Epigenetic Mechanisms in the Transcriptional Regulation of Circadian Rhythm in Mammals
Simple Summary
Abstract
1. Introduction
1.1. Circadian Rhythm
1.2. Core Regulatory Circuits of Circadian Rhythms
1.3. Circadian Rhythm and Diseases
1.3.1. Circadian Rhythm and Cancer
1.3.2. Circadian Rhythm and Metabolism
1.3.3. Circadian Rhythm and Neurodegenerative Diseases
1.3.4. Circadian Rhythm and Neurodevelopmental Disorders
1.3.5. Circadian Rhythm and Musculoskeletal Disorders
1.3.6. Circadian Rhythm and Cardiovascular Disorders (CVDs)
1.4. Transcriptional Regulatory Basis of Circadian Rhythms
2. Circadian Rhythm and Histone Modifications
2.1. Histone Methylation
2.2. Histone Acetylation
2.3. Histone Phosphorylation
2.4. Histone Adenylation and Ubiquitination
2.5. Histone Sumoylation and Redox Modifications
3. Circadian Rhythm and Chromatin Remodeling
3.1. Chromatin Structure
3.2. Chromatin State
3.3. Nucleosome Changes
4. Circadian Rhythm and Pol II Pausing Control
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosbash, M. The implications of multiple circadian clock origins. PLoS Biol. 2009, 7, e62. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C.S. Temporal organization: Reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 1993, 55, 16–54. [Google Scholar] [CrossRef] [PubMed]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef]
- Bargiello, T.A.; Young, M.W. Molecular genetics of a biological clock in Drosophila. Proc. Natl. Acad. Sci. USA 1984, 81, 2142–2146. [Google Scholar] [CrossRef]
- Bargiello, T.A.; Jackson, F.R.; Young, M.W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 1984, 312, 752–754. [Google Scholar] [CrossRef]
- Reddy, P.; Zehring, W.A.; Wheeler, D.A.; Pirrotta, V.; Hadfield, C.; Hall, J.C.; Rosbash, M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 1984, 38, 701–710. [Google Scholar] [CrossRef]
- Zehring, W.A.; Wheeler, D.A.; Reddy, P.; Konopka, R.J.; Kyriacou, C.P.; Rosbash, M.; Hall, J.C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 1984, 39, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Aryal, R.P.; Kwak, P.B.; Tamayo, A.G.; Gebert, M.; Chiu, P.L.; Walz, T.; Weitz, C.J. Macromolecular Assemblies of the Mammalian Circadian Clock. Mol. Cell 2017, 67, 770–782.e6. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E.; Panda, S. Circadian timekeeping and output mechanisms in animals. Curr. Opin. Neurobiol. 2013, 23, 724–731. [Google Scholar] [CrossRef]
- Bray, M.S.; Shaw, C.A.; Moore, M.W.; Garcia, R.A.; Zanquetta, M.M.; Durgan, D.J.; Jeong, W.J.; Tsai, J.Y.; Bugger, H.; Zhang, D.; et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1036–H1047. [Google Scholar] [CrossRef]
- Zheng, X.; Sehgal, A. Speed control: Cogs and gears that drive the circadian clock. Trends Neurosci. 2012, 35, 574–585. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Selby, C.P.; Todo, T.; Niwa, H.; Thompson, C.; Fruechte, E.M.; Hitomi, K.; Thresher, R.J.; Ishikawa, T.; Miyazaki, J.; et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. USA 1999, 96, 12114–12119. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef] [PubMed]
- Todd, W.D.; Venner, A.; Anaclet, C.; Broadhurst, R.Y.; De Luca, R.; Bandaru, S.S.; Issokson, L.; Hablitz, L.M.; Cravetchi, O.; Arrigoni, E.; et al. Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun. 2020, 11, 4410. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef]
- Hirano, A.; Fu, Y.H.; Ptáček, L.J. The intricate dance of post-translational modifications in the rhythm of life. Nat. Struct. Mol. Biol. 2016, 23, 1053–1060. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Klein, P.S.; Lazar, M.A. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 2006, 311, 1002–1005. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, L.; Yang, X.; Zhang, X.; Wang, L.; Xie, Q. Circadian clock in plants: Linking timing to fitness. J. Integr. Plant Biol. 2022, 64, 792–811. [Google Scholar] [CrossRef] [PubMed]
- Mezhnina, V.; Ebeigbe, O.P.; Poe, A.; Kondratov, R.V. Circadian Control of Mitochondria in Reactive Oxygen Species Homeostasis. Antioxid. Redox Signal. 2022, 37, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Vieira, T.K.; Jurema da Rocha Leão, M.; Pereira, L.X.; Alves da Silva, L.C.; Pereira da Paz, B.B.; Santos Ferreira, R.J.; Feitoza, C.C.; Fernandes Duarte, A.K.; Barros Ferreira Rodrigues, A.K.; Cavalcanti de Queiroz, A.; et al. Correlation between circadian rhythm related genes, type 2 diabetes, and cancer: Insights from metanalysis of transcriptomics data. Mol. Cell Endocrinol. 2021, 526, 111214. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, Z.; Ji, L.; Wang, Y.; Yang, M.; Lai, R.; Zhong, Y.; Zhang, X.; Wang, L. ARID1A-dependent permissive chromatin accessibility licenses estrogen-receptor signaling to regulate circadian rhythms genes in endometrial cancer. Cancer Lett. 2020, 492, 162–173. [Google Scholar] [CrossRef]
- Albuquerque, T.; Neves, A.R.; Quintela, T.; Costa, D. Exploring the link between chronobiology and drug delivery: Effects on cancer therapy. J. Mol. Med. 2021, 99, 1349–1371. [Google Scholar] [CrossRef]
- Lee, Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp. Mol. Med. 2021, 53, 1529–1538. [Google Scholar] [CrossRef]
- Hadadi, E.; Taylor, W.; Li, X.M.; Aslan, Y.; Villote, M.; Rivière, J.; Duvallet, G.; Auriau, C.; Dulong, S.; Raymond-Letron, I.; et al. Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat. Commun. 2020, 11, 3193. [Google Scholar] [CrossRef]
- Pham, T.T.; Lee, E.S.; Kong, S.Y.; Kim, J.; Kim, S.Y.; Joo, J.; Yoon, K.A.; Park, B. Night-shift work, circadian and melatonin pathway related genes and their interaction on breast cancer risk: Evidence from a case-control study in Korean women. Sci. Rep. 2019, 9, 10982. [Google Scholar] [CrossRef] [PubMed]
- Aiello, I.; Fedele, M.L.M.; Román, F.; Marpegan, L.; Caldart, C.; Chiesa, J.J.; Golombek, D.A.; Finkielstein, C.V.; Paladino, N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020, 6, eaaz4530. [Google Scholar] [CrossRef]
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.C.; Paterson, J.L.; Ferguson, S.A.; Stanley, D.; Wright, K.P., Jr.; Dawson, D. The shift work and health research agenda: Considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep. Med. Rev. 2017, 34, 3–9. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol. Clin. Exp. Res. 2016, 40, 335–347. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, W.; Zhang, E.E. Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer’s disease-risk genes. Sci. Rep. 2016, 6, 36035. [Google Scholar] [CrossRef] [PubMed]
- Weissová, K.; Bartoš, A.; Sládek, M.; Nováková, M.; Sumová, A. Moderate Changes in the Circadian System of Alzheimer’s Disease Patients Detected in Their Home Environment. PLoS ONE 2016, 11, e0146200. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, B.; Zhang, Y.B.; Ding, H.; Zhang, Y.; Yu, J.; Gu, M.; Chan, P.; Cai, Y. Association of ARNTL and PER1 genes with Parkinson’s disease: A case-control study of Han Chinese. Sci. Rep. 2015, 5, 15891. [Google Scholar] [CrossRef]
- Benedict, C.; Blennow, K.; Zetterberg, H.; Cedernaes, J. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology 2020, 94, e1181–e1189. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Richdale, A.L.; Baker, E.K.; Peiró, A.M. Sleep in autism: A biomolecular approach to aetiology and treatment. Sleep. Med. Rev. 2020, 54, 101357. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Martínez, M.J.; Javaloyes, A.; Inda, M.D.; Fernández, N.; Gázquez, P.; Aguilar, V.; Pérez, A.; Hernández, L.; Richdale, A.L.; et al. Sleep problems in adults with autism spectrum disorder and intellectual disability. Autism Res. 2019, 12, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Rauch, T.A.; Pfeifer, G.P.; Hu, V.W. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010, 24, 3036–3051. [Google Scholar] [CrossRef]
- Wang, Y.; Billon, C.; Walker, J.K.; Burris, T.P. Therapeutic Effect of a Synthetic RORα/γ Agonist in an Animal Model of Autism. ACS Chem. Neurosci. 2016, 7, 143–148. [Google Scholar] [CrossRef]
- Nicholas, B.; Rudrasingham, V.; Nash, S.; Kirov, G.; Owen, M.J.; Wimpory, D.C. Association of Per1 and Npas2 with autistic disorder: Support for the clock genes/social timing hypothesis. Mol. Psychiatry 2007, 12, 581–592. [Google Scholar] [CrossRef]
- Yang, Z.; Matsumoto, A.; Nakayama, K.; Jimbo, E.F.; Kojima, K.; Nagata, K.; Iwamoto, S.; Yamagata, T. Circadian-relevant genes are highly polymorphic in autism spectrum disorder patients. Brain Dev. 2016, 38, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Dintwa, L.; Hughes, C.E.; Blain, E.J. Importance of mechanical cues in regulating musculoskeletal circadian clock rhythmicity: Implications for articular cartilage. Physiol. Rep. 2023, 11, e15780. [Google Scholar] [CrossRef]
- Snelling, S.J.; Forster, A.; Mukherjee, S.; Price, A.J.; Poulsen, R.C. The chondrocyte-intrinsic circadian clock is disrupted in human osteoarthritis. Chronobiol. Int. 2016, 33, 574–579. [Google Scholar] [CrossRef]
- Gossan, N.; Zeef, L.; Hensman, J.; Hughes, A.; Bateman, J.F.; Rowley, L.; Little, C.B.; Piggins, H.D.; Rattray, M.; Boot-Handford, R.P.; et al. The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum. 2013, 65, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ming, J. The role of circadian rhythm in osteoporosis; a review. Front. Cell Dev. Biol. 2022, 10, 960456. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef]
- Maronde, E.; Schilling, A.F.; Seitz, S.; Schinke, T.; Schmutz, I.; van der Horst, G.; Amling, M.; Albrecht, U. The clock genes Period 2 and Cryptochrome 2 differentially balance bone formation. PLoS ONE 2010, 5, e11527. [Google Scholar] [CrossRef]
- Takarada, T.; Xu, C.; Ochi, H.; Nakazato, R.; Yamada, D.; Nakamura, S.; Kodama, A.; Shimba, S.; Mieda, M.; Fukasawa, K.; et al. Bone Resorption Is Regulated by Circadian Clock in Osteoblasts. J. Bone Miner. Res. 2017, 32, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Samsa, W.E.; Vasanji, A.; Midura, R.J.; Kondratov, R.V. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 2016, 84, 194–203. [Google Scholar] [CrossRef]
- Xu, C.; Ochi, H.; Fukuda, T.; Sato, S.; Sunamura, S.; Takarada, T.; Hinoi, E.; Okawa, A.; Takeda, S. Circadian Clock Regulates Bone Resorption in Mice. J. Bone Miner. Res. 2016, 31, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Cheng, Y.; Kapoor, S.; Reilly, D.; Price, T.S.; Fitzgerald, G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. USA 2007, 104, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Brewer, R.A.; Peliciari-Garcia, R.A.; Collins, H.E.; He, L.; Birky, T.L.; Peden, B.W.; Thompson, E.G.; Ammons, B.J.; Bray, M.S.; et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J. Biol. Rhythms 2014, 29, 257–276. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, G.R.; Tang, Y.; Brewer, R.A.; Brahma, M.K.; Stanley, H.L.; Shanmugam, G.; Rajasekaran, N.S.; Rowe, G.C.; Frank, S.J.; Wende, A.R.; et al. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. J. Mol. Cell Cardiol. 2017, 110, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Pulinilkunnil, T.; Villegas-Montoya, C.; Garvey, M.E.; Frangogiannis, N.G.; Michael, L.H.; Chow, C.W.; Dyck, J.R.; Young, M.E. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ. Res. 2010, 106, 546–550. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Alon, U. Network motifs: Theory and experimental approaches. Nat. Rev. Genet. 2007, 8, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Etchegaray, J.P.; Cagampang, F.R.; Loudon, A.S.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lazar, M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020, 41, 707–732. [Google Scholar] [CrossRef]
- Yu, X.; Rollins, D.; Ruhn, K.A.; Stubblefield, J.J.; Green, C.B.; Kashiwada, M.; Rothman, P.B.; Takahashi, J.S.; Hooper, L.V. TH17 cell differentiation is regulated by the circadian clock. Science 2013, 342, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.P.; Machida, K.K.; Noton, E.; Constance, C.M.; Dallmann, R.; Di Napoli, M.N.; DeBruyne, J.P.; Lambert, C.M.; Yu, E.A.; Reppert, S.M.; et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell Biol. 2009, 29, 3853–3866. [Google Scholar] [CrossRef]
- Anafi, R.C.; Lee, Y.; Sato, T.K.; Venkataraman, A.; Ramanathan, C.; Kavakli, I.H.; Hughes, M.E.; Baggs, J.E.; Growe, J.; Liu, A.C.; et al. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014, 12, e1001840. [Google Scholar] [CrossRef]
- Goriki, A.; Hatanaka, F.; Myung, J.; Kim, J.K.; Yoritaka, T.; Tanoue, S.; Abe, T.; Kiyonari, H.; Fujimoto, K.; Kato, Y.; et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 2014, 12, e1001839. [Google Scholar] [CrossRef] [PubMed]
- Annayev, Y.; Adar, S.; Chiou, Y.Y.; Lieb, J.D.; Sancar, A.; Ye, R. Gene model 129 (Gm129) encodes a novel transcriptional repressor that modulates circadian gene expression. J. Biol. Chem. 2014, 289, 5013–5024. [Google Scholar] [CrossRef]
- Du, N.H.; Arpat, A.B.; De Matos, M.; Gatfield, D. MicroRNAs shape circadian hepatic gene expression on a transcriptome-wide scale. Elife 2014, 3, e02510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Z.; Zhao, M.; Li, J.; Lu, M.; Liu, W.; Ying, H.; Liu, M.; Yan, J. Oscillating primary transcripts harbor miRNAs with circadian functions. Sci. Rep. 2016, 6, 21598. [Google Scholar] [CrossRef]
- Na, Y.J.; Sung, J.H.; Lee, S.C.; Lee, Y.J.; Choi, Y.J.; Park, W.Y.; Shin, H.S.; Kim, J.H. Comprehensive analysis of microRNA-mRNA co-expression in circadian rhythm. Exp. Mol. Med. 2009, 41, 638–647. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Papp, J.W.; Varlamova, O.; Dziema, H.; Russell, B.; Curfman, J.P.; Nakazawa, T.; Shimizu, K.; Okamura, H.; Impey, S.; et al. microRNA modulation of circadian-clock period and entrainment. Neuron 2007, 54, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Gatfield, D.; Le Martelot, G.; Vejnar, C.E.; Gerlach, D.; Schaad, O.; Fleury-Olela, F.; Ruskeepää, A.L.; Oresic, M.; Esau, C.C.; Zdobnov, E.M.; et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes. Dev. 2009, 23, 1313–1326. [Google Scholar] [CrossRef]
- Kojima, S.; Sher-Chen, E.L.; Green, C.B. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes. Dev. 2012, 26, 2724–2736. [Google Scholar] [CrossRef]
- Gendreau, K.L.; Unruh, B.A.; Zhou, C.; Kojima, S. Identification and Characterization of Transcripts Regulated by Circadian Alternative Polyadenylation in Mouse Liver. G3 2018, 8, 3539–3548. [Google Scholar] [CrossRef]
- Xu, W.; Li, X. Regulation of Pol II Pausing during Daily Gene Transcription in Mouse Liver. Biology 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Z.; Shliaha, P.V.; Miele, M.; Hendrickson, R.C.; Jiang, X.; Helin, K. H3K4me3 regulates RNA polymerase II promoter-proximal pause-release. Nature 2023, 615, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, C.; Schmitz, R.J.; Nathanson, J.; Yeo, G.; Ecker, J.R.; Panda, S. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 2012, 16, 833–845. [Google Scholar] [CrossRef]
- Du, S.; Chen, L.; Ge, L.; Huang, W. A Novel Loop: Mutual Regulation Between Epigenetic Modification and the Circadian Clock. Front. Plant Sci. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, J.; Huang, F.Y.; Yang, S.; Wu, K. The Plant Circadian Clock and Chromatin Modifications. Genes 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Hung, F.Y.; Chen, F.F.; Li, C.; Chen, C.; Lai, Y.C.; Chen, J.H.; Cui, Y.; Wu, K. The Arabidopsis LDL1/2-HDA6 histone modification complex is functionally associated with CCA1/LHY in regulation of circadian clock genes. Nucleic Acids Res. 2018, 46, 10669–10681. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- Heintzman, N.D.; Hon, G.C.; Hawkins, R.D.; Kheradpour, P.; Stark, A.; Harp, L.F.; Ye, Z.; Lee, L.K.; Stuart, R.K.; Ching, C.W.; et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 2009, 459, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.G.; Levine, S.S.; Boyer, L.A.; Jaenisch, R.; Young, R.A. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 2007, 130, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef]
- Lachner, M.; O’Carroll, D.; Rea, S.; Mechtler, K.; Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 2001, 410, 116–120. [Google Scholar] [CrossRef]
- Bannister, A.J.; Zegerman, P.; Partridge, J.F.; Miska, E.A.; Thomas, J.O.; Allshire, R.C.; Kouzarides, T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 2001, 410, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.A.; Weitz, C.J. Temporal orchestration of repressive chromatin modifiers by circadian clock Period complexes. Nat. Struct. Mol. Biol. 2014, 21, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rada-Iglesias, A.; Bajpai, R.; Swigut, T.; Brugmann, S.A.; Flynn, R.A.; Wysocka, J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 2011, 470, 279–283. [Google Scholar] [CrossRef]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Filippakopoulos, P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 246–262. [Google Scholar] [CrossRef]
- Belkina, A.C.; Denis, G.V. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 2012, 12, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. Nuclear receptor corepressors. Nucl. Recept. Signal. 2003, 1, e001. [Google Scholar] [CrossRef]
- Watson, P.J.; Fairall, L.; Santos, G.M.; Schwabe, J.W. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature 2012, 481, 335–340. [Google Scholar] [CrossRef]
- Guenther, M.G.; Barak, O.; Lazar, M.A. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell Biol. 2001, 21, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.G.; Lane, W.S.; Fischle, W.; Verdin, E.; Lazar, M.A.; Shiekhattar, R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes. Dev. 2000, 14, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liu, T.; Sun, Z.; Bugge, A.; Mullican, S.E.; Alenghat, T.; Liu, X.S.; Lazar, M.A. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 2011, 331, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, B.; Emmett, M.J.; Damle, M.; Sun, Z.; Feng, D.; Armour, S.M.; Remsberg, J.R.; Jager, J.; Soccio, R.E.; et al. Gene Regulation. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 2015, 348, 1488–1492. [Google Scholar] [CrossRef]
- Yin, L.; Lazar, M.A. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol. Endocrinol. 2005, 19, 1452–1459. [Google Scholar] [CrossRef]
- Sun, Z.; Feng, D.; Fang, B.; Mullican, S.E.; You, S.H.; Lim, H.W.; Everett, L.J.; Nabel, C.S.; Li, Y.; Selvakumaran, V.; et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol. Cell 2013, 52, 769–782. [Google Scholar] [CrossRef]
- You, S.H.; Lim, H.W.; Sun, Z.; Broache, M.; Won, K.J.; Lazar, M.A. Nuclear receptor co-repressors are required for the histone-deacetylase activity of HDAC3 in vivo. Nat. Struct. Mol. Biol. 2013, 20, 182–187. [Google Scholar] [CrossRef]
- Alenghat, T.; Meyers, K.; Mullican, S.E.; Leitner, K.; Adeniji-Adele, A.; Avila, J.; Bućan, M.; Ahima, R.S.; Kaestner, K.H.; Lazar, M.A. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 2008, 456, 997–1000. [Google Scholar] [CrossRef]
- Rutter, J.; Reick, M.; Wu, L.C.; McKnight, S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 2001, 293, 510–514. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Sahar, S.; Grimaldi, B.; Tamaru, T.; Takamatsu, K.; Nakahata, Y.; Sassone-Corsi, P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007, 450, 1086–1090. [Google Scholar] [CrossRef]
- Zullo, J.M.; Demarco, I.A.; Piqué-Regi, R.; Gaffney, D.J.; Epstein, C.B.; Spooner, C.J.; Luperchio, T.R.; Bernstein, B.E.; Pritchard, J.K.; Reddy, K.L.; et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 2012, 149, 1474–1487. [Google Scholar] [CrossRef]
- Poleshko, A.; Shah, P.P.; Gupta, M.; Babu, A.; Morley, M.P.; Manderfield, L.J.; Ifkovits, J.L.; Calderon, D.; Aghajanian, H.; Sierra-Pagán, J.E.; et al. Genome-Nuclear Lamina Interactions Regulate Cardiac Stem Cell Lineage Restriction. Cell 2017, 171, 573–587.e14. [Google Scholar] [CrossRef] [PubMed]
- Somech, R.; Shaklai, S.; Geller, O.; Amariglio, N.; Simon, A.J.; Rechavi, G.; Gal-Yam, E.N. The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J. Cell Sci. 2005, 118, 4017–4025. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hanover, J.A.; Krause, M.W.; Love, D.C. Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 2012, 13, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, K.; Kivimäe, S.; Allen, J.J.; Chalkley, R.J.; Huang, Y.; Baer, K.; Kissel, H.; Burlingame, A.L.; Shokat, K.M.; Ptáček, L.J.; et al. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 2013, 17, 291–302. [Google Scholar] [CrossRef]
- Li, M.D.; Ruan, H.B.; Hughes, M.E.; Lee, J.S.; Singh, J.P.; Jones, S.P.; Nitabach, M.N.; Yang, X. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Padiath, Q.S.; Shapiro, R.E.; Jones, C.R.; Wu, S.C.; Saigoh, N.; Saigoh, K.; Ptácek, L.J.; Fu, Y.H. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 2005, 434, 640–644. [Google Scholar] [CrossRef]
- Lee, H.M.; Chen, R.; Kim, H.; Etchegaray, J.P.; Weaver, D.R.; Lee, C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc. Natl. Acad. Sci. USA 2011, 108, 16451–16456. [Google Scholar] [CrossRef]
- Nam, H.J.; Boo, K.; Kim, D.; Han, D.H.; Choe, H.K.; Kim, C.R.; Sun, W.; Kim, H.; Kim, K.; Lee, H.; et al. Phosphorylation of LSD1 by PKCα is crucial for circadian rhythmicity and phase resetting. Mol. Cell 2014, 53, 791–805. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Calorie restriction: Is AMPK a key sensor and effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.P.; Carling, D.; Munday, M.R.; Hardie, D.G. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur. J. Biochem. 1992, 203, 615–623. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef]
- Um, J.H.; Yang, S.; Yamazaki, S.; Kang, H.; Viollet, B.; Foretz, M.; Chung, J.H. Activation of 5’-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J. Biol. Chem. 2007, 282, 20794–20798. [Google Scholar] [CrossRef]
- Wang, J.; Symul, L.; Yeung, J.; Gobet, C.; Sobel, J.; Lück, S.; Westermark, P.O.; Molina, N.; Naef, F. Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc. Natl. Acad. Sci. USA 2018, 115, E1916–E1925. [Google Scholar] [CrossRef]
- Yoo, S.H.; Mohawk, J.A.; Siepka, S.M.; Shan, Y.; Huh, S.K.; Hong, H.K.; Kornblum, I.; Kumar, V.; Koike, N.; Xu, M.; et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 2013, 152, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Siepka, S.M.; Yoo, S.H.; Park, J.; Song, W.; Kumar, V.; Hu, Y.; Lee, C.; Takahashi, J.S. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 2007, 129, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Godinho, S.I.; Maywood, E.S.; Shaw, L.; Tucci, V.; Barnard, A.R.; Busino, L.; Pagano, M.; Kendall, R.; Quwailid, M.M.; Romero, M.R.; et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 2007, 316, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Busino, L.; Bassermann, F.; Maiolica, A.; Lee, C.; Nolan, P.M.; Godinho, S.I.; Draetta, G.F.; Pagano, M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 2007, 316, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Local, A.; Huang, H.; Albuquerque, C.P.; Singh, N.; Lee, A.Y.; Wang, W.; Wang, C.; Hsia, J.E.; Shiau, A.K.; Ge, K.; et al. Identification of H3K4me1-associated proteins at mammalian enhancers. Nat. Genet. 2018, 50, 73–82. [Google Scholar] [CrossRef]
- Rickels, R.; Herz, H.M.; Sze, C.C.; Cao, K.; Morgan, M.A.; Collings, C.K.; Gause, M.; Takahashi, Y.H.; Wang, L.; Rendleman, E.J.; et al. Histone H3K4 monomethylation catalyzed by Trr and mammalian COMPASS-like proteins at enhancers is dispensable for development and viability. Nat. Genet. 2017, 49, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Rada-Iglesias, A. Is H3K4me1 at enhancers correlative or causative? Nat. Genet. 2018, 50, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Cardone, L.; Hirayama, J.; Giordano, F.; Tamaru, T.; Palvimo, J.J.; Sassone-Corsi, P. Circadian clock control by SUMOylation of BMAL1. Science 2005, 309, 1390–1394. [Google Scholar] [CrossRef]
- Tamaru, T.; Takamatsu, K. Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues. Neurochem. Int. 2018, 119, 11–16. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, W.; Jiang, Y.; Zou, L.; Chen, F.; Xiao, W.; Zhang, X.; Cao, Y.; Xu, L.; Zhu, Y. Bmal1 Regulates the Redox Rhythm of HSPB1, and Homooxidized HSPB1 Attenuates the Oxidative Stress Injury of Cardiomyocytes. Oxid. Med. Cell Longev. 2021, 2021, 5542815. [Google Scholar] [CrossRef]
- Stangherlin, A.; Reddy, A.B. Regulation of circadian clocks by redox homeostasis. J. Biol. Chem. 2013, 288, 26505–26511. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Misteli, T. Functional implications of genome topology. Nat. Struct. Mol. Biol. 2013, 20, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Arnal, L.; Hakim, O.; Patel, V.R.; Baldi, P.; Hager, G.L.; Sassone-Corsi, P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat. Struct. Mol. Biol. 2013, 20, 1206–1213. [Google Scholar] [CrossRef]
- Zhao, H.; Sifakis, E.G.; Sumida, N.; Millán-Ariño, L.; Scholz, B.A.; Svensson, J.P.; Chen, X.; Ronnegren, A.L.; Mallet de Lima, C.D.; Varnoosfaderani, F.S.; et al. PARP1- and CTCF-Mediated Interactions between Active and Repressed Chromatin at the Lamina Promote Oscillating Transcription. Mol. Cell 2015, 59, 984–997. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, W.; Li, P.; Zhang, Y.; Zhao, M.; Fan, Z.; Zhao, Z.; Yan, J. Long-Range Chromosome Interactions Mediated by Cohesin Shape Circadian Gene Expression. PLoS Genet. 2016, 12, e1005992. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Marhon, S.A.; Zhang, Y.; Steger, D.J.; Won, K.J.; Lazar, M.A. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science 2018, 359, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Mermet, J.; Yeung, J.; Hurni, C.; Mauvoisin, D.; Gustafson, K.; Jouffe, C.; Nicolas, D.; Emmenegger, Y.; Gobet, C.; Franken, P.; et al. Clock-dependent chromatin topology modulates circadian transcription and behavior. Genes. Dev. 2018, 32, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef]
- Kornberg, R.D. Chromatin structure: A repeating unit of histones and DNA. Science 1974, 184, 868–871. [Google Scholar] [CrossRef]
- Durrin, L.K.; Mann, R.K.; Grunstein, M. Nucleosome loss activates CUP1 and HIS3 promoters to fully induced levels in the yeast Saccharomyces cerevisiae. Mol. Cell Biol. 1992, 12, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Workman, J.L.; Kingston, R.E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 1998, 67, 545–579. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Smith, E.; Shilatifard, A. The chromatin signaling pathway: Diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Mol. Cell 2010, 40, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Reinberg, D. Chromatin higher-order structures and gene regulation. Curr. Opin. Genet. Dev. 2011, 21, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Fischle, W.; Wang, Y.; Allis, C.D. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 2003, 15, 172–183. [Google Scholar] [CrossRef]
- Narlikar, G.J.; Fan, H.Y.; Kingston, R.E. Cooperation between complexes that regulate chromatin structure and transcription. Cell 2002, 108, 475–487. [Google Scholar] [CrossRef] [PubMed]
- de la Serna, I.L.; Ohkawa, Y.; Imbalzano, A.N. Chromatin remodelling in mammalian differentiation: Lessons from ATP-dependent remodellers. Nat. Rev. Genet. 2006, 7, 461–473. [Google Scholar] [CrossRef]
- Wang, B.; Kettenbach, A.N.; Gerber, S.A.; Loros, J.J.; Dunlap, J.C. Neurospora WC-1 recruits SWI/SNF to remodel frequency and initiate a circadian cycle. PLoS Genet. 2014, 10, e1004599. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Chen, S.; Shi, G.; Guo, J.; Xu, Y.; Liu, C. SWItch/sucrose nonfermentable (SWI/SNF) complex subunit BAF60a integrates hepatic circadian clock and energy metabolism. Hepatology 2011, 54, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Yudkovsky, N.; Logie, C.; Hahn, S.; Peterson, C.L. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes. Dev. 1999, 13, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhao, M.; Joshi, P.D.; Li, P.; Zhang, Y.; Guo, W.; Xu, Y.; Wang, H.; Zhao, Z.; Yan, J. A class of circadian long non-coding RNAs mark enhancers modulating long-range circadian gene regulation. Nucleic Acids Res. 2017, 45, 5720–5738. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.A.; Robles, M.S.; Knutti, D.; Weitz, C.J. A molecular mechanism for circadian clock negative feedback. Science 2011, 332, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Everett, L.J.; Jager, J.; Briggs, E.; Armour, S.M.; Feng, D.; Roy, A.; Gerhart-Hines, Z.; Sun, Z.; Lazar, M.A. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell 2014, 159, 1140–1152. [Google Scholar] [CrossRef]
- Denslow, S.A.; Wade, P.A. The human Mi-2/NuRD complex and gene regulation. Oncogene 2007, 26, 5433–5438. [Google Scholar] [CrossRef]
- Xue, Y.; Wong, J.; Moreno, G.T.; Young, M.K.; Côté, J.; Wang, W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell 1998, 2, 851–861. [Google Scholar] [CrossRef]
- Belden, W.J.; Loros, J.J.; Dunlap, J.C. Execution of the Circadian Negative Feedback Loop in Neurospora Requires the Atp-Dependent Chromatin-Remodeling Enzyme Clockswitch. Mol. Cell 2007, 25, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Zhou, M.; Liu, Y. CATP is a critical component of the Neurospora circadian clock by regulating the nucleosome occupancy rhythm at the frequency locus. EMBO Rep. 2013, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Beytebiere, J.R.; Greenwell, B.J.; Sahasrabudhe, A.; Menet, J.S. Clock-controlled rhythmic transcription: Is the clock enough and how does it work? Transcription 2019, 10, 212–221. [Google Scholar] [CrossRef]
- Petkau, N.; Budak, H.; Zhou, X.; Oster, H.; Eichele, G. Acetylation of BMAL1 by TIP60 controls BRD4-P-TEFb recruitment to circadian promoters. Elife 2019, 8, e43235. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Roeder, R.G. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 2010, 11, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Chiang, C.M. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 105–178. [Google Scholar] [CrossRef]
- Roeder, R.G. 50+ years of eukaryotic transcription: An expanding universe of factors and mechanisms. Nat. Struct. Mol. Biol. 2019, 26, 783–791. [Google Scholar] [CrossRef]
- Hsieh, T.S.; Cattoglio, C.; Slobodyanyuk, E.; Hansen, A.S.; Darzacq, X.; Tjian, R. Enhancer-promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat. Genet. 2022, 54, 1919–1932. [Google Scholar] [CrossRef]
- Richter, W.F.; Nayak, S.; Iwasa, J.; Taatjes, D.J. The Mediator complex as a master regulator of transcription by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2022, 23, 732–749. [Google Scholar] [CrossRef]
- Suter, D.M.; Molina, N.; Gatfield, D.; Schneider, K.; Schibler, U.; Naef, F. Mammalian genes are transcribed with widely different bursting kinetics. Science 2011, 332, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Larson, D.R. Transcription in Living Cells: Molecular Mechanisms of Bursting. Annu. Rev. Biochem. 2020, 89, 189–212. [Google Scholar] [CrossRef]
- Lim, B.; Levine, M.S. Enhancer-promoter communication: Hubs or loops? Curr. Opin. Genet. Dev. 2021, 67, 5–9. [Google Scholar] [CrossRef] [PubMed]
- McSwiggen, D.T.; Mir, M.; Darzacq, X.; Tjian, R. Evaluating phase separation in live cells: Diagnosis, caveats, and functional consequences. Genes. Dev. 2019, 33, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.; Lis, J.T. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2015, 16, 167–177. [Google Scholar] [CrossRef]
- Chiu, A.C.; Suzuki, H.I.; Wu, X.; Mahat, D.B.; Kriz, A.J.; Sharp, P.A. Transcriptional Pause Sites Delineate Stable Nucleosome-Associated Premature Polyadenylation Suppressed by U1 snRNP. Mol. Cell 2018, 69, 648–663.e7. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Shibata, H.; Handa, H. Transcription elongation factors DSIF and NELF: Promoter-proximal pausing and beyond. Biochim. Biophys. Acta 2013, 1829, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lin, C.; Shilatifard, A. The super elongation complex (SEC) family in transcriptional control. Nat. Rev. Mol. Cell Biol. 2012, 13, 543–547. [Google Scholar] [CrossRef]
- Chen, Y.; Vos, S.M.; Dienemann, C.; Ninov, M.; Urlaub, H.; Cramer, P. Allosteric transcription stimulation by RNA polymerase II super elongation complex. Mol. Cell 2021, 81, 3386–3399.e10. [Google Scholar] [CrossRef] [PubMed]
- Price, D.H. Transient pausing by RNA polymerase II. Proc. Natl. Acad. Sci. USA 2018, 115, 4810–4812. [Google Scholar] [CrossRef] [PubMed]
- Kamieniarz-Gdula, K.; Proudfoot, N.J. Transcriptional Control by Premature Termination: A Forgotten Mechanism. Trends Genet. 2019, 35, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, W.; Ni, T.; Tang, Z.; Nakadai, T.; Zhu, J.; Roeder, R.G. RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science 2015, 350, 1383–1386. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.A.; McGinty, R.K.; Nguyen, U.T.; Muir, T.W.; Allis, C.D.; Roeder, R.G. The n-SET domain of Set1 regulates H2B ubiquitylation-dependent H3K4 methylation. Mol. Cell 2013, 49, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Valekunja, U.K.; Edgar, R.S.; Oklejewicz, M.; van der Horst, G.T.; O'Neill, J.S.; Tamanini, F.; Turner, D.J.; Reddy, A.B. Histone methyltransferase MLL3 contributes to genome-scale circadian transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Yoo, S.H.; Huang, H.C.; Kumar, V.; Lee, C.; Kim, T.K.; Takahashi, J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Poolman, T.; Cunningham, P.; Hunter, L.; Voronkov, M.; Kitchen, G.B.; Goosey, L.; Begley, N.; Kay, D.; Hespe, A.; et al. Circadian clock function does not require the histone methyltransferase MLL3. FASEB J. 2022, 36, e22356. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Schade, A.E.; Branigan, T.B.; Müller, G.A.; DeCaprio, J.A. Coordinating gene expression during the cell cycle. Trends Biochem. Sci. 2022, 47, 1009–1022. [Google Scholar] [CrossRef]
- Hsiung, C.C.; Bartman, C.R.; Huang, P.; Ginart, P.; Stonestrom, A.J.; Keller, C.A.; Face, C.; Jahn, K.S.; Evans, P.; Sankaranarayanan, L.; et al. A hyperactive transcriptional state marks genome reactivation at the mitosis-G1 transition. Genes. Dev. 2016, 30, 1423–1439. [Google Scholar] [CrossRef] [PubMed]
- Palozola, K.C.; Donahue, G.; Liu, H.; Grant, G.R.; Becker, J.S.; Cote, A.; Yu, H.; Raj, A.; Zaret, K.S. Mitotic transcription and waves of gene reactivation during mitotic exit. Science 2017, 358, 119–122. [Google Scholar] [CrossRef]
- Gaucher, J.; Montellier, E.; Sassone-Corsi, P. Molecular Cogs: Interplay between Circadian Clock and Cell Cycle. Trends Cell Biol. 2018, 28, 368–379. [Google Scholar] [CrossRef]
- Bieler, J.; Cannavo, R.; Gustafson, K.; Gobet, C.; Gatfield, D.; Naef, F. Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Mol. Syst. Biol. 2014, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Yamaguchi, S.; Mitsui, S.; Emi, A.; Shimoda, F.; Okamura, H. Control mechanism of the circadian clock for timing of cell division in vivo. Science 2003, 302, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hirota, T.; Han, X.; Cho, H.; Chong, L.W.; Lamia, K.; Liu, S.; Atkins, A.R.; Banayo, E.; Liddle, C.; et al. Circadian Amplitude Regulation via FBXW7-Targeted REV-ERBα Degradation. Cell 2016, 165, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Bartman, C.R.; Hamagami, N.; Keller, C.A.; Giardine, B.; Hardison, R.C.; Blobel, G.A.; Raj, A. Transcriptional Burst Initiation and Polymerase Pause Release Are Key Control Points of Transcriptional Regulation. Mol. Cell 2019, 73, 519–532.e4. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.G.; Schwalb, B.; Mackowiak, S.D.; Velychko, T.; Hanzl, A.; Imrichova, H.; Brand, M.; Agerer, B.; Chorn, S.; Nabet, B.; et al. Selective Mediator dependence of cell-type-specifying transcription. Nat. Genet. 2020, 52, 719–727. [Google Scholar] [CrossRef] [PubMed]
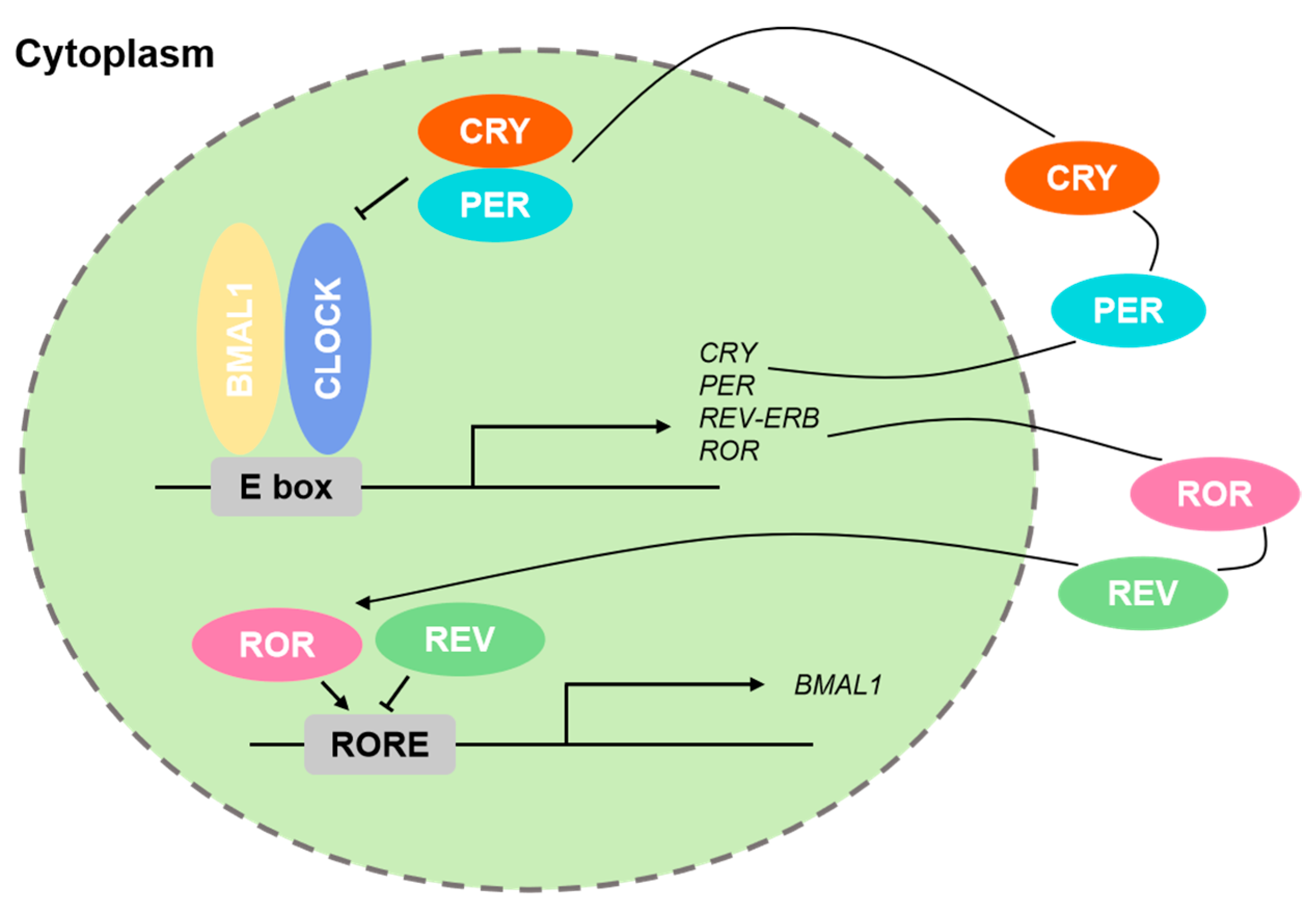
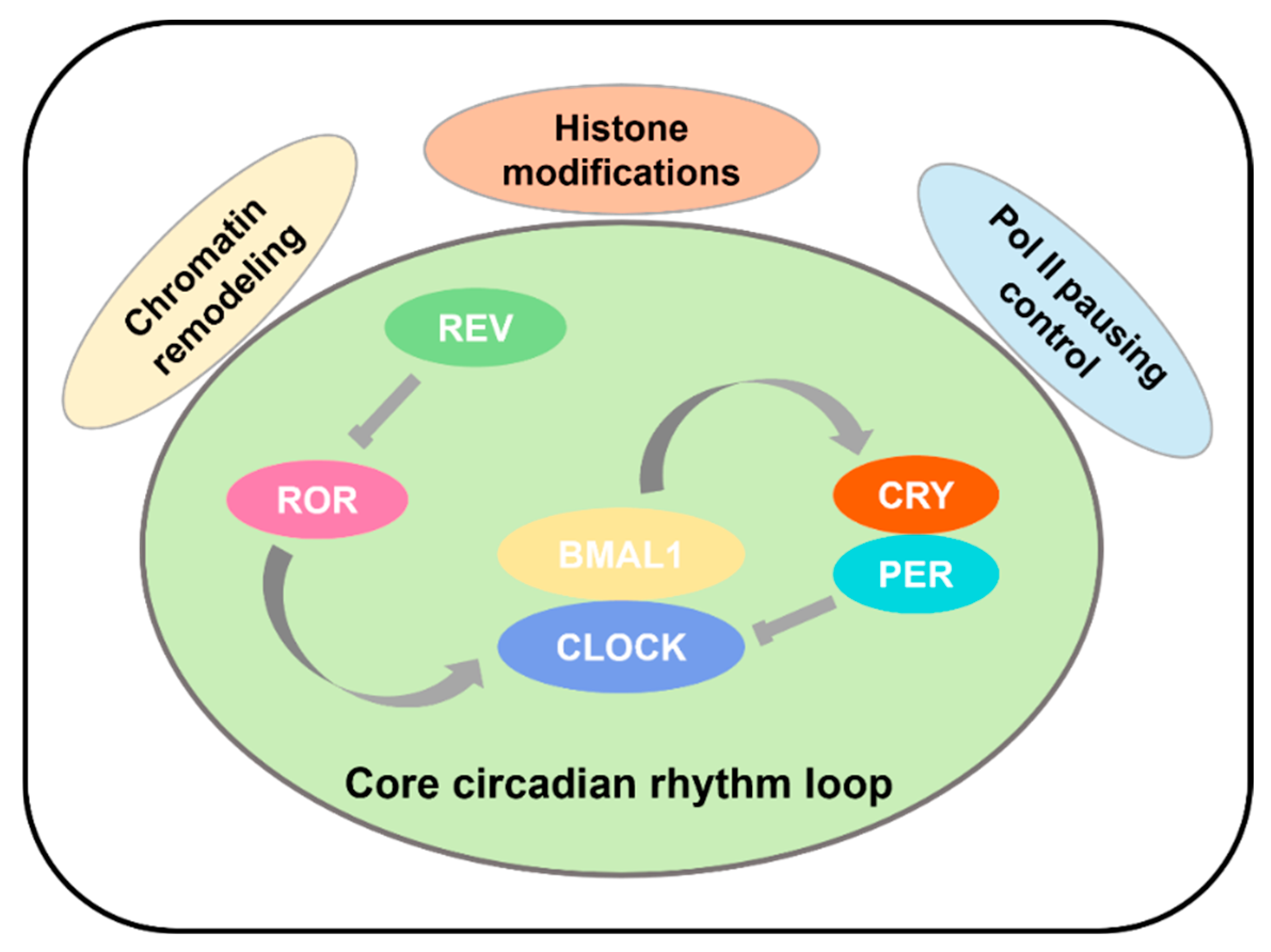
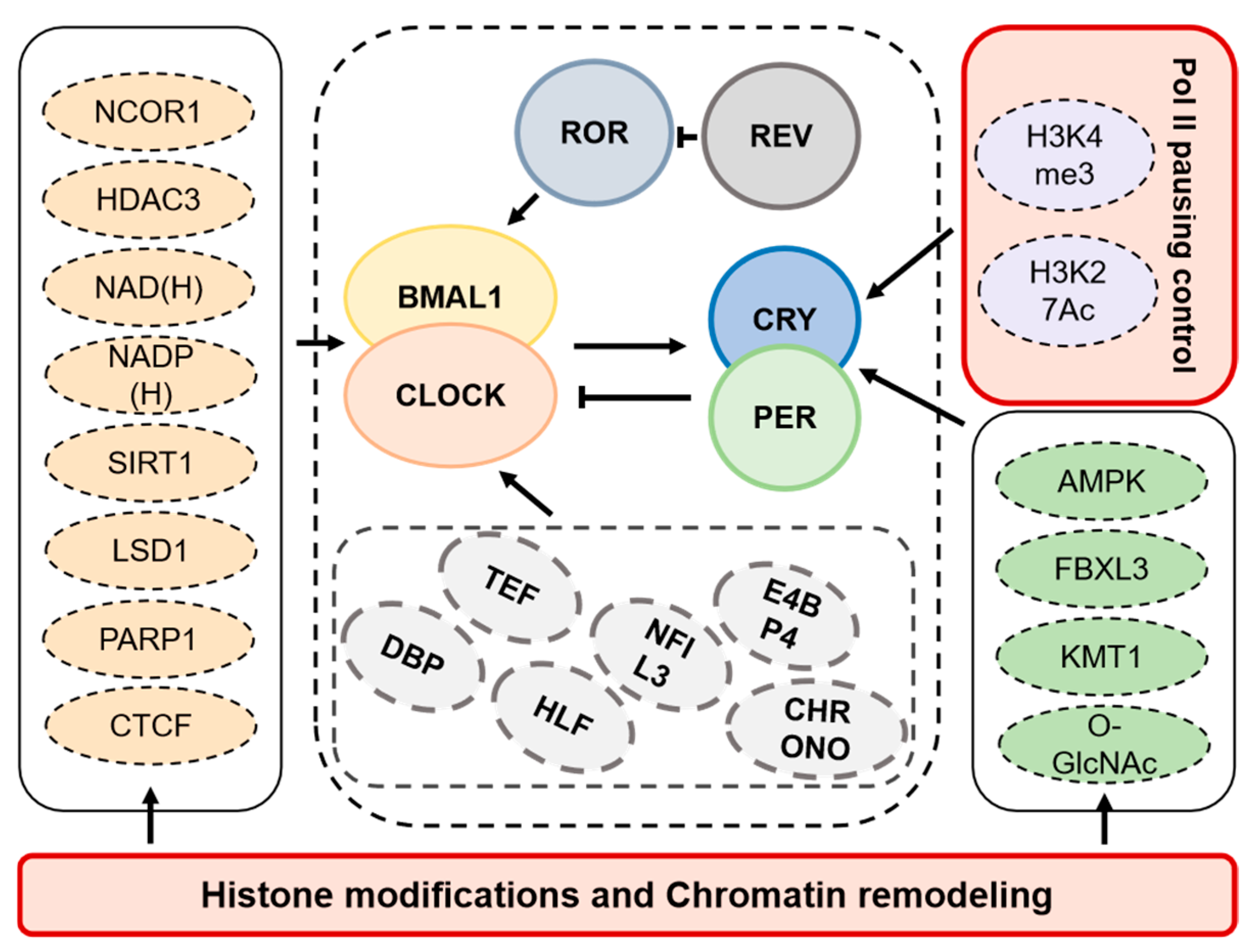
| Protein | Type | Role in Circadian Rhythm | Expression Locations | Conservation Across Species |
|---|---|---|---|---|
| BMAL1 | Transcription factor (bHLH) | Forms a complex with CLOCK to drive circadian gene expression | SCN, liver, heart, and peripheral tissues [9] | Highly conserved across eukaryotes |
| CLOCK | Transcription factor (bHLH) | Forms a complex with BMAL1 to activate circadian genes | SCN, liver, brain, and peripheral tissues [12] | Highly conserved across eukaryotes |
| PER | Transcription factor (bHLH) | Represses CLOCK–BMAL1 activity in the feedback loop | SCN, liver, and various peripheral tissues [13] | Highly conserved from flies to mammals |
| CRY | Photoreceptor/Transcription factor | Binds PER to mediate degradation and regulate the feedback loop | SCN, retina, and peripheral tissues [14] | Highly conserved from flies to mammals |
| Method of Clock Disturbance | Effects | Type of Diseases | Data Sources | Impact | Refs |
|---|---|---|---|---|---|
| Environmental changes | Abnormal cell cycles, intracellular inflammation, activation of carcinogenic pathways | Multiple cancer | Human data | Whole-body | [24,25,26] |
| Irregular light exposure | Increased cancer cell proliferation and metastasis | BC, melanoma | Human data and mouse models | Whole-body and cell-specific | [27,28,29] |
| Shift work | Increased risk of breast cancer, particularly with certain genotypes | BC | Human data and mouse models | Whole-body | [27,28,29] |
| Chronic jet lag | Promoted tumor growth by regulating immune responses and cell cycle regulatory factors | Melanoma | Mouse models | Cell-specific | [30] |
| Bmal1 deficiency | Weight gain, increased adipose tissue weight, decreased glucose tolerance, reduced insulin secretion | Metabolic disorders, diabetes | Mouse models | Whole-body and cell-specific | [31,32] |
| Per2 and CRY deficiency | Alterations in lipid metabolism and gluconeogenesis | Metabolic disorders | Mouse models | Whole-body and cell-specific | [33,34] |
| Sleep disruption | Physiological and psychological stress, impaired gut microbiota, inflammation | Metabolic diseases | Mouse models | Whole-body | [35,36,37] |
| Circadian-related gene alterations | Potential risk genes for AD, altered rhythmic expression of specific genes | AD | Human data and mouse models | Whole-body and cell-specific | [38,39] |
| Clock gene polymorphisms | Associated with the risk of PD | PD | Human data | Whole-body | [40] |
| Circadian rhythm disruption | Increased frequency of napping, atypical circadian rhythms of melatonin release | AD | Human data | Whole-body | [39] |
| Circadian rhythm disruption | Significant disruptions in sleep rhythms, altered gene methylation levels | ASD | Human data and mouse models | Whole-body and cell-specific | [41,42,43,44,45,46,47] |
| Circadian rhythm gene mutations | Specific gene mutations associated with ASD | ASD | Human data | Whole-body | [46,47] |
| Intrinsic circadian clock disruption in chondrocytes | Increased MMP13 transcription, altered matrix composition | OA | Human data and mouse models | Whole-body and cell-specific | [48,49,50] |
| Cry and Per gene disruption in osteoblasts/knockout | Increased bone mass/Influenced bone formation rates and structure | Osteoporosis | Mouse models | Whole-body | [51,52,53] |
| BMAL1 knockout/deficiency | Elevated RANKL expression, increased bone resorption, impaired bone density/impaired bone density and marrow differentiation, maintained bone integrity | Osteoporosis | Mouse models | Whole-body | [54,55,56] |
| BMAL1 overexpression | Reduced bone loss by modulating NFATc1 signaling | Osteoporosis | Mouse models | Whole-body | [57] |
| Clock gene mutations | Lower mean arterial pressure, impaired sympathoadrenal responses to stress | CVD | Human data and mouse models | Whole-body | [58,59] |
| Cardiomyocyte-specific BMAL1 knockout | Impaired glucose utilization, accelerated dilated cardiomyopathy, reduced longevity | CVD | Mouse models | Whole-body | [60] |
| Light/dark cycle manipulations | Profound impact on cardiovascular and immune functions | CVD | Mouse models | Whole-body | [61] |
| Circadian period mutations | Disrupted heart rate rhythms, reduced heart rate | CVD | Human data and mouse models | Whole-body and cell-specific | [62] |
| SCN lesions | Mirrored human data on circadian misalignment effects | CVD | Human data and mouse models | Whole-body and cell-specific | [63] |
| Transcription Factors/Regulatory Genes | Description | Organisms Studied |
|---|---|---|
| CLOCK and BMAL1 | Form a heterodimer that binds to E-box elements, promoting expression of downstream genes like Per1, Per2, CRY1, CRY2, and Rev-Erba. | Mammals |
| PERs and CRYs | Form heterodimers and inhibit BMAL1 transcriptional activity. | Mammals |
| Rev-Erba | Inhibits the transcription of Bmal1. | Mammals |
| RORa | Promotes the transcription of Bmal1. | Mammals |
| DBP, TEF, HLF | Acidic amino acid and basic leucine zipper transcription factors that recognize D-box sequences on promoters and enhancers. | Mammals |
| NFIL3/E4BP4 | Recognizes D-box sequences and functions as a transcriptional repressor. | Mammals |
| CHRONO | Inhibits Bmal1 target genes by interacting with BMAL1–CLOCK and PER proteins. | Mammals |
| HMTs | Catalyze the methylation of histones, associated with transcriptional activation or repression. | Mammals |
| Histone demethylases | Remove methyl groups from histones, affecting transcriptional regulation. | Mammals |
| H3K4me3, H3K4me1 | Marks associated with active promoters and enhancers, respectively. | Mammals |
| H3K27me3 | A repressive mark catalyzed by PRC2, associated with transcriptional repression. | Mammals |
| H3K9me2/3 | Associated with the repression of the chromatin state. | Mammals |
| HATs | Catalyze the acetylation of histones, promoting transcription and chromatin accessibility. | Mammals |
| HDACs | Removal of acetyl groups from histones leads to chromatin condensation and transcriptional repression. | Mammals |
| SIRT1 | Deacetylates PER2 and BMAL1, influencing circadian rhythm. | Mammals |
| AMPK | Phosphorylates CRY1 and PER2, affecting their stability and activity. | Mammals |
| Pol II | Involved in transcription initiation, pausing, and elongation. | Mammals |
| SWI/SNF, CHD family | ATP-dependent chromatin remodeling enzymes are essential for circadian gene expression. | Mammals, Neurospora crassa, fruit flies |
| CTCF | Mediates chromatin interactions and regulation. | Mammals |
| PARP1 | Mediates circadian regulation of chromatin by mobilizing clock-controlled genes to LADs. | Mammals |
| LncRNAs | Involved in regulating the interaction of distal enhancers to promote circadian gene expression. | Mammals |
| eRNAs | Enhancer RNAs involved in circadian regulation. | Mammals |
| Nuclear PER complex | Guides H3K9 methyltransferase to promote deacetylation and recruitment of Per1 and Per2 promoters. | Mammals |
| E3 ubiquitin ligase (FBXL3) | Mediates degradation of CRY1, influencing circadian loop activation. | Mammals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, W.; Ge, X.; Chen, Q.; Li, J.-D. Epigenetic Mechanisms in the Transcriptional Regulation of Circadian Rhythm in Mammals. Biology 2025, 14, 42. https://doi.org/10.3390/biology14010042
Mao W, Ge X, Chen Q, Li J-D. Epigenetic Mechanisms in the Transcriptional Regulation of Circadian Rhythm in Mammals. Biology. 2025; 14(1):42. https://doi.org/10.3390/biology14010042
Chicago/Turabian StyleMao, Wei, Xingnan Ge, Qianping Chen, and Jia-Da Li. 2025. "Epigenetic Mechanisms in the Transcriptional Regulation of Circadian Rhythm in Mammals" Biology 14, no. 1: 42. https://doi.org/10.3390/biology14010042
APA StyleMao, W., Ge, X., Chen, Q., & Li, J.-D. (2025). Epigenetic Mechanisms in the Transcriptional Regulation of Circadian Rhythm in Mammals. Biology, 14(1), 42. https://doi.org/10.3390/biology14010042






