caused approximately 1.27 million deaths in 2019, with projections indicating potential annual casualties exceeding 10 million by 2050 [2]. The World Health Organization (WHO) has emphasized the risk of AMR developing into a gradual pandemic without significant changes in antibiotic stewardship [39]. This situation has intensified research into natural antimicrobial alternatives [24], with particular attention to honeybee venom (BV) and Monascus red dye (RD) as potential therapeutic agents against pathogens.
Chemical characterization of BV through GC–MS analysis revealed several bioactive compounds with therapeutic potential. Key identified molecules included astaxanthin, hycanthone, and fucoxanthin, which demonstrate broad-spectrum antimicrobial activity alongside antiviral, anti-aging, and anticancer properties. These compounds have already found applications in commercial therapeutic products [40]. Additional bioactive components identified in BV, such as tetraneurin-A-diol and dotriacontane, are documented for their antimicrobial and antiviral efficacy [41,42]. The chemical profile of BV showed a distinct influence from the bees’ citrus-based diet, evidenced by the presence of citrus-associated compounds including flavone 4′-OH,5-OH,7-dI-O-Glucoside, dotriacontane, and aralionine [43].
The main compound in BV, melittin, is capable of penetrating the peptidoglycan layer of bacterial cell walls, which may explain its strong antimicrobial activity, particularly against Gram-positive bacteria like E. faecalis [44]. BV melittin exhibits its antibacterial activity primarily through membrane-targeted mechanisms. The positively charged peptides strongly interact with the negatively charged membrane phospholipids, leading to membrane destabilization and eventual rupture. This interaction results in the formation of transmembrane pores, compromising bacterial cell integrity [45].
In parallel, the fatty acids present in RD extract demonstrate multiple mechanisms of antimicrobial action. They primarily target cellular bioenergetics by disrupting energy production pathways and interfering with essential enzyme activities and nutrient transport systems. At the membrane level, these fatty acids interact with bacterial phospholipids to form lipid micelle aggregates, which sequester membrane components and severely compromise membrane integrity. This leads to the collapse of the proton gradient, resulting in critical energy losses and eventual cell death [46,47].
Similarly, GC–MS analysis of RD revealed a range of bioactive molecules, including aspidospermidin-17-ol, 1-acetyl-16-methoxy; octanoic acid; hexadecanoic acid methyl ester; and cyclopentaneundecanoic acid. These compounds are known for their broad-spectrum antimicrobial properties and are also recognized for their antiviral, anti-inflammatory, immunomodulatory, and anticancer activities [48,49]. The analysis also indicated a high concentration of free fatty acids, particularly oleic acid, which constituted 62% of the RD extract’s chemical composition. Free fatty acids are well known for their antibacterial properties and are used by organisms to defend against pathogenic bacteria [46].
Our GC–MS analysis identified 23 metabolites in the extract of RD. Among the metabolites, fatty acids and their derivatives were dominant, with oleic acid being the most abundant compound. It was reported previously that one of the most distinguished biological activities of fatty acids (FAs) is their ability to kill or inhibit the growth of microorganisms [50]. However, the direct contributions of fatty acids to antimicrobial activity are poorly understood. It was suggested that the most common target of action is the cell membrane [50,51,52]. For instance, fatty acids negatively affected bacterial viability and biofilm production [51] and disrupted the bacterial membranes in S. aureus [52]. This aligns with our SEM observations of membrane damage. Moreover, FAs may increase permeability and succeeding cell lysis, disrupting the electron transport chain and inhibiting enzymatic activity and nutrient intake [50]. Moreover, they can disrupt the microorganisms’ metabolic pathways, inhibit DNA/RNA replication, and affect the expression of virulence genes [50]. Further studies are required to better explain the potential antibacterial role(s) of fatty acids against MDR human pathogenic bacteria.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) results demonstrated strong antibacterial activity of BV and RD extracts against three bacterial strains, with particularly effective results against S. aureus. Previous studies have reported similar findings for BV, especially against E. coli and S. aureus. For example, Maitip et al. [53] observed significant antimicrobial effects of BV against these pathogens, and Bakhiet et al. [54] further confirmed the antibacterial properties of BV against E. coli and S. aureus. In the current study, we initially tested serial concentrations of the BV and RD extracts (up to 200 μg·mL−1) against the three bacterial strains in Mueller–Hinton broth; however, we decided to limit the concentration to the highest concentration (200 μg·mL−1), since it showed the highest inhibition based on preliminary experiments. We believe that testing higher concentrations could help identify the plateau of antibacterial efficacy and provide a clearer picture of the full dose–response curve. It is worth mentioning that although the IC50 values presented (e.g., S. aureus: 1.183, and 3.131 µg.mL−1 for BV and RD, respectively) are promising in vitro; however, the translation of these concentrations to in vivo settings requires further investigation. Achieving these concentrations in vivo depends on factors such as bioavailability, pharmacokinetics, and the route of administration. Further investigations are required to explore the effective dose and the potential for these compounds to reach therapeutic levels in clinical settings.
