Research Progress on Natural Products That Regulate miRNAs in the Treatment of Osteosarcoma
Simple Summary
Abstract
1. Introduction
2. The Role of miRNA Molecules in Tumor Biological Processes and Therapy
3. Interaction Between Natural Products and miRNAs in Osteosarcoma Treatment
4. Natural Products Mediate the Inhibition of Osteosarcoma Cell Proliferation, Differentiation, and Metastasis Through miRNA Regulation
| Natural Products | miRNAs | Cancer Type | Target | Effect | Cell Models | Reference |
|---|---|---|---|---|---|---|
| Pomegranate-derived peptide PG2 | miR-339-5p | Leukemia | CDK2/miR-339-5p/caspase-3 | Induce cell apoptosis | NB4 MOLT-4 | [52] |
| Baicalein | miR-7 | Gastric cancer | miR-7/FAK/AKT | Mediate cell proliferation, metastasis, and angiogenesis | HGC-27 SGC-7901 MGC-803 BGC-823 | [53] |
| Elemene | miRNA-145-5p | Non-small cell lung cancer | miR-145-5p/MAP3K3/NF-κB | Suppress tumor growth | A549 H460 H322 H1299 293T | [54] |
| Schisandrin B | miR-708-5p | Osteosarcoma | PI3K/AKT | Inhibit cell viability and migration, induce cell apoptosis | SaOS2 U2OS | [55] |
| Rutin | miR-877-3p | Pancreatic cancer | Bcl-2 | Induce cell apoptosis | PANC-1 SW1990 MIA PaCa-2 | [56] |
| Homoharringtonine | miR-18a-3p | Breast cancer | miR-18a-3p/AKT/mTOR | Suppress cell growth and promote apoptosis | MDA-MB-231 MCF-7 T47D HCC1937 MCF-10A | [57] |
| Andrographolide | miR-21-5p | Breast cancer | NF-κB/miR-21-5p/PDCD4 | Suppress the growth and metastasis | MCF-7 | [58] |
| Maackiain | miR-374a | Triple-negative breast cancer | miR-374a/GADD45A | Promote cell proliferation, migration, and invasion | MDA-MB-231 BT549 FBS | [59] |
| Ursolic acid | miR-140-5p | Colorectal cancer | TGF-β3 | Inhibit proliferation and cell cycle and promote apoptosis | SCSP-5032 | [60] |
5. Natural Products Mediate miRNA to Promote Apoptosis in Osteosarcoma Cells
6. Natural Products Mediate miRNAs to Promote Ferroptosis in Osteosarcoma Cells
7. Natural Products That Modulate miRNAs Influence Autophagy in Osteosarcoma Cells
| miRNA | Expression | Expression Effect | Target Gene | Type of Cell Line | Reference |
|---|---|---|---|---|---|
| miR-375 | up | Suppress Cell Proliferation and Autophagy | ATG2B | hFOB1.19 U2OS MG63 | [115] |
| miR-19 | up | Promote Cell Proliferation, Invasion, Migration, and EMT | SPRED2 | hFOB MG-63 SOSP-9607 Saos-2 U2OS | [116] |
| miR 22 | up | Inhibit Autophagy and Induce Apoptosis | ATG5 beclin1 LC3 | MG-63, U2OS, Saos2 | [117] |
| miR-579-3p | down | Promote Autophagy | MSH6 | U-2OS MG63 | [118] |
| miR-495-3p | up | Induce Mitophagy and Apoptosis | Sphk1 | BEAS2B A549 H1299 | [119] |
| miR-29a-3p | up | Aggravate Apoptosis; Dampen Cell Proliferation, Colony Formation, Migration, and Invasion; and Promote Autophagy | IGF1 | 143B MG-63 HOS SJSA-1 hFOB 1.19 | [120] |
| miR-488-3p | down | Repress Malignant Behaviors and Facilitate Autophagy | Neurensin-2 | U2OS Saos2 OS 99-1 | [111] |
| miR-22 | up | Suppress Proliferation and Promote Sensitivity; Mediate Autophagy | MTDH | MG-63 | [121] |
| miR-193b | up | Induce Autophagy and Apoptosis | FEN1 | MG-63 U2OS 143B | [122] |
| miR-506-3p | up | Suppress Cell Invasion | SPHK1 | MG63 143B MNNG/HOS SaOS-2 U-2OS | [123] |
8. Natural Products Modulate miRNA to Regulate Drug Resistance in Tumor Cell
| Natural Products | Tumor Type | Corresponding Products | miRNAs | Dysregulation | Pathway/Target | Reference |
|---|---|---|---|---|---|---|
| DET | CC | 5-FU | miR-205/Bcl2 | Upregulated | miR-205/Bcl2 | [142] |
| Curcumol | TNBC | DOX | miR-181b-2-3p | Upregulated | miR-181b-2-3p-ABCC3 | [143] |
| Quercetin | CRC | 5-FU | miR-27a | Downregulated | miR-27a/Wnt/β-catenin | [144] |
| Terpenoids | CC | 5-FU | miR-495-3p | Upregulated | P-gp | [145] |
| Rutin | HCC | SFN | miRNA-590-5P | Upregulated | BANCR/miRNA-590-5P/OLR1 | [146] |
| Cur | CRC | DDP | miR-137 | Upregulated | GLS | [147] |
| AC | CC | 5-FU | miR-142-3p | Upregulated | ABCG2 | [148] |
| Tan IIA | CRC | OXA | miR-30b-5p | Upregulated | microRNA-30b-5p/AVEN | [149] |
| Cur | CRC | OXA | miR-409-3p | Upregulated | ERCC1 | [150] |
| Y6 | HCC | OXA | MiR-338-3p | Upregulated | MiR-338-3p/HIF-1α/TWIST | [151] |
| Cur | AML | DOX | miR-20a-5p | Downregulated | lncRNA HOTAIR/miR-20a-5p/WT1 | [152] |
| G-Rg3 | GC | DDP | miR-429 | Upregulated | SOX2 PI3K/AKT/mTOR | [153] |
9. The Potential of Natural Products to Exert Antitumor Effects by Targeting m6A Modifications
10. Discussion
11. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smrke, A.; Anderson, P.M.; Gulia, A.; Gennatas, S.; Huang, P.H.; Jones, R.L. Future Directions in the Treatment of Osteosarcoma. Cells 2021, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K. Challenges of Systemic Therapy Investigations for Bone Sarcomas. Int. J. Mol. Sci. 2022, 23, 3540. [Google Scholar] [CrossRef] [PubMed]
- Xiaobo, Z.; Xidan, G.; Jing, X.; Zhuoya, Z.; Tingtong, L.; Xueyan, Z.; Xin, K. The role of lncRNA and miRNA on the effects of occurrence and development of osteosarcoma. Int. Immunopharmacol. 2024, 144, 113726. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Righi, A.; Kido, A.; Honoki, K.; Tanaka, Y.; Fujii, H.; Mavrogenis, A.F.; Tanaka, Y.; Errani, C. Effect of adjuvant chemotherapy on periosteal osteosarcoma: A systematic review. Jpn. J. Clin. Oncol. 2022, 52, 896–904. [Google Scholar] [CrossRef]
- Timilsina, S.; Saad, M.A.; Lang, R.T.; Hasan, T.; Spring, B.Q. Methods for assessing and removing non-specific photoimmunotherapy damage in patient-derived tumor cell culture models. Photochem. Photobiol. 2024, 1–17. [Google Scholar] [CrossRef]
- Mukaida, N.; Nakamoto, Y. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 1839–1858. [Google Scholar] [CrossRef]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2020, 68, e28352. [Google Scholar] [CrossRef]
- Whelan, J.S.; Davis, L.E. Osteosarcoma, Chondrosarcoma, and Chordoma. J. Clin. Oncol. 2018, 36, 2. [Google Scholar] [CrossRef]
- Bortoletto, S.; Nunes-Souza, E.; Marchi, R.; Ruthes, M.O.; Okano, L.M.; Tofolo, M.V.; Centa, A.; Fonseca, A.S.; Rosolen, D.; Cavalli, L.R. MicroRNAs role in telomere length maintenance and telomerase activity in tumor cells. J. Mol. Med. 2024, 102, 1089–1100. [Google Scholar] [CrossRef]
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review). Int. J. Oncol. 2016, 49, 5–32. [Google Scholar] [CrossRef]
- Lopacinska-Jørgensen, J.; Oliveira, D.V.N.P.; Wayne Novotny, G.; Høgdall, C.K.; Høgdall, E.V. Integrated microRNA and mRNA signatures associated with overall survival in epithelial ovarian cancer. PLoS ONE 2021, 19, e0315859. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, M.; Li, S.; Geng, Z.; Jin, Y.; Liu, D. Natural Products Treat Colorectal Cancer by Regulating miRNA. Pharmaceuticals 2023, 16, 1122. [Google Scholar] [CrossRef] [PubMed]
- Otoukesh, B.; Abbasi, M.; Gorgani, H.O.; Farahini, H.; Moghtadaei, M.; Boddouhi, B.; Kaghazian, P.; Hosseinzadeh, S.; Alaee, A. MicroRNAs signatures, bioinformatics analysis of miRNAs, miRNA mimics and antagonists, and miRNA therapeutics in osteosarcoma. Cancer Cell Int. 2020, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Meng, W.; Guo, Z.; Liu, M.; He, Y.; Li, Y.; Ma, Z. The miR-183 Cluster: Biogenesis, Functions, and Cell Communication via Exosomes in Cancer. Cells 2023, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Fridman, M.V.; Braga, E.A. Molecular Mechanisms and microRNAs in Osteosarcoma Pathogenesis. Biochemistry 2016, 81, 315–328. [Google Scholar] [CrossRef]
- Ma, H.; Pan, J.S.; Jin, L.X.; Wu, J.; Ren, Y.D.; Chen, P.; Xiao, C.; Han, J. MicroRNA-17~92 inhibits colorectal cancer progression by targeting angiogenesis. Cancer Lett. 2016, 376, 293–302. [Google Scholar] [CrossRef]
- Sun, X.; Lin, F.; Sun, W.; Zhu, W.; Fang, D.; Luo, L.; Li, S.; Zhang, W.; Jiang, L. Exosome-transmitted miRNA-335-5p promotes colorectal cancer invasion and metastasis by facilitating EMT via targeting RASA1. Mol. Ther. Nucleic Acids 2021, 24, 164–174. [Google Scholar] [CrossRef]
- Xu, S.J.; Hu, H.T.; Li, H.L.; Chang, S. The Role of miRNAs in Immune Cell Development, Immune Cell Activation, and Tumor Immunity: With a Focus on Macrophages and Natural Killer Cells. Cells 2019, 10, 1140. [Google Scholar] [CrossRef]
- Alcantara, K.M.M.; Garcia, R.L. MicroRNA-92a promotes cell proliferation, migration and survival by directly targeting the tumor suppressor gene NF2 in colorectal and lung cancer cells. Oncol. Rep. 2019, 41, 2103–2116. [Google Scholar] [CrossRef]
- Persson, R.; Hodges, M.; King, B.D.; Chen, A.; Zeh, K.; Levine, A.A. 1204 pharmacokinetics of miravirsen, a mir-122 inhibitor, predict the prolonged viral load reduction in treatment naive genotype 1 hcv infected patients. J. Hepatol. 2012, 56, S477. [Google Scholar] [CrossRef]
- Kamel, A.; Owen, T.; Cole, I.; Valencia, T.; Lee, E.C. Pharmacokinetics and Absorption, Distribution, Metabolism and Excretion of RGLS4326 in Mouse and Monkey, an Anti–miR-17 Oligonucleotide for the Treatment of Polycystic Kidney Disease. Drug Metab. Dispos. 2023, 51, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Bao, Y.; Tian, M.; Ren, Q.; Zhang, W. miR-29 family inhibited the proliferation and migration of lung cancer cells by targeting SREBP-1. Mol. Cell. Toxicol. 2021, 18, 165–175. [Google Scholar] [CrossRef]
- Meng, D.; Dong, Y.; Shang, Q.; Sun, Z. Anti-tumor effect and hepatotoxicity mechanisms of psoralen. Front. Pharmacol. 2024, 15, 142700. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Li, Y.; Sarkar, F.H. Regulating miRNA by natural agents as a new strategy for cancer treatment. Curr. Drug Targets 2013, 16, 1167–1174. [Google Scholar] [CrossRef]
- Xie, W.; Melzig, M.F. The Stability of Medicinal Plant microRNAs in the Herb Preparation Process. Molecules 2018, 23, 919. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Huang, H.-W.; Shu, C.-W.; Hou, M.-F.; Yuan, S.-S.F.; Wang, H.-R.; Chang, Y.-T.; Farooqi, A.A.; Tang, J.-Y.; Chang, H.-W. DNA methylation, histone acetylation and methylation of epigenetic modifications as a therapeutic approach for cancers. Cancer Lett. 2016, 373, 185–192. [Google Scholar] [CrossRef]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Shi, Q.; Shen, L.; Gan, J.; He, L.; Lin, J.; Guo, S.; Xiong, Z.; Lin, J.; Zhang, S. Integrative analysis identifies DNMTs against immune-infiltrating neutrophils and dendritic cells in colorectal cancer. Epigenetics 2019, 14, 392–404. [Google Scholar] [CrossRef]
- Li, S.G.; Shi, Q.W.; Yuan, L.Y.; Qin, L.P.; Wang, Y.; Miao, Y.Q.; Chen, Z.; Ling, C.Q.; Qin, W.X. C-Myc-dependent repression of two oncogenic miRNA clusters contributes to triptolide-induced cell death in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 51. [Google Scholar] [CrossRef]
- Sun, A.Q.; Ju, X.L. Advances in Research on Anticancer Properties of Salidroside. Chin. J. Integr. Med. 2021, 27, 153–160. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, S.; Chen, B.; Du, C.; Xiao, P.; Luo, X.; Wei, L.; Lei, Y.; Zhao, C.; Huang, W. Psoralidin inhibits osteosarcoma growth and metastasis by downregulating ITGB1 expression via the FAK and PI3K/Akt signaling pathways. Chin. Med. 2023, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gao, X.; Zou, L.; Lei, M.; Feng, J.; Hu, Z. Bavachin Induces Ferroptosis through the STAT3/P53/SLC7A11 Axis in Osteosarcoma Cells. Oxidative Med. Cell. Longev. 2021, 2021, 1783485. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chang, J.; Ruan, H.; Zhi, W.; Wang, X.; Zhao, F.; Ma, X.; Sun, X.; Liang, Q.; Xu, H.; et al. Cantharidin inhibits osteosarcoma proliferation and metastasis by directly targeting miR-214-3p/DKK3 axis to inactivate β-catenin nuclear translocation and LEF1 translation. Int. J. Biol. Sci. 2021, 17, 2504–2522. [Google Scholar] [CrossRef]
- Lin, H.; Hao, Y.; Wan, X.; He, J.; Tong, Y. Baicalein inhibits cell development, metastasis and EMT and induces apoptosis by regulating ERK signaling pathway in osteosarcoma. J. Recept. Signal Transduct. 2020, 40, 49–57. [Google Scholar] [CrossRef]
- Yingang, L.; Xizhuang, B. Naringenin induces ferroptosis in osteosarcoma cells through the STAT3-MGST2 signaling pathway. J. Bone Oncol. 2024, 50, 100657. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Z.; Ni, X.; Lv, C. Piperlongumine induces apoptosis and G2/M phase arrest in human osteosarcoma cells by regulating ROS/PI3K/Akt pathway. Toxicol. In Vitr. 2020, 65, 104775. [Google Scholar] [CrossRef]
- Shen, M.; Yu, H.; Jin, Y.; Mo, J.; Sui, J.; Qian, X.; Chen, T. Metformin Facilitates Osteoblastic Differentiation and M2 Macrophage Polarization by PI3K/AKT/mTOR Pathway in Human Umbilical Cord Mesenchymal Stem Cells. Stem Cells Int. 2022, 2022, 9498876. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, C.; Yang, P.; Li, C.; Li, M. Eldecalcitol induces apoptosis and autophagy in human osteosarcoma MG-63 cells by accumulating ROS to suppress the PI3K/Akt/mTOR signaling pathway. Cell. Signal. 2020, 73, 109841. [Google Scholar] [CrossRef]
- Chang, R.M.; Xiao, S.; Lei, X.; Yang, H.; Fang, F.; Yang, L.Y. miRNA-487a Promotes Proliferation and Metastasis in Hepatocellular Carcinoma. Clin. Cancer Res. 2017, 23, 2593–2604. [Google Scholar] [CrossRef]
- Xie, F.; Yuan, Y.; Xie, L.; Ran, P.; Xiang, X.; Huang, Q.; Qi, G.; Guo, X.; Xiao, C.; Zheng, S. miRNA-320a inhibits tumor proliferation and invasion by targeting c-Myc in human hepatocellular carcinoma. OncoTargets Ther. 2017, 10, 885–894. [Google Scholar] [CrossRef]
- Lin, C.-P.; Liu, C.-R.; Lee, C.-N.; Chan, T.-S.; Liu, H.E. Targeting c-Myc as a novel approach for hepatocellular carcinoma. World J. Hepatol. 2010, 2, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Navarro, F.; Maher, C.A.; Maliszewski, L.E.; Yan, N.; O’Day, E.; Chowdhury, D.; Dykxhoorn, D.M.; Tsai, P.; Hofmann, O.; et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3’UTR microRNA recognition elements. Mol. Cell 2009, 35, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-M.; Lu, H. Autoregulatory suppression of c-Myc by miR-185-3p. J. Biol. Chem. 2011, 286, 33901–33909. [Google Scholar] [CrossRef] [PubMed]
- Jarboe, T.; DeSouza, N.; Singh, S.; Moscatello, A.; Geliebter, J.; Tiwari, R.K.; Li, X.-M. Abstract 317: Berberine-mediated reprogramming of the inflammatory environment in anaplastic thyroid cancer. Cancer Res. 2021, 81, 317. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, W.; Li, F.; Yu, D.; Xu, C.; Hu, H. Triptolide Inhibits Breast Cancer Cell Metastasis Through Inducing the Expression of miR-146a, a Negative Regulator of Rho GTPase. Oncol. Res. 2019, 27, 1043–1050. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted miR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641. [Google Scholar] [CrossRef]
- Örenlili Yaylagül, E.; Ülger, C. The effect of baicalein on Wnt/β-catenin pathway and miR-25 expression in Saos-2 osteosarcoma cell line. Turk. J. Med. Sci. 2020, 50, 1168–1179. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Zhou, Y.B.; Xiang, Y.X.; Wang, L.S.; Hu, W.K.; Wang, W.J. Baicalein inhibits osteosarcoma cell proliferation and invasion through the miR-183/Ezrin pathway. Mol. Med. Rep. 2018, 18, 1104–1112. [Google Scholar] [CrossRef]
- Yang, S.F.; Lee, W.J.; Tan, P.; Tang, C.H.; Hsiao, M.; Hsieh, F.K.; Chien, M.H. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget 2015, 6, 2736–2753. [Google Scholar] [CrossRef]
- Bertozzi, D.; Marinello, J.; Manzo, S.G.; Fornari, F.; Gramantieri, L.; Capranico, G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1α activity by changing miR expression patterns in human cancer cells. Mol. Cancer Ther. 2014, 13, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kantorn, C.; Chosita, N.; Mashima, N.; Wasinee, K.; Sittiruk, R.; Dalina, T. Anticancer effects of pomegranate-derived peptide PG2 on CDK2 and miRNA-339-5p-mediated apoptosis via extracellular vesicles in acute leukemia. Sci. Rep. 2024, 14, 27367. [Google Scholar] [CrossRef]
- Qiao, D.; Xing, J.; Duan, Y.; Wang, S.; Yao, G.; Zhang, S.; Jin, J.; Lin, Z.; Chen, L.; Piao, Y. The molecular mechanism of baicalein repressing progression of gastric cancer mediating miR-7/FAK/AKT signaling pathway. Phytomedicine 2022, 100, 154046. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, J.; Peng, Y.; Tian, X.; Zhang, W.; Chen, J.; Wang, Y.; Wang, Y.; Yang, Y.; Zhang, Y.; et al. Elemene as a binding stabilizer of microRNA-145-5p suppresses the growth of non-small cell lung cancer. J. Pharm. Anal. 2024, 101118, in press. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Tong, X.; Zhang, Y. Schisandrin B Inhibits Cell Viability and Migration, and Induces Cell Apoptosis by circ_0009112/miR-708-5p Axis Through PI3K/AKT Pathway in Osteosarcoma. Front. Genet. 2020, 11, 588670. [Google Scholar] [CrossRef]
- Huo, M.; Xia, A.; Cheng, W.; Zhou, M.; Wang, J.; Shi, T.; Cai, C.; Jin, W.; Zhou, M.; Liao, Y.; et al. Rutin Promotes Pancreatic Cancer Cell Apoptosis by Upregulating miRNA-877-3p Expression. Molecules 2022, 27, 2293. [Google Scholar] [CrossRef]
- Wang, L.B.; Wang, D.N.; Wu, L.G.; Cao, J.; Tian, J.H.; Liu, R.; Ma, R.; Yu, J.J.; Wang, J.; Huang, Q.; et al. Homoharringtonine inhibited breast cancer cells growth via miR-18a-3p/AKT/mTOR signaling pathway. Int. J. Biol. Sci. 2021, 17, 995–1009. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; He, Z.; Chen, M.; Ding, Y.; Yao, Y.; Duan, Y.; Zixuan, L.; Qi, C.; Zheng, L.; et al. Andrographolide suppresses the growth and metastasis of luminal-like breast cancer by inhibiting the NF-κB/miR-21-5p/PDCD4 signaling pathway. Front. Cell Dev. Biol. 2021, 9, 643525. [Google Scholar] [CrossRef]
- Peng, F.; Wang, L.; Xiong, L.; Tang, H.; Du, J.; Peng, C. Maackiain Modulates miR-374a/GADD45A Axis to Inhibit Triple-Negative Breast Cancer Initiation and Progression. Front. Pharmacol. 2022, 13, 806869. [Google Scholar] [CrossRef]
- Zhang, T.; Xiang, F.; Li, X.; Chen, Z.; Wang, J.; Guo, J.; Zhu, S.; Zhou, J.; Kang, X.; Wu, R. Mechanistic study on ursolic acid inhibiting the growth of colorectal cancer cells through the downregulation of TGF-β3 by miR-140-5p. J. Biochem. Mol. Toxicol. 2024, 38, e23581. [Google Scholar] [CrossRef]
- Fulda, S. Mechanisms of Deregulation of Apoptosis in Myeloma. Blood 2019, 134, SCI-10. [Google Scholar] [CrossRef]
- Cai, X.; Yin, W.; Tang, C.; Lu, Y.; He, Y. Molecular mechanism of microRNAs regulating apoptosis in osteosarcoma. Mol. Biol. Rep. 2022, 49, 6945–6956. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Kim, G.S. Differences of Key Proteins between Apoptosis and Necroptosis. BioMed Res. Int. 2021, 2021, 3420168. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.; Mirzaie-asl, A.; Saidijam, M.; Moradi, M. Methanolic extract of Artemisia absinthium prompts apoptosis, enhancing expression of Bax/Bcl-2 ratio, cell cycle arrest, caspase-3 activation and mitochondrial membrane potential destruction in human colorectal cancer HCT-116 cells. Mol. Biol. Rep. 2020, 47, 8831–8840. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, T.; Yang, L.; Liu, T.; Song, Z.; Liu, S.; Zhang, D.; Tang, C. Polysaccharide from Cordyceps cicadae inhibit mitochondrial apoptosis to ameliorate drug-induced kidney injury via Bax/Bcl-2/Caspase-3 pathway. J. Funct. Foods 2022, 97, 105244. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, G.; Yan, Z.; Wang, L.; Wang, D. Hydrogen gas promotes apoptosis of lung adenocarcinoma A549 cells through X-linked inhibitor of apoptosis and baculoviral inhibitor of apoptosis protein repeat-containing 3. J. Cancer Res. Ther. 2022, 18, 1380–1386. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.; Pan, W.; Chen, F. miR-139-mediated NOTCH1 regulation is crucial for the inhibition of osteosarcoma progression caused by resveratrol. Life Sci. 2019, 242, 117215. [Google Scholar] [CrossRef]
- Jia, S.; He, D.; Liang, X.; Cheng, P.; Liu, J.; Chen, M.; Wang, C.; Zhang, H.; Meng, C. Corilagin induces apoptosis and inhibits autophagy of HL-60 cells by regulating miR-451/HMGB1 axis. Mol. Med. Rep. 2021, 25, 34. [Google Scholar] [CrossRef]
- Al Subeh, Z.Y.; Poschel, D.B.; Redd, P.S.; Klement, J.D.; Merting, A.D.; Yang, D.; Mehta, M.; Shi, H.; Colson, Y.L.; Oberlies, N.H.; et al. Lipid Nanoparticle Delivery of Fas Plasmid Restores Fas Expression to Suppress Melanoma Growth In Vivo. ACS Nano 2022, 16, 12695–12710. [Google Scholar] [CrossRef]
- Yuan, L.; Cai, Y.; Zhang, L.; Liu, S.; Li, P.; Li, X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: A Comprehensive Review. Front. Pharmacol. 2022, 12, 801662. [Google Scholar] [CrossRef]
- Hu, T.; Fei, Z.; Wei, N. Chemosensitive effects of Astragaloside IV in osteosarcoma cells via induction of apoptosis and regulation of caspase-dependent Fas/FasL signaling. Pharmacol. Rep. 2017, 69, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.R.M.; da Silva Gomes, P.R.; Romão, P.; Maluf, F.C.; Guimarães, V.R.; Candido, P.; Gonçalves, G.L.; de Camargo, J.A.; Dos Santos, G.A.; Silva, I.; et al. Enhancing RECK Expression Through miR-21 Inhibition: A Promising Strategy for Bladder Carcinoma Control. Biochem. Genet. 2024, 51, 101534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, Y.; Liu, J.-S.; Long, S.-Z.; Liu, H.-L.; Zhang, J.; Zhang, T. The role of miR-21/RECK in the inhibition of osteosarcoma by curcumin. Mol. Cell. Probes 2020, 51, 101534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Q.; Yu, L.; Zhu, D.; Li, Y.; Xue, Z.; Hua, Z.; Luo, X.; Song, Z.; Lu, C.; et al. The Role of miRNA in Tumor Immune Escape and miRNA-Based Therapeutic Strategies. Front. Immunol. 2022, 12, 807895. [Google Scholar] [CrossRef]
- Shi, C.; Cao, P.; Wang, Y.; Zhang, Q.; Zhang, D.; Wang, Y.; Wang, L.; Gong, Z. PANoptosis: A Cell Death Characterized by Pyroptosis, Apoptosis, and Necroptosis. J. Inflamm. Res. 2023, 16, 1523–1532. [Google Scholar] [CrossRef]
- Hongxin, T.; Ziqi, S.; Xiaohua, W.; Sicheng, S.; Jie, D.; Li, L.; Ziyan, F.; Danni, H.; Pu, C.; Xi, C.; et al. Endoplasmic reticulum-targeted biomimetic nanoparticles induce apoptosis and ferroptosis by regulating endoplasmic reticulum function in colon cancer. J. Control. Release 2024, 375, 422–437. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Li, H.; Ma, H.; Xu, F.; Qu, B. Effects of lead exposure on placental cellular apoptosis and endoplasmic reticulum stress in rats. Chin. Med. J. 2014, 127, 1744–1748. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, P.; Lu, L.; Yi, T.; Li, Y.; Mao, W.; Zhou, Q.; Lin, K. A study on expression of GRP78 and CHOP in neutrophil endoplasmic reticulum and their relationship with neutrophil apoptosis in the development of sepsis. J. Biosci. 2024, 49, 49. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Li, F.; Lv, X.; Fu, X.; Gu, H.; Liu, H.; Liu, J.; Dai, M.; Zhang, B. Efficacy of celastrol combined with cisplatin in enhancing the apoptosis of U-2OS osteosarcoma cells via the mitochondrial and endoplasmic reticulum pathways of apoptosis. Oncol. Lett. 2019, 17, 3305–3313. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Narula, A.; Liu, L.; Ahn, K.S. Molecular targets and anticancer potential of evodiamine. Phytochem. Lett. 2022, 52, 92–103. [Google Scholar] [CrossRef]
- Haga, S.; Kanno, A.; Morita, N.; Jin, S.; Matoba, K.; Ozawa, T.; Ozaki, M. Poly(ADP-ribose) Polymerase (PARP) is Critically Involved in Liver Ischemia/Reperfusion-injury. J. Surg. Res. 2021, 270, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Huang, C.; Tian, X.; Sun, W.; Jiang, S. Research Advances in Antitumor Mechanism of Evodiamine. J. Chem. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Ma, X.; Zhang, B.; Feng, H. Targeting ferroptosis in osteosarcoma. J. Bone Oncol. 2021, 30, 100380. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Al-Shibli, R.; AlSuleimani, M.; Ahmed, I.; Al Lawati, A.; Das, S. Association of MiRNA and Bone Tumors: Future Therapeutic Inroads. Curr. Med. Chem. 2024, 31, 1103–1120. [Google Scholar] [CrossRef]
- Elsakka, E.G.; Midan, H.M.; Abulsoud, A.I.; Fathi, D.; Abdelmaksoud, N.M.; Mageed, S.S.A.; Zaki, M.B.; Abd-Elmawla, M.A.; Rizk, N.I.; Elrebehy, M.A.; et al. Emerging insights: miRNA modulation of ferroptosis pathways in lung cancer. Exp. Cell Res. 2024, 442, 114272. [Google Scholar] [CrossRef]
- Jin, S.; Liu, P.-S.; Zheng, D.; Xie, X. The interplay of miRNAs and ferroptosis in diseases related to iron overload. Apoptosis 2023, 29, 45–65. [Google Scholar] [CrossRef]
- Mahmoudi-Lamouki, R.; Kadkhoda, S.; Hussen, B.M.; Ghafouri-Fard, S. Emerging role of miRNAs in the regulation of ferroptosis. Front. Mol. Biosci. 2023, 10, 1115996. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Liang, X.-L.; Xiong, G.-F.; Xing, X.-L.; Zhang, Q.-J.; Zhang, B.-R.; Liu, M.-W. Analysis and identification of oxidative stress-ferroptosis related biomarkers in ischemic stroke. Sci. Rep. 2024, 14, 3803. [Google Scholar] [CrossRef]
- Habaxi, K.; Wang, W.; Taximaimaiti, M.; Wang, L. Methylation Regulation of LPCAT3 Improves Osteoarthritis by Regulating ACSL4 to Inhibit Chondrocyte Ferroptosis. Crit. Rev. Eukaryot. Gene Expr. 2024, 34, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-M.; Li, F.-J.; Long, H.-Z.; Zhou, Z.-W.; Luo, H.-Y.; Xu, S.-G.; Gao, L.-C. Relationship between miRNA and ferroptosis in tumors. Front. Pharmacol. 2022, 13, 977062. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ge, J.; Song, X.; Li, F.; Sun-Waterhouse, D.; Li, D. Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ. Toxicol. 2021, 37, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Chuang, Y.-T.; Yen, C.-Y.; Chien, T.-M.; Chang, F.-R.; Tsai, Y.-H.; Wu, K.-C.; Tang, J.-Y.; Chang, H.-W. Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis. Int. J. Mol. Sci. 2024, 25, 6083. [Google Scholar] [CrossRef]
- Liu, M.; Xu, C.; Yang, H.; Jiang, Q.; Chen, G.; Wang, W.; Shao, T.; Deng, T.; Yuan, F.; Xie, P.; et al. Pro-oncogene FBI-1 inhibits the ferroptosis of prostate carcinoma PC-3 cells via the microRNA-324-3p/GPX4 axis. J. Cancer 2024, 15, 4097–4112. [Google Scholar] [CrossRef]
- Ma, X.; Xu, M.; Zhang, X.; Wang, X.; Su, K.; Xu, Z.; Wang, X.; Yang, Y. Gambogenic acid inhibits proliferation and ferroptosis by targeting the miR-1291/FOXA2 and AMPKα/SLC7A11/GPX4 axis in colorectal cancer. Cell Biol. Int. 2023, 47, 1813–1824. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Nan, J.; Wan, S.; Luo, J.; Li, X.; Chen, W. High glucose elevates intracellular calcium level and induces ferroptosis in glomerular endothelial cells through the miR-223-3p/ITPR3 pathway. Mol. Cell. Endocrinol. 2024, 594, 112384. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, Y.; Yang, N.; Liu, M.; Luo, Y.; Zhang, Y.; Zhou, J.; Liu, H.; Li, J. Oridonin-induced ferroptosis and apoptosis: A dual approach to suppress the growth of osteosarcoma cells. BMC Cancer 2024, 24, 198. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Benthani, F.A.; Wu, J.; Liang, D.; Bian, Z.-X.; Jiang, X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2019, 27, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Wagner, A.E.; Wolffram, S.; Rimbach, G. Effect of quercetin on inflammatory gene expression in mice liver in vivo—Role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol. Res. 2012, 65, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhong, Q.; Ma, R.; Ni, Z.; Thakur, K.; Zhang, J.; Wei, Z. Apigenin, a natural flavonoid, promotes autophagy and ferroptosis in human endometrial carcinoma Ishikawa cells in vitro and in vivo. Food Sci. Hum. Wellness 2023, 12, 2242–2251. [Google Scholar] [CrossRef]
- Wollert, T. Autophagy. Curr. Biol. 2019, 29, R671–R677. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and Autophagy-Related Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Tedesco, G.; Santarosa, M.; Maestro, R. Beyond self-eating: Emerging autophagy-independent functions for the autophagy molecules in cancer (Review). Int. J. Oncol. 2024, 64, 57. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, X.; Ji, T.; Qi, C.; Gao, X.; Wei, R. The emerging roles of miRNA-mediated autophagy in ovarian cancer. Cell Death Dis. 2024, 15, 314. [Google Scholar] [CrossRef]
- Shahverdi, M.; Hajiasgharzadeh, K.; Sorkhabi, A.D.; Jafarlou, M.; Shojaee, M.; Tabrizi, N.J.; Alizadeh, N.; Santarpia, M.; Brunetti, O.; Safarpour, H.; et al. The regulatory role of autophagy-related miRNAs in lung cancer drug resistance. Biomed. Pharmacother. 2022, 148, 112735. [Google Scholar] [CrossRef]
- Yun, C.; Zhang, J.; Morigele. miR-488-3p Represses Malignant Behaviors and Facilitates Autophagy of Osteosarcoma Cells by Targeting Neurensin-2. Curr. Pharm. Biotechnol. 2024, 25, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Gioti, K.; Papachristodoulou, A.; Benaki, D.; Aligiannis, N.; Skaltsounis, A.-L.; Mikros, E.; Tenta, R. Assessment of the Nutraceutical Effects of Oleuropein and the Cytotoxic Effects of Adriamycin, When Administered Alone and in Combination, in MG-63 Human Osteosarcoma Cells. Nutrients 2021, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhong, X.; Song, Y.; Zhong, W.; Wang, S.; Wang, J.; Huang, P.; Niu, Y.; Yang, W.; Ding, Z.; et al. Triptolide induces apoptosis and cytoprotective autophagy by ROS accumulation via directly targeting peroxiredoxin 2 in gastric cancer cells. Cancer Lett. 2024, 587, 216622. [Google Scholar] [CrossRef]
- Jamali, Z.; Taheri-Anganeh, M.; Shabaninejad, Z.; Keshavarzi, A.; Taghizadeh, H.; Razavi, Z.S.; Mottaghi, R.; Abolhassan, M.; Movahedpour, A.; Mirzaei, H. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. IUBMB Life 2020, 72, 1306–1321. [Google Scholar] [CrossRef]
- Gao, S.; Wang, K.; Wang, X. miR-375 targeting autophagy-related 2B (ATG2B) suppresses autophagy and tumorigenesis in cisplatