Antibacterial Potential of Honeybee Venom and Monascus purpureus Extracellular Metabolites Against Multidrug-Resistant Pathogenic Bacteria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Extraction of Honeybee Venom
2.2. Source and Extraction of Red Dye
2.3. Chemical Profiling of the Honeybee Venom and Red Dye Extracts
2.4. Antibacterial Activity
2.4.1. Bacterial Strains
2.4.2. Determination of Minimum Inhibitory Concentration (MIC) and the Half-Maximal Inhibitory Concentration (IC50)
2.4.3. Comparison of the Antibacterial Activity of the Extracts and Traditional Antibiotics
2.4.4. Cellular Structure of the Tested Bacterial Strains
2.5. Statistical Analysis
3. Results
3.1. Honeybee Venom (BV) Extract Exhibited Notable Antibacterial Activity Against MDR Human Pathogenic Bacteria
3.2. Monascus Red Dye (RD) Extract Exhibited Strong Antibacterial Activity Against MDR Human Pathogenic Bacteria
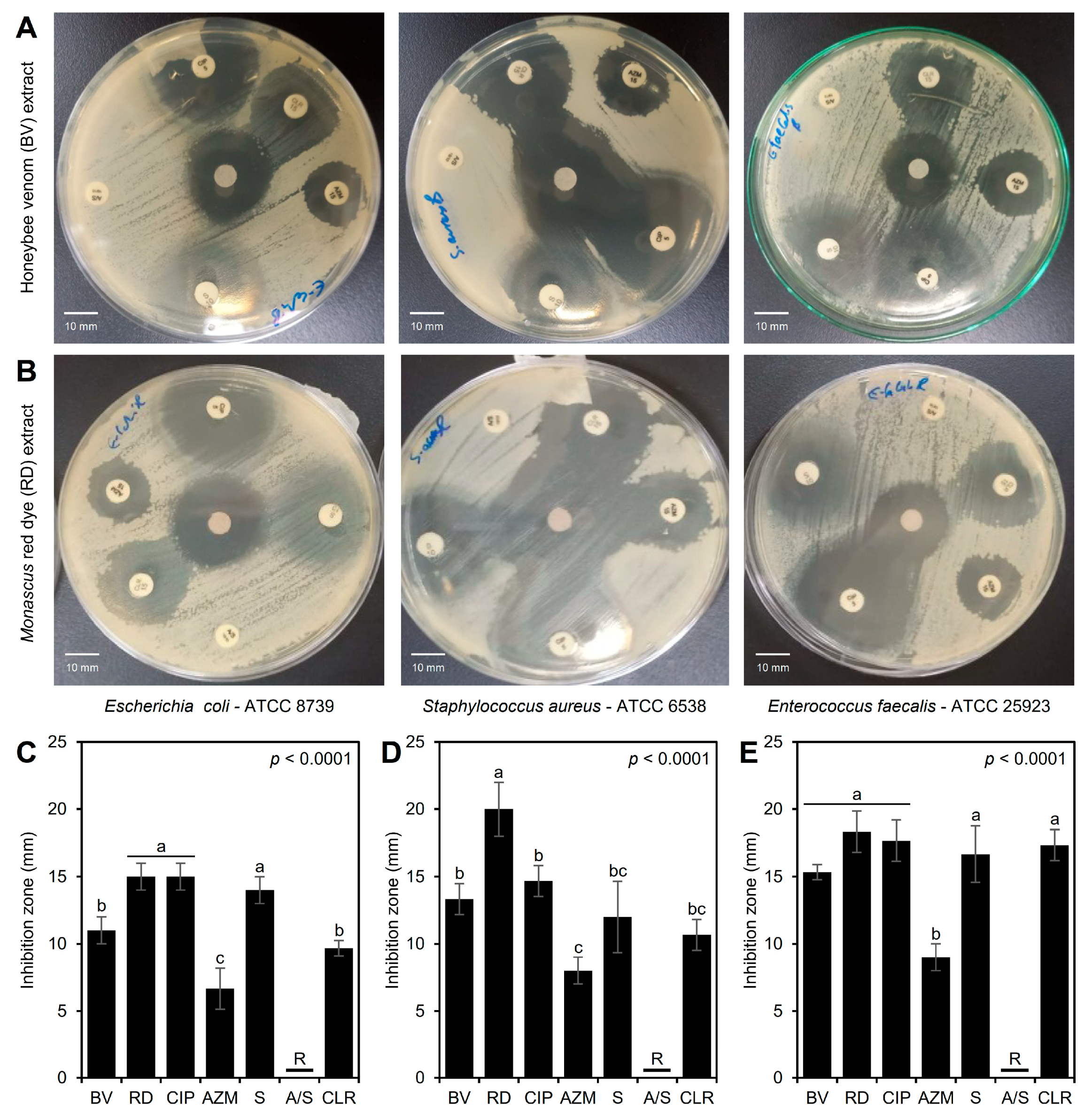
3.3. Antibacterial Activity of the BV and RD Extracts Against Traditional Antibiotics
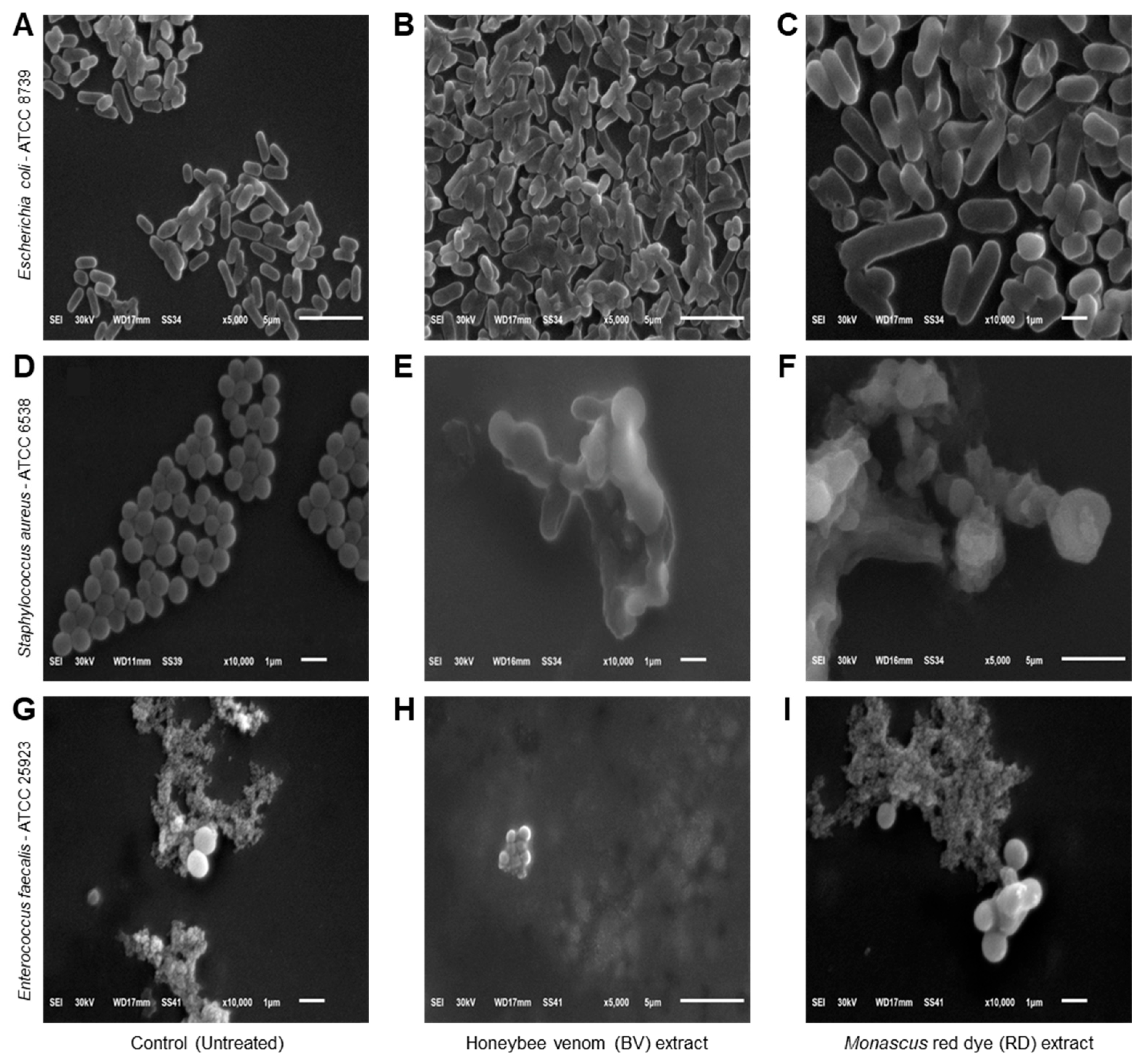
3.4. Effects of BV and RD Extracts on Cellular Morphology of Tested Strains
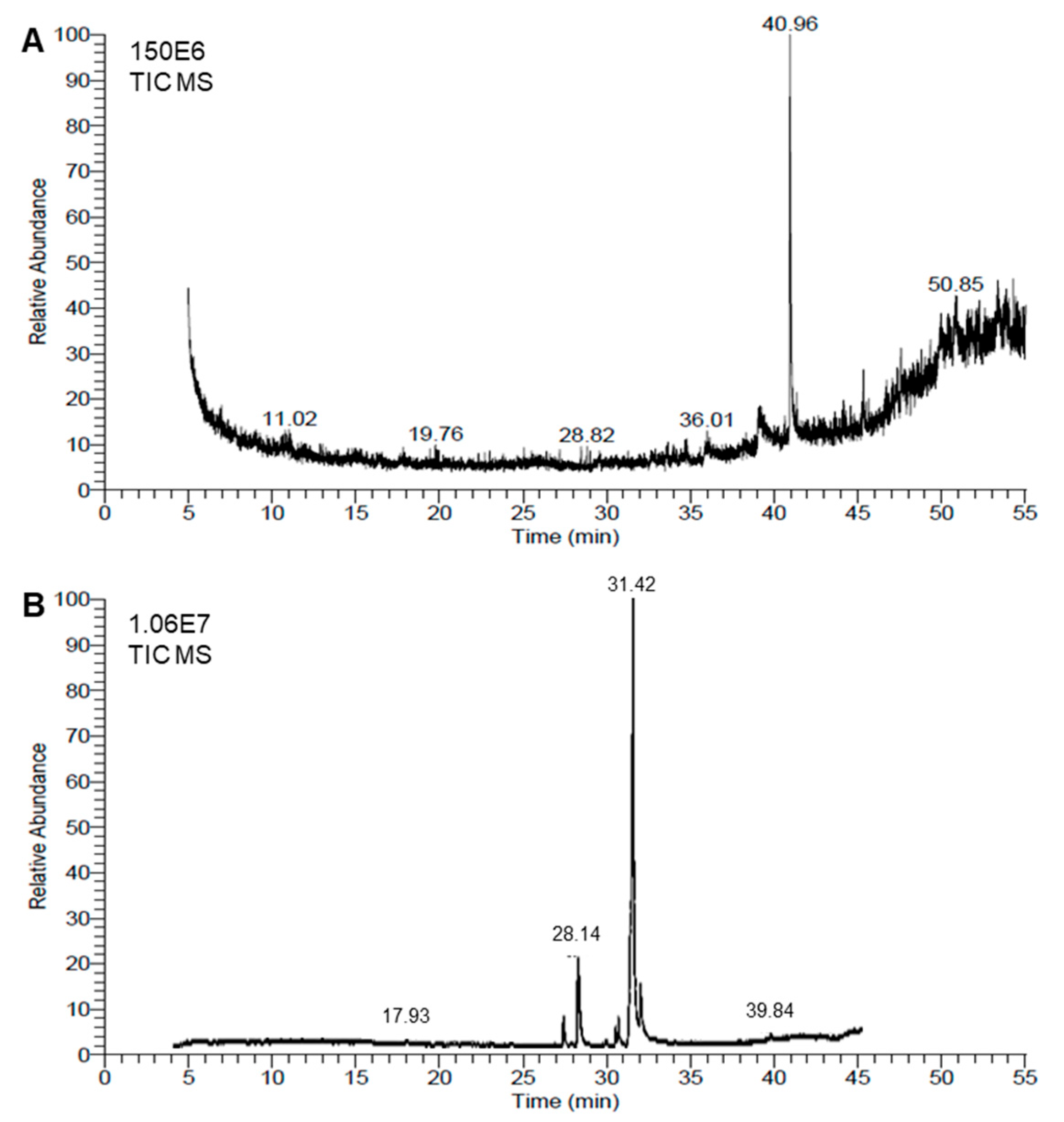
3.5. Chemical Characterizations of the BV and RD Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Maarouf, L.; Amin, M.; Evans, B.A.; Abouelfetouh, A. Knowledge, Attitudes and Behaviour of Egyptians towards Antibiotic Use in the Community: Can We Do Better? Antimicrob. Resist. Infect. Control 2023, 12, 50. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and Socioeconomic Factors Contributing to Global Antimicrobial Resistance: A Univariate and Multivariable Analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in Understanding Antimicrobial Resistance across Domesticated Animal, Human, and Environmental Systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz Abdelmoneim, S.; Mohamed Ghazy, R.; Anwar Sultan, E.; Hassaan, M.A.; Anwar Mahgoub, M. Antimicrobial Resistance Burden Pre and Post-COVID-19 Pandemic with Mapping the Multidrug Resistance in Egypt: A Comparative Cross-Sectional Study. Sci. Rep. 2024, 14, 7176. [Google Scholar] [CrossRef]
- Teiba, I.I.; El-Bilawy, E.H.; Elsheery, N.I.; Rastogi, A. Microbial Allies in Agriculture: Harnessing Plant Growth-Promoting Microorganisms as Guardians against Biotic and Abiotic Stresses. Horticulturae 2024, 10, 12. [Google Scholar] [CrossRef]