Data on the antibacterial activity of Monascus red dye against the specific bacterial strains tested here are limited; however, other studies report similar antibacterial effects of RD, especially against B. subtilis ATCC 6633 and E. coli MG1655 [55]. Chaudhary et al. [56] also found that RD exhibited antibacterial activity against B. cereus and E. coli.
To assess the potential of BV and RD as alternatives to traditional antibiotics, their antibacterial activities were compared to five commonly used antibiotics using the disc diffusion method. The bacterial strains were sensitive to four of the antibiotics but resistant to ampicillin-sulbactam. RD showed the largest inhibition zone against S. aureus (20 ± 0.22 mm), followed by a notable inhibition zone against E. faecalis (18 ± 0.15 mm). BV also exhibited significant antibacterial effects, with the largest inhibition zone observed against E. faecalis (15 ± 0.22 mm), followed by S. aureus (13 ± 0.15 mm). Among the antibiotics, ciprofloxacin (CIP) produced the largest inhibition zones, while azithromycin (AZM) had the smallest zones. These findings are consistent with previous studies [57,58]. Overall, both BV and RD exhibited potent antibacterial effects, comparable to several antibiotics tested.
Microscopic examination of the bacterial cells provided additional insight into the antibacterial mechanisms of BV and RD. Scanning electron microscopy (SEM) revealed significant structural changes in the bacterial cells treated with these extracts. Treated strains showed rough, shrunken, and wrinkled surfaces compared to the smooth, well-defined surfaces of untreated control cells. These results suggest that BV and RD disrupt the bacterial cell membrane, increasing permeability and leading to ATP loss.
We believe that evaluating the cytotoxicity of BV and RD extracts is crucial before proposing them as potential therapeutic agents. Although cellular toxicity and biological activities of BV were well-reported previously [59,60,61,62], our knowledge about the cytotoxicity of Monascus fermentation products is still limited [63]. For example, BV induced cell death in normal human lymphocytes and HL-60 cells in a time-dependent manner till 24 h post-treatment (hpt); however, these cytotoxic effects ended thereafter, which may have been due to the half-life of BV [59]. Likewise, BV showed cytotoxic, genotoxic, and mutagenic potentials to HepG2 cells at 3 hpt; however, low concentrations of 0.1, 0.05, and 0.01 μg·mL−1 were not cytotoxic [60]. Similarly, BV application exhibited significant antiproliferative and cytotoxic effects on several tumorigenic cell lines and nontumorigenic cells [61]. Likewise, it displayed antibacterial activities against MDR human pathogens such as Extended Spectrum Beta-Lactamases producing E. coli and vancomycin-resistant Enterococcus faecium [61]. It is worth mentioning that detoxification of BV via hydrolyzation of melittin, the main metabolite of BV, significantly decreased its cytotoxicity and allergenic activity in MCF 10A and RAW 264.7 cells [62].
On the other hand, it was reported previously that the presence of the secondary metabolite of Monascus species, citrinin (CTN), in fermentation products, is a potential threat to public health. CTN was detected in lipid extracts of Monascus species but was not found in aqueous extracts [63]. Accordingly, when human embryonic kidney cells (HEK293) were incubated for 72 h with Monascus extracts, the concentrations causing 50% cell death by all lipid extracts were in the range of 1.8–4.7 mg.mL−1, whereas aqueous extracts showed a lower cytotoxicity [63]. Collectively, these findings suggest that aqueous extracts of BV and RD have promising antibacterial properties and might be potential alternatives to conventional antibiotics. However, future investigations are required to assess the cytotoxicity of both extracts and to determine safe and effective dosage ranges.
5. Conclusions
In response to the global challenge of antimicrobial resistance (AMR) and declining antibiotic efficacy, this study investigated novel antimicrobial agents from natural sources. We evaluated two natural products: honeybee venom (BV) collected from honeybee colonies at the Faculty of Agriculture apiary, Tanta University, and red dye (RD) extracted from Monascus purpureus. GC–MS analysis of both extracts revealed diverse bioactive metabolites with documented biological activities, particularly antimicrobial properties. Both extracts demonstrated robust antibacterial activity against all tested bacterial strains, with MIC and MBC values indicating potent antimicrobial effects. Notably, both BV and RD produced larger inhibition zones compared to conventional antibiotics, suggesting their potential as therapeutic alternatives. Nevertheless, while the current study focused on MDR bacterial cultures to establish baseline antimicrobial activity and mechanisms of BV and RD, evaluating their efficacy and performance in complex systems such as mixed microbial communities or biofilms in biofilms is required, which is more representative of clinical and environmental settings. Additionally, further studies on evaluating the synergistic effects of RD extracts with existing traditional antibiotics could enhance their applicability against MDR pathogens. Finally, further pharmacological studies are required to elucidate their precise mechanisms of action, evaluate potential toxicity and side effects, determine optimal dosing regimens, develop standardized pharmaceutical formulations, and assess stability and shelf-life parameters.
Author Contributions
Conceptualization, I.I.T. and Y.N.; data curation, I.I.T., A.H.M. and Y.N.; formal analysis, I.I.T., Y.N., A.E.M. and E.H.E.-B.; funding acquisition, Y.S.A.M. and A.H.M.; investigation, I.I.T., A.E.M., A.M.A., I.M. and E.H.E.-B.; methodology, I.I.T., A.E.M., A.M.A., I.M. and E.H.E.-B.; resources, I.I.T., Y.S.A.M., A.H.M., Y.N., A.M.A. and I.M.; software, Y.S.A.M., Y.N. and A.E.M.; validation, I.I.T., A.E.M., A.M.A., I.M. and E.H.E.-B.; visualization, Y.N.; writing—original draft, I.I.T. and Y.N.; writing—review and editing, I.I.T., Y.S.A.M., A.H.M., Y.N., A.E.M., A.M.A., I.M. and E.H.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Deanship of Research and Graduate Studies at King Khalid University through a Large Research Project under grant number RGP2/249/45.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this research through a Large Research Project under grant number RGP2/249/45. We also gratefully acknowledge the members of our laboratories for their valuable suggestions and comments concerning this study. Additionally, I.I.T. and Y.N. would like to acknowledge the Graduate Student and Research Affairs Sector of Tanta University, Egypt.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Maarouf, L.; Amin, M.; Evans, B.A.; Abouelfetouh, A. Knowledge, Attitudes and Behaviour of Egyptians towards Antibiotic Use in the Community: Can We Do Better? Antimicrob. Resist. Infect. Control 2023, 12, 50. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and Socioeconomic Factors Contributing to Global Antimicrobial Resistance: A Univariate and Multivariable Analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in Understanding Antimicrobial Resistance across Domesticated Animal, Human, and Environmental Systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz Abdelmoneim, S.; Mohamed Ghazy, R.; Anwar Sultan, E.; Hassaan, M.A.; Anwar Mahgoub, M. Antimicrobial Resistance Burden Pre and Post-COVID-19 Pandemic with Mapping the Multidrug Resistance in Egypt: A Comparative Cross-Sectional Study. Sci. Rep. 2024, 14, 7176. [Google Scholar] [CrossRef]
- Teiba, I.I.; El-Bilawy, E.H.; Elsheery, N.I.; Rastogi, A. Microbial Allies in Agriculture: Harnessing Plant Growth-Promoting Microorganisms as Guardians against Biotic and Abiotic Stresses. Horticulturae 2024, 10, 12. [Google Scholar] [CrossRef]
- Prosser, J.I.; Bohannan, B.J.M.; Curtis, T.P.; Ellis, R.J.; Firestone, M.K.; Freckleton, R.P.; Green, J.L.; Green, L.E.; Killham, K.; Lennon, J.J.; et al. The Role of Ecological Theory in Microbial Ecology. Nat. Rev. Microbiol. 2007, 5, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Cook, H.A.; Furuya, E.Y.; Bhat, M.; Lee, M.-H.; Vavagiakis, P.; Visintainer, P.; Vasquez, G.; Larson, E.; Lowy, F.D. Staphylococcus aureus in the Community: Colonization versus Infection. PLoS ONE 2009, 4, e6708. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus Infection; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tong, S.Y.C.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel Staphylococcal Species That Form Part of a Staphylococcus aureus-Related Complex: The Non-Pigmented Staphylococcus argenteus Sp. Nov. and the Non-Human Primate-Associated Staphylococcus schweitzeri Sp. Nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef]
- Boucher, H.W.; Corey, G.R. Epidemiology of Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, S344–S349. [Google Scholar] [CrossRef] [PubMed]
- Gambushe, S.M.; Zishiri, O.T.; El Zowalaty, M.E. Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect. Drug Resist. 2022, 15, 4645–4673. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia Coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Ameer, M.A.; Wasey, A.; Salen, P. Escherichia coli (e Coli 0157 H7); StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, 10-1128. [Google Scholar] [CrossRef]
- Repoila, F.; Le Bohec, F.; Guérin, C.; Lacoux, C.; Tiwari, S.; Jaiswal, A.K.; Santana, M.P.; Kennedy, S.P.; Quinquis, B.; Rainteau, D.; et al. Adaptation of the Gut Pathobiont Enterococcus faecalis to Deoxycholate and Taurocholate Bile Acids. Sci. Rep. 2022, 12, 8485. [Google Scholar] [CrossRef]
- Comerlato, C.B.; de Resende, M.C.C.; Caierão, J.; d’Azevedo, P.A. Presence of Virulence Factors in Enterococcus faecalis and Enterococcus faecium Susceptible and Resistant to Vancomycin. Mem. Inst. Oswaldo Cruz 2013, 108, 590–595. [Google Scholar] [CrossRef]
- Hancock, L.E.; Murray, B.E.; Sillanpää, J. Enterococcal Cell Wall Components and Structures; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Boccella, M.; Santella, B.; Pagliano, P.; De Filippis, A.; Casolaro, V.; Galdiero, M.; Borrelli, A.; Capunzo, M.; Boccia, G.; Franci, G. Prevalence and Antimicrobial Resistance of Enterococcus Species: A Retrospective Cohort Study in Italy. Antibiotics 2021, 10, 1552. [Google Scholar] [CrossRef]
- El Basuini, M.F.; El-Bilawy, E.H.; Kari, Z.A.; Raza, S.H.A.; Tapingkae, W.; Van Doan, H.; Dawood, M.A.O. Pharmacotherapeutic Potential of Astaxanthin: Human and Animal Targeting Roles—A Review. Ann. Anim. Sci. 2022, 22, 829–838. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic Application of Anti-Arthritis, Pain-Releasing, and Anti-Cancer Effects of Bee Venom and Its Constituent Compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee Venom Therapy: Potential Mechanisms and Therapeutic Applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.J.; Mendez-Lnocencio, J.I.; Omidvar, B.; Omidvar, J.; Santilli, J.; Nielsen, H.S.J.; Pavot, A.P.; Richert, J.R.; Bellanti, J.A. A Phase I Study of the Safety of Honeybee Venom Extract as a Possible Treatment for Patients with Progressive Forms of Multiple Sclerosis. Allergy Asthma Proc. 2005, 26, 470–476. [Google Scholar]
- Kang, D.W.; Choi, J.G.; Kim, J.; Park, J.B.; Lee, J.H.; Kim, H.W. Bee Venom Reduces Burn-Induced Pain via the Suppression of Peripheral and Central Substance P Expression in Mice. J. Vet. Sci. 2021, 22, e9. [Google Scholar] [CrossRef]
- Haque, M.A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of Bakery Waste for Biocolorant and Enzyme Production by Monascus Purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef]
- Kalaivani, M.; Sabitha, R.; Kalaiselvan, V.; Rajasekaran, A. Health Benefits and Clinical Impact of Major Nutrient, Red Yeast Rice: A Review. Food Bioprocess Technol. 2010, 3, 333–339. [Google Scholar] [CrossRef]
- Kaur, M.; Goel, M.; Mishra, R.C.; Lahane, V.; Yadav, A.K.; Barrow, C.J. Characterization of the Red Biochromes Produced by the Endophytic Fungus Monascus Purpureus CPEF02 with Antimicrobial and Antioxidant Activities. Fermentation 2023, 9, 328. [Google Scholar] [CrossRef]
- Embaby, A.M.; Hussein, M.N.; Hussein, A. Monascus Orange and Red Pigments Production by Monascus Purpureus ATCC16436 through Co-Solid State Fermentation of Corn Cob and Glycerol: An Eco-Friendly Environmental Low Cost Approach. PLoS ONE 2018, 13, e0207755. [Google Scholar] [CrossRef] [PubMed]
- Srianta, I.; Kusdiyantini, E.; Zubaidah, E.; Ristiarini, S.; Nugerahani, I.; Alvin, A.; Iswanto, N.; Zhang, B.-B. Utilization of Agro-Industrial by-Products in Monascus Fermentation: A Review. Bioresour. Bioprocess. 2021, 8, 129. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-State Fermentation for the Production of Monascus Pigments from Jackfruit Seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef]
- Bento de Carvalho, T.; Barbosa, J.B.; Teixeira, P. Assessing Antimicrobial Efficacy on Plastics and Other Non-Porous Surfaces: A Closer Look at Studies Using the ISO 22196:2011 Standard. Biology 2024, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Pearson, J. Susceptibility Testing: Accurate and Reproducible Minimum Inhibitory Concentration (MIC) and Non-inhibitory Concentration (NIC) Values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Surendra, N.S.; Jayaram, G.N.; Reddy, M.S. Antimicrobial Activity of Crude Venom Extracts in Honeybees (Apis Cerana, Apis Dorsata, Apis Florea) Tested against Selected Pathogens. African J. Microbiol. Res. 2011, 5, 2765–2772. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Mbuagbaw, L.; Mertz, D.; Burrows, L.L.; Bowdish, D.M.E.; Moja, L.; Wright, G.D.; Schünemann, H.J. Coronavirus Disease 2019 and Antimicrobial Resistance: Parallel and Interacting Health Emergencies. Clin. Infect. Dis. 2021, 72, 1657–1659. [Google Scholar] [CrossRef]
- Henderson, M.J.; Trychta, K.A.; Yang, S.-M.; Bäck, S.; Yasgar, A.; Wires, E.S.; Danchik, C.; Yan, X.; Yano, H.; Shi, L.; et al. A Target-Agnostic Screen Identifies Approved Drugs to Stabilize the Endoplasmic Reticulum-Resident Proteome. Cell Rep. 2021, 35, 109040. [Google Scholar] [CrossRef]
- Hu, J.-F.; Patel, R.; Li, B.; Garo, E.; Hough, G.W.; Goering, M.G.; Yoo, H.-D.; O’neil-Johnson, M.; Eldridge, G.R. Anti-HCV Bioactivity of Pseudoguaianolides from Parthenium Hispitum. J. Nat. Prod. 2007, 70, 604–607. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Omar, M.O.A.; Mahmoud, M.E. Phytochemical Analysis, Antioxidant Capacities, and in Vitro Biological Activities of the Extract of Seed Coat as by-Products of Pea. BMC Chem. 2023, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Izhar, M.P.; Hafeez, A.; Kushwaha, P. Simrah Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications. J. Clust. Sci. 2023, 34, 2257–2273. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Hwang, S.J.; Jang, C.H.; Chenoweth, P.J.; Pak, S.C. Effects of Bee Venom Treatment on Growth Performance of Young Pigs. Am. J. Chin. Med. 2009, 37, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Delgado, O.; Espinoza-Culupú, A.O.; López-López, E. Antimicrobial Activity of Apis Mellifera Bee Venom Collected in Northern Peru. Antibiotics 2023, 12, 779. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Katyal, P.; Kaur, J.; Bhatia, S.; Singh, S.; Poonia, A.K.; Puniya, A.K.; Raposo, A.; Yoo, S.; Han, H.; et al. Bioactive Activity and Safety Analysis of Monascus Red Biopigment. Food Biosci. 2024, 57, 103523. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Wei, C.; Yan, Q.; Sun, Y.-Z.; Yi, G.; Li, D.; Fu, W. Monascus Purpureus M-32 Improves Growth Performance, Immune Response, Intestinal Morphology, Microbiota and Disease Resistance in Litopenaeus vannamei. Aquaculture 2021, 530, 735947. [Google Scholar] [CrossRef]
- Obukhova, E.S.; Murzina, S.A. Mechanisms of the Antimicrobial Action of Fatty Acids: A Review. Appl. Biochem. Microbiol. 2024, 60, 1035–1043. [Google Scholar] [CrossRef]
- Stenz, L.; François, P.; Fischer, A.; Huyghe, A.; Tangomo, M.; Hernandez, D.; Cassat, J.; Linder, P.; Schrenzel, J. Impact of Oleic Acid (Cis-9-Octadecenoic Acid) on Bacterial Viability and Biofilm Production in Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 287, 149–155. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane Disruption by Antimicrobial Fatty Acids Releases Low-Molecular-Weight Proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef]
- Maitip, J.; Mookhploy, W.; Khorndork, S.; Chantawannakul, P. Comparative Study of Antimicrobial Properties of Bee Venom Extracts and Melittins of Honey Bees. Antibiotics 2021, 10, 1503. [Google Scholar] [CrossRef] [PubMed]
- K Bakhiet, E.; A M Hussien, H.; Elshehaby, M. Apis Mellifera Venom Inhibits Bacterial and Fungal Pathogens in Vitro. Pakistan J. Biol. Sci. 2022, 25, 875–884. [Google Scholar] [CrossRef]
- Husakova, M.; Orlandi, V.T.; Bolognese, F.; Branska, B.; Patakova, P. Screening Antibacterial Photodynamic Effect of Monascus Red Yeast Rice (Hong-Qu) and Mycelium Extracts. Curr. Microbiol. 2024, 81, 183. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Katyal, P.; Panwar, H.; Kaur, J.; Aluko, R.E.; Puniya, A.K.; Poonia, A.K. Antioxidative, Anti-Inflammatory, and Anticancer Properties of the Red Biopigment Extract from Monascus Purpureus (MTCC 369). J. Food Biochem. 2022, 46, e14249. [Google Scholar] [CrossRef]
- Tanuwidjaja, I.; Svečnjak, L.; Gugić, D.; Levanić, M.; Jurić, S.; Vinceković, M.; Mrkonjić Fuka, M. Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom. Molecules 2021, 26, 3049. [Google Scholar] [CrossRef] [PubMed]
- Čujová, S.; Bednárová, L.; Slaninová, J.; Straka, J.; Čeřovský, V. Interaction of a Novel Antimicrobial Peptide Isolated from the Venom of Solitary Bee Colletes Daviesanus with Phospholipid Vesicles and Escherichia coli Cells. J. Pept. Sci. 2014, 20, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, S.J.; Kim, B.M.; Kim, Y.J.; Woo, H.D.; Chung, H.W. Cytotoxicity of Honeybee (Apis mellifera) Venom in Normal Human Lymphocytes and HL-60 Cells. Chem. Biol. Interact. 2007, 169, 189–197. [Google Scholar] [CrossRef]
- Hoshina, M.M.; Marin-Morales, M.A. Anti-Genotoxicity and Anti-Mutagenicity of Apis mellifera Venom. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 762, 43–48. [Google Scholar] [CrossRef]
- Gülmez, Y.; Aydın, A.; Can, İ.; Tekin, Ş.; Cacan, E. Cellular Toxicity and Biological Activities of Honey Bee (Apis mellifera L.) Venom. Marmara Pharm. J. 2017, 21, 251–260. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.S.; Lee, K.S.; Seo, H.S.; Lee, C.Y.; Kim, K.K. Detoxification of Bee Venom Increases Its Anti-Inflammatory Activity and Decreases Its Cytotoxicity and Allergenic Activity. Appl. Biochem. Biotechnol. 2021, 193, 4068–4082. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Wu, T.S.; Su, M.C.; Ping Chung, C.; Yu, F.Y. Evaluation of Citrinin Occurrence and Cytotoxicity in Monascus Fermentation Products. J. Agric. Food Chem. 2004, 53, 170–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).