- Prosser, J.I.; Bohannan, B.J.M.; Curtis, T.P.; Ellis, R.J.; Firestone, M.K.; Freckleton, R.P.; Green, J.L.; Green, L.E.; Killham, K.; Lennon, J.J.; et al. The Role of Ecological Theory in Microbial Ecology. Nat. Rev. Microbiol. 2007, 5, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Cook, H.A.; Furuya, E.Y.; Bhat, M.; Lee, M.-H.; Vavagiakis, P.; Visintainer, P.; Vasquez, G.; Larson, E.; Lowy, F.D. Staphylococcus aureus in the Community: Colonization versus Infection. PLoS ONE 2009, 4, e6708. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus Infection; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tong, S.Y.C.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel Staphylococcal Species That Form Part of a Staphylococcus aureus-Related Complex: The Non-Pigmented Staphylococcus argenteus Sp. Nov. and the Non-Human Primate-Associated Staphylococcus schweitzeri Sp. Nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef]
- Boucher, H.W.; Corey, G.R. Epidemiology of Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, S344–S349. [Google Scholar] [CrossRef] [PubMed]
- Gambushe, S.M.; Zishiri, O.T.; El Zowalaty, M.E. Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect. Drug Resist. 2022, 15, 4645–4673. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia Coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Ameer, M.A.; Wasey, A.; Salen, P. Escherichia coli (e Coli 0157 H7); StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, 10-1128. [Google Scholar] [CrossRef]
- Repoila, F.; Le Bohec, F.; Guérin, C.; Lacoux, C.; Tiwari, S.; Jaiswal, A.K.; Santana, M.P.; Kennedy, S.P.; Quinquis, B.; Rainteau, D.; et al. Adaptation of the Gut Pathobiont Enterococcus faecalis to Deoxycholate and Taurocholate Bile Acids. Sci. Rep. 2022, 12, 8485. [Google Scholar] [CrossRef]
- Comerlato, C.B.; de Resende, M.C.C.; Caierão, J.; d’Azevedo, P.A. Presence of Virulence Factors in Enterococcus faecalis and Enterococcus faecium Susceptible and Resistant to Vancomycin. Mem. Inst. Oswaldo Cruz 2013, 108, 590–595. [Google Scholar] [CrossRef]
- Hancock, L.E.; Murray, B.E.; Sillanpää, J. Enterococcal Cell Wall Components and Structures; Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Boccella, M.; Santella, B.; Pagliano, P.; De Filippis, A.; Casolaro, V.; Galdiero, M.; Borrelli, A.; Capunzo, M.; Boccia, G.; Franci, G. Prevalence and Antimicrobial Resistance of Enterococcus Species: A Retrospective Cohort Study in Italy. Antibiotics 2021, 10, 1552. [Google Scholar] [CrossRef]
- El Basuini, M.F.; El-Bilawy, E.H.; Kari, Z.A.; Raza, S.H.A.; Tapingkae, W.; Van Doan, H.; Dawood, M.A.O. Pharmacotherapeutic Potential of Astaxanthin: Human and Animal Targeting Roles—A Review. Ann. Anim. Sci. 2022, 22, 829–838. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic Application of Anti-Arthritis, Pain-Releasing, and Anti-Cancer Effects of Bee Venom and Its Constituent Compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee Venom Therapy: Potential Mechanisms and Therapeutic Applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.J.; Mendez-Lnocencio, J.I.; Omidvar, B.; Omidvar, J.; Santilli, J.; Nielsen, H.S.J.; Pavot, A.P.; Richert, J.R.; Bellanti, J.A. A Phase I Study of the Safety of Honeybee Venom Extract as a Possible Treatment for Patients with Progressive Forms of Multiple Sclerosis. Allergy Asthma Proc. 2005, 26, 470–476. [Google Scholar]
- Kang, D.W.; Choi, J.G.; Kim, J.; Park, J.B.; Lee, J.H.; Kim, H.W. Bee Venom Reduces Burn-Induced Pain via the Suppression of Peripheral and Central Substance P Expression in Mice. J. Vet. Sci. 2021, 22, e9. [Google Scholar] [CrossRef]
- Haque, M.A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of Bakery Waste for Biocolorant and Enzyme Production by Monascus Purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef]
- Kalaivani, M.; Sabitha, R.; Kalaiselvan, V.; Rajasekaran, A. Health Benefits and Clinical Impact of Major Nutrient, Red Yeast Rice: A Review. Food Bioprocess Technol. 2010, 3, 333–339. [Google Scholar] [CrossRef]
- Kaur, M.; Goel, M.; Mishra, R.C.; Lahane, V.; Yadav, A.K.; Barrow, C.J. Characterization of the Red Biochromes Produced by the Endophytic Fungus Monascus Purpureus CPEF02 with Antimicrobial and Antioxidant Activities. Fermentation 2023, 9, 328. [Google Scholar] [CrossRef]
- Embaby, A.M.; Hussein, M.N.; Hussein, A. Monascus Orange and Red Pigments Production by Monascus Purpureus ATCC16436 through Co-Solid State Fermentation of Corn Cob and Glycerol: An Eco-Friendly Environmental Low Cost Approach. PLoS ONE 2018, 13, e0207755. [Google Scholar] [CrossRef] [PubMed]
- Srianta, I.; Kusdiyantini, E.; Zubaidah, E.; Ristiarini, S.; Nugerahani, I.; Alvin, A.; Iswanto, N.; Zhang, B.-B. Utilization of Agro-Industrial by-Products in Monascus Fermentation: A Review. Bioresour. Bioprocess. 2021, 8, 129. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-State Fermentation for the Production of Monascus Pigments from Jackfruit Seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef]
- Bento de Carvalho, T.; Barbosa, J.B.; Teixeira, P. Assessing Antimicrobial Efficacy on Plastics and Other Non-Porous Surfaces: A Closer Look at Studies Using the ISO 22196:2011 Standard. Biology 2024, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Pearson, J. Susceptibility Testing: Accurate and Reproducible Minimum Inhibitory Concentration (MIC) and Non-inhibitory Concentration (NIC) Values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Surendra, N.S.; Jayaram, G.N.; Reddy, M.S. Antimicrobial Activity of Crude Venom Extracts in Honeybees (Apis Cerana, Apis Dorsata, Apis Florea) Tested against Selected Pathogens. African J. Microbiol. Res. 2011, 5, 2765–2772. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Mbuagbaw, L.; Mertz, D.; Burrows, L.L.; Bowdish, D.M.E.; Moja, L.; Wright, G.D.; Schünemann, H.J. Coronavirus Disease 2019 and Antimicrobial Resistance: Parallel and Interacting Health Emergencies. Clin. Infect. Dis. 2021, 72, 1657–1659. [Google Scholar] [CrossRef]
- Henderson, M.J.; Trychta, K.A.; Yang, S.-M.; Bäck, S.; Yasgar, A.; Wires, E.S.; Danchik, C.; Yan, X.; Yano, H.; Shi, L.; et al. A Target-Agnostic Screen Identifies Approved Drugs to Stabilize the Endoplasmic Reticulum-Resident Proteome. Cell Rep. 2021, 35, 109040. [Google Scholar] [CrossRef]
- Hu, J.-F.; Patel, R.; Li, B.; Garo, E.; Hough, G.W.; Goering, M.G.; Yoo, H.-D.; O’neil-Johnson, M.; Eldridge, G.R. Anti-HCV Bioactivity of Pseudoguaianolides from Parthenium Hispitum. J. Nat. Prod. 2007, 70, 604–607. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Omar, M.O.A.; Mahmoud, M.E. Phytochemical Analysis, Antioxidant Capacities, and in Vitro Biological Activities of the Extract of Seed Coat as by-Products of Pea. BMC Chem. 2023, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Izhar, M.P.; Hafeez, A.; Kushwaha, P. Simrah Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications. J. Clust. Sci. 2023, 34, 2257–2273. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Hwang, S.J.; Jang, C.H.; Chenoweth, P.J.; Pak, S.C. Effects of Bee Venom Treatment on Growth Performance of Young Pigs. Am. J. Chin. Med. 2009, 37, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Delgado, O.; Espinoza-Culupú, A.O.; López-López, E. Antimicrobial Activity of Apis Mellifera Bee Venom Collected in Northern Peru. Antibiotics 2023, 12, 779. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Katyal, P.; Kaur, J.; Bhatia, S.; Singh, S.; Poonia, A.K.; Puniya, A.K.; Raposo, A.; Yoo, S.; Han, H.; et al. Bioactive Activity and Safety Analysis of Monascus Red Biopigment. Food Biosci. 2024, 57, 103523. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Wei, C.; Yan, Q.; Sun, Y.-Z.; Yi, G.; Li, D.; Fu, W. Monascus Purpureus M-32 Improves Growth Performance, Immune Response, Intestinal Morphology, Microbiota and Disease Resistance in Litopenaeus vannamei. Aquaculture 2021, 530, 735947. [Google Scholar] [CrossRef]
- Obukhova, E.S.; Murzina, S.A. Mechanisms of the Antimicrobial Action of Fatty Acids: A Review. Appl. Biochem. Microbiol. 2024, 60, 1035–1043. [Google Scholar] [CrossRef]
- Stenz, L.; François, P.; Fischer, A.; Huyghe, A.; Tangomo, M.; Hernandez, D.; Cassat, J.; Linder, P.; Schrenzel, J. Impact of Oleic Acid (Cis-9-Octadecenoic Acid) on Bacterial Viability and Biofilm Production in Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 287, 149–155. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane Disruption by Antimicrobial Fatty Acids Releases Low-Molecular-Weight Proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef]
- Maitip, J.; Mookhploy, W.; Khorndork, S.; Chantawannakul, P. Comparative Study of Antimicrobial Properties of Bee Venom Extracts and Melittins of Honey Bees. Antibiotics 2021, 10, 1503. [Google Scholar] [CrossRef] [PubMed]
- K Bakhiet, E.; A M Hussien, H.; Elshehaby, M. Apis Mellifera Venom Inhibits Bacterial and Fungal Pathogens in Vitro. Pakistan J. Biol. Sci. 2022, 25, 875–884. [Google Scholar] [CrossRef]
- Husakova, M.; Orlandi, V.T.; Bolognese, F.; Branska, B.; Patakova, P. Screening Antibacterial Photodynamic Effect of Monascus Red Yeast Rice (Hong-Qu) and Mycelium Extracts. Curr. Microbiol. 2024, 81, 183. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Katyal, P.; Panwar, H.; Kaur, J.; Aluko, R.E.; Puniya, A.K.; Poonia, A.K. Antioxidative, Anti-Inflammatory, and Anticancer Properties of the Red Biopigment Extract from Monascus Purpureus (MTCC 369). J. Food Biochem. 2022, 46, e14249. [Google Scholar] [CrossRef]
- Tanuwidjaja, I.; Svečnjak, L.; Gugić, D.; Levanić, M.; Jurić, S.; Vinceković, M.; Mrkonjić Fuka, M. Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom. Molecules 2021, 26, 3049. [Google Scholar] [CrossRef] [PubMed]
- Čujová, S.; Bednárová, L.; Slaninová, J.; Straka, J.; Čeřovský, V. Interaction of a Novel Antimicrobial Peptide Isolated from the Venom of Solitary Bee Colletes Daviesanus with Phospholipid Vesicles and Escherichia coli Cells. J. Pept. Sci. 2014, 20, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, S.J.; Kim, B.M.; Kim, Y.J.; Woo, H.D.; Chung, H.W. Cytotoxicity of Honeybee (Apis mellifera) Venom in Normal Human Lymphocytes and HL-60 Cells. Chem. Biol. Interact. 2007, 169, 189–197. [Google Scholar] [CrossRef]
- Hoshina, M.M.; Marin-Morales, M.A. Anti-Genotoxicity and Anti-Mutagenicity of Apis mellifera Venom. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 762, 43–48. [Google Scholar] [CrossRef]
- Gülmez, Y.; Aydın, A.; Can, İ.; Tekin, Ş.; Cacan, E. Cellular Toxicity and Biological Activities of Honey Bee (Apis mellifera L.) Venom. Marmara Pharm. J. 2017, 21, 251–260. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.S.; Lee, K.S.; Seo, H.S.; Lee, C.Y.; Kim, K.K. Detoxification of Bee Venom Increases Its Anti-Inflammatory Activity and Decreases Its Cytotoxicity and Allergenic Activity. Appl. Biochem. Biotechnol. 2021, 193, 4068–4082. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Wu, T.S.; Su, M.C.; Ping Chung, C.; Yu, F.Y. Evaluation of Citrinin Occurrence and Cytotoxicity in Monascus Fermentation Products. J. Agric. Food Chem. 2004, 53, 170–175. [Google Scholar] [CrossRef]
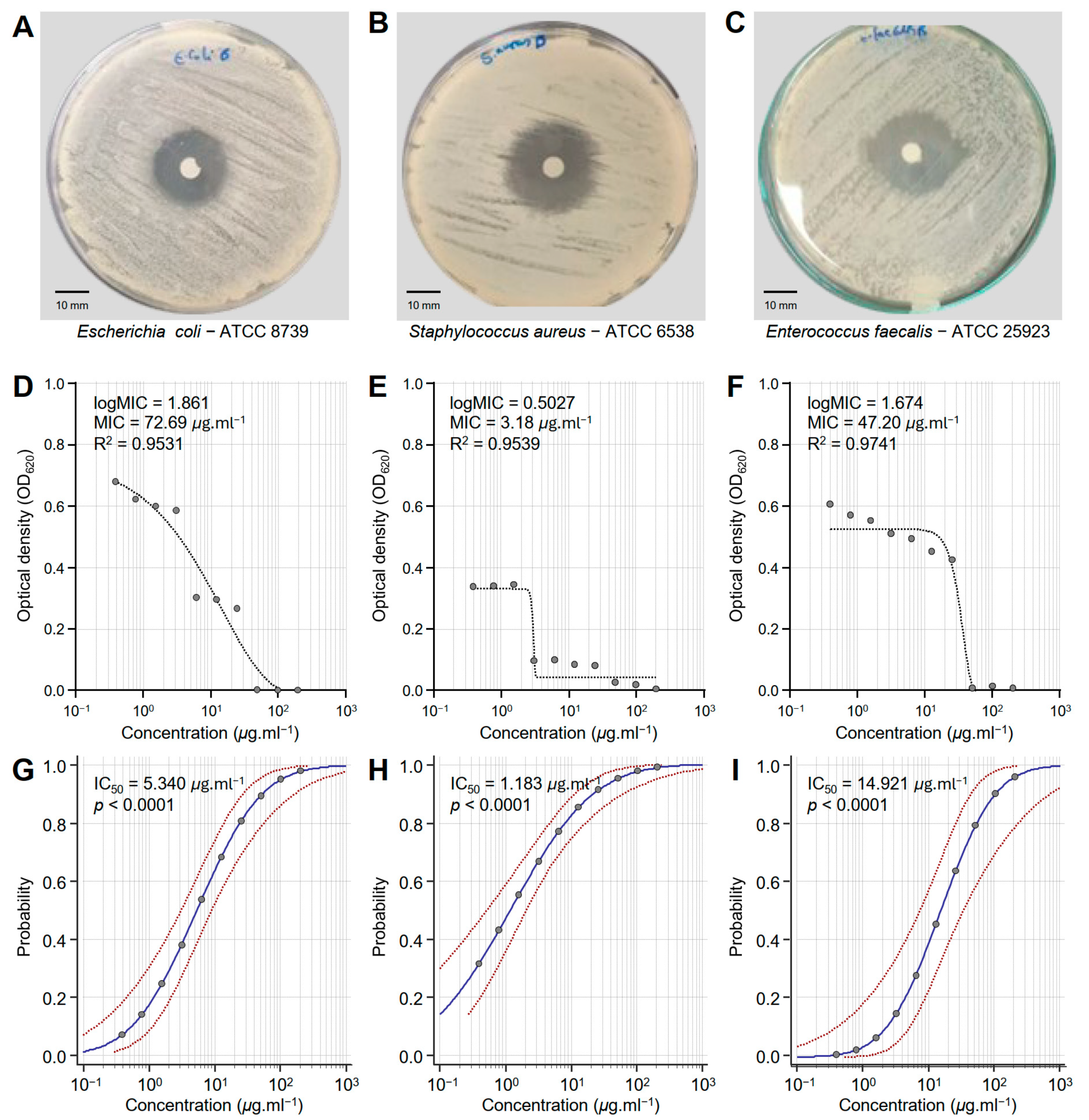
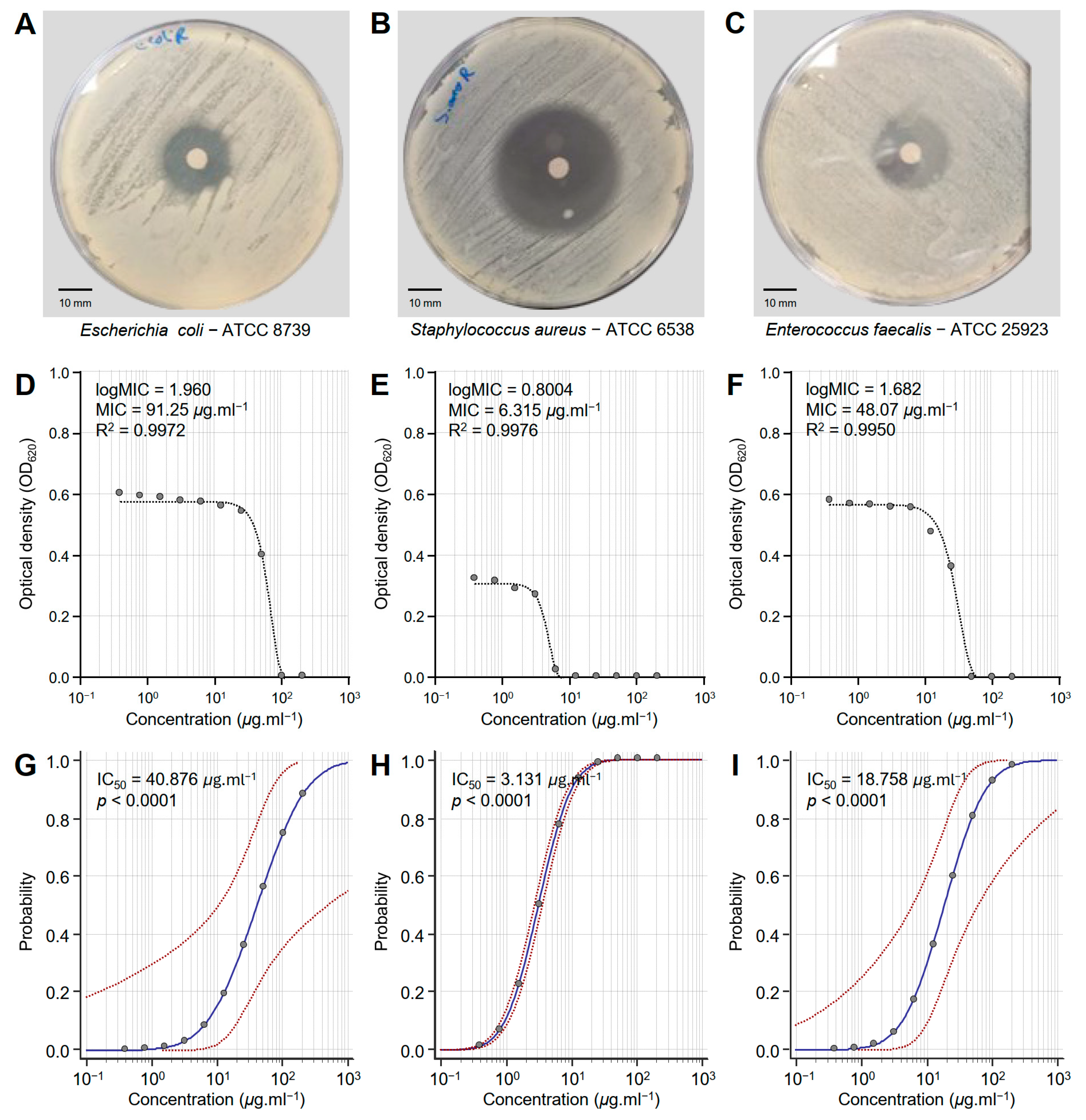
| Bacterial Strain | IC50 (μg·mL−1) | 95% Confidence Interval | Overall Model Fit | ||||
|---|---|---|---|---|---|---|---|
| Lower | Upper | χ2 | p-Value | Cox & Snell R2 | Nagelkerke R2 | ||
| Honeybee venom (BV) extract | |||||||
| E. coli ATCC 8739 | 5.340 | 3.080 | 8.929 | 501.384 | <0.0001 | 0.3943 | 0.5287 |
| S. aureus ATCC 6538 | 1.183 | 0.524 | 2.069 | 290.859 | <0.0001 | 0.2524 | 0.3696 |
| E. faecalis ATCC 25923 | 14.921 | 7.181 | 32.692 | 615.206 | <0.0001 | 0.4595 | 0.6186 |
| Monascus red dye (RD) extract | |||||||
| E. coli ATCC 8739 | 40.876 | 10.632 | 491.037 | 567.824 | <0.0001 | 0.4329 | 0.6256 |
| S. aureus ATCC 6538 | 3.131 | 2.729 | 3.592 | 846.946 | <0.0001 | 0.5709 | 0.7843 |
| E. faecalis ATCC 25923 | 18.758 | 6.201 | 61.248 | 770.928 | <0.0001 | 0.5371 | 0.7303 |
| No | Compound | RT | %Area | Molecular Formula | Mass |
|---|---|---|---|---|---|
| 1 | 5-HCyclopropa[3,4]benz[1,2-e]azulen-5-one,4,9,9-atris(acetyloxy)3[(acetyloxy)methyl]1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-4a,7-bdihydroxy1,1,6,8-tetramethyl | 6.88 | 0.78 | C28H36O11 | 549 |
| 2 | 2,2Bis[4[(4,6dichloro1,3,5triazin2yl)oxy]phenyl]1,1,1,3,3,3hexafluoropropane | 7.82 | 0.82 | C21H8Cl4F6N6O2 | 632 |
| 3 | Hycanthone | 9.26 | 0.81 | C20H24N2O2S | 356 |
| 4 | 4,5,6,7Tetrakis(pchlorophenoxy)1,2diiminoisoindoline | 10.79 | 1.11 | C32H19Cl4N3O4 | 10.79 |
| 5 | Copper tetraphenylporphyrin | 11.11 | 0.9 | C44H28CuN4 | 676 |
| 6 | 3,4,10,11tetrakis(Dimethylamino)7,14bis(trifluoromethyl)7,14epoxydinaphtho[1,8ab:1′,8′ef]cyclooctane | 11.79 | 0.78 | C32H32F6N4O | 603 |
| 7 | Decanoicacid,1,1a,1b,4,4a,5,7a,7b,8,9decahydro4a,7bdihydroxy1,1,6,8tetramethyl5oxo3[[(1oxodecyl)oxy]methyl]9aHc yclopropa[3,4]benz[1,2e]azulene9,9adiylester | 11.95 | 1.05 | C50H82O9 | 827 |
| 8 | 2-Myristynoyl pantetheine | 17.83 | 1.2 | C25H44N2O5S | 484 |
| 9 | 2,4bis(áchloroethyl)6,7bis[ámethoxycarbonylethyl]8formyl1,3,5trimethylporphyrin | 19.76 | 1.03 | C36H38Cl2N4O5 | 677 |
| 10 | Tetraneurin-A-diol | 32.66 | 0.83 | C15H20O5 | 280 |
| 11 | Tristrimethylsilyl ether derivative of 1,25-dihydroxyvit amine d2 | 33.55 | 1.16 | C37H68O3Si3 | 645 |
| 12 | Pregn-4-ene-3,11,20trione,6,17,21-tris[(trimethylsilyl)oxy]-,3,20-bis(O-methyloxime), (6á)- | 33.65 | 0.91 | C32H58N2O6Si3 | 651 |
| 13 | 16-Oxapentacyclo [13.2.2.0(1,13).0(2,10).0(5,9)] nonadecane | 35.58 | 0.74 | C22H34D2O3 | 346 |
| 14 | trans-2-Phenyl-1,3-dioxolane4methyloctadec-9,12,15-trienoate | 35.88 | 0.88 | C28H40O4 | 440 |
| 15 | 2-Cyclohexyl-4a,7-dimethyl-3,4,4a,5,6,8a-hexahydro-2H-benzo[e][1,2]oxazine-3-carbonitrile | 38.28 | 0.81 | C17H26N2O | 274 |
| 16 | Butanoicacid,4-chloro,1,1a,1b,4,4a,5,7a,7b,8,9-decahydro-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-9a-Hcyclopropa[3,4]benz[1,2-e]azulene9,9a-diylester | 39.03 | 0.76 | C28H38Cl2O8 | 573 |
| 17 | 4-(-1-hydroxyethyl)-1,6,7-tris-(2-methoxycarbonylethyl)-2,3,5,8-tetramethylporphyrin | 39.16 | 1.23 | C38H44N4O7 | 668 |
| 18 | 9-Octadecen-1-ol,(Z)-(CAS) | 40.96 | 28.35 | C18H36O | 268 |
| 19 | 6-C-Xylosyl-8-C-glucosylapigenin-permethylated derivative | 42.38 | 1.43 | C33H36O17 | 704 |
| 20 | 5á-Pregnan-20-one,3à,11á,17,21-tetrakis(trimethylsiloxy)-,O-methyloxime | 43.64 | 1.2 | C34H69NO5Si4 | 684 |
| 21 | (22S)-21-Acetoxy-6à-,11ádihydroxy16à,17à-propylmethylenedioxypregna-1,4-diene-3,20-dione | 44.13 | 1.15 | C27H36O8 | 488 |
| 22 | N,N’-Dicyclohexyl-1,7-dipyrrolidinylperylene-3,4:9,10-tetracarboxylicacid bisimide | 44.2 | 0.73 | C44H44N4O4 | 692 |
| 23 | Dotriacontane (CAS) | 44.56 | 1.55 | C32H66 | 450 |
| 24 | Isochiapin B | 44.81 | 1.01 | C19H22O6 | 346 |
| 25 | 1,2-Benzenedicarboxylic acid, di isooctyl ester(CAS) | 45.34 | 4.45 | C24H38O4 | 390 |
| 26 | (5,10,15,20-tetraphenyl[2-(2)H1]prophyrinato)zinc(II) | 45.65 | 1.14 | C44H27DN4Zn | 677 |
| 27 | 3,5,9-Trioxa-5-phosphaheptacos-18-en-1-aminium,4-hydroxy-N,N,N-trimethyl-10-oxo-7-[(1-oxo-9-octadecenyl)oxy]-,hydroxide, inner salt,4-oxide,(R) | 46.64 | 1.35 | C44H84NO8P | 786 |
| 28 | 3-Hydroxy-1-(4{13-[4-(3-hydroxy-3-phenylacryloyl)phenyl]tridecyl}-phenyl)-3-phenylprop-2-en-1-one | 46.69 | 1.06 | C43H48O4 | 628 |
| 29 | Pregn-4-ene-3,20-dione, 17,21-dihydroxy-,bis(Omethyloxime) | 46.73 | 0.88 | C23H36N2O4 | 404 |
| 30 | Corynan-17-ol,18,19-didehydro-10-methoxy-,acetate (ester) | 47.09 | 2.19 | C22H28N2O3 | 368 |
| 31 | Flavone4′-oh,5-oh,7-di-o-glucoside | 47.34 | 3.43 | C27H30O15 | 594 |
| 32 | 4,25-Secoobscurinervan-21-deoxy-16-methoxy-22-methyl-,(22à)-(CAS) | 47.58 | 3.89 | C23H32N2O2 | 368 |
| 33 | Fucoxanthin | 47.86 | 1.06 | C42H58O6 | 658 |
| 34 | 4H-Cyclopropa[5′,6′]benz[1′,2′:7,8]azuleno[5,6-b]oxiren-4-one,8,8abis(acetyloxy)-2a-[(acetyloxy)methy-l]1,1a,1b,1c,2a,3,3a,6a,6b,7,8,8a-dodecahydro-3,3a,6b-trihydroxy-1,1,5,7-tetramethyl- | 48.08 | 3.07 | C26H34O11 | 522 |
| 35 | Astaxanthin | 48.23 | 1.6 | C40H52O4 | 596 |
| 36 | Benzene,2-(1-decyl-1-undecenyl)-1,4-dimethyl-(CAS) | 48.29 | 1.72 | C29H50 | 398 |
| 37 | 9-Octadecenoicacid,(2-phenyl-1,3-dioxolan-4-yl)methyl ester, cis-(CAS) | 48.35 | 1.77 | C28H44O4 | 444 |
| 38 | (2-hydroxy-5,10,15,20-tetraphenylporphinato)zinc(II) | 48.53 | 2.81 | C44H28N4OZn | 694 |
| 39 | Ethyl iso-allocholate | 49.02 | 1.46 | C26H44O5 | 436 |
| 40 | Tetraphenylporphyrinat odibromotitanium(IV) | 49.34 | 2.07 | C44H28Br2N4Ti | 820 |
| 41 | Stigmast-5-en-3-ol,(3á,24S)-(CAS) | 49.83 | 1.19 | C29H50O | 414 |
| 42 | Aralionine | 49.94 | 5.16 | C34H38N4O5 | 582 |
| No | Compound | RT | %Area | Molecular Formula | Mass |
|---|---|---|---|---|---|
| 1 | Aspidospermidin-17-ol,1-acetyl-16-methoxy- | 5.23 | 0.32 | C22H30N2O3 | 370 |
| 2 | Ethanimidothioic acid, 2-(dimethylamino)-n-[[(methylamino)carbonyl]oxy]-2-oxo-, methyl ester | 5.35 | 0.55 | C7H13N3O3S | 219 |
| 3 | Octanoic acid, 7-oxo- | 5.56 | 0.2 | C8H14O3 | 158 |
| 4 | Hexadecanoic acid, methyl ester | 27.26 | 2.76 | C17H34O2 | 270 |
| 5 | n-Hexadecanoic acid | 28.14 | 12.76 | C16H32O2 | 256 |
| 6 | 17-Octadecynoic acid, TMS derivative | 29.83 | 0.52 | C21H40O2Si | 352 |
| 7 | 9,12-Octadecadienoic acid, methyl ester, (E,E)- | 30.38 | 1.55 | C19H34O2 | 294 |
| 8 | 9-Octadecenoic acid (Z)-, methyl ester | 30.56 | 2.1 | C19H36O2 | 296 |
| 9 | Oleic Acid | 31.42 | 62.48 | C18H34O2 | 288 |
| 10 | Octadecanoic acid | 31.87 | 10.38 | C18H36O2 | 282 |
| 11 | 9-Octadecenoic acid (z) | 32.25 | 0.72 | C18H34O2 | 282 |
| 12 | 9,12-Octadecadienoyl chloride, (Z,Z) | 32.32 | 0.69 | C18H31ClO | 298 |
| 13 | Hi-oleic safflower oil | 32.46 | 0.44 | C21H22O11 | 450 |
| 14 | 2-Aminoethanethiol hydrogen sulfate (ester) | 32.53 | 0.38 | C2H7NO3S2 | 157 |
| 15 | 13,16-Octadecadienoic acid, methyl ester | 32.6 | 0.4 | C19H34O2 | 294 |
| 16 | 17-Octadecynoic acid | 32.72 | 0.54 | C18H32O2 | 280 |
| 17 | Cyclopentaneundecanoic acid | 32.84 | 0.37 | C16H30O2 | 254 |
| 18 | 8,11,14-Eicosatrienoic acid, (Z,Z,Z)- | 33.03 | 0.37 | C20H34O2 | 306 |
| 19 | cis-5,8,11,14,17-Eicosapentaenoicacid | 39.65 | 0.87 | C20H30O2 | 302 |
| 20 | 9,12,15-Octadecatrienoic acid, | 40.88 | 0.22 | C27H52O4Si2 | 496 |
| 21 | Trideuteriomethyl10-epoxy-7-ethyl-3,11-dimethyltrideca-2,6-dienoate | 41.13 | 0.18 | C18H27D3O3 | 297 |
| 22 | 1,25-Dihydroxyvitamin D3, TMS derivative | 41.41 | 0.3 | C30H52O3Si | 488 |
| 23 | Cholest-5-en-3-yl(9z)-9-octadecenoate | 41.51 | 0.25 | C45H78O2 | 650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teiba, I.I.; Mazrou, Y.S.A.; Makhlouf, A.H.; Nehela, Y.; Mohamed, A.E.; Abbas, A.M.; Mamdouh, I.; El-Bilawy, E.H. Antibacterial Potential of Honeybee Venom and Monascus purpureus Extracellular Metabolites Against Multidrug-Resistant Pathogenic Bacteria. Biology 2025, 14, 21. https://doi.org/10.3390/biology14010021
Teiba II, Mazrou YSA, Makhlouf AH, Nehela Y, Mohamed AE, Abbas AM, Mamdouh I, El-Bilawy EH. Antibacterial Potential of Honeybee Venom and Monascus purpureus Extracellular Metabolites Against Multidrug-Resistant Pathogenic Bacteria. Biology. 2025; 14(1):21. https://doi.org/10.3390/biology14010021
Chicago/Turabian StyleTeiba, Islam I., Yasser S. A. Mazrou, Abeer H. Makhlouf, Yasser Nehela, Abdallah E. Mohamed, Ahmed M. Abbas, Islam Mamdouh, and Emad H. El-Bilawy. 2025. "Antibacterial Potential of Honeybee Venom and Monascus purpureus Extracellular Metabolites Against Multidrug-Resistant Pathogenic Bacteria" Biology 14, no. 1: 21. https://doi.org/10.3390/biology14010021
APA StyleTeiba, I. I., Mazrou, Y. S. A., Makhlouf, A. H., Nehela, Y., Mohamed, A. E., Abbas, A. M., Mamdouh, I., & El-Bilawy, E. H. (2025). Antibacterial Potential of Honeybee Venom and Monascus purpureus Extracellular Metabolites Against Multidrug-Resistant Pathogenic Bacteria. Biology, 14(1), 21. https://doi.org/10.3390/biology14010021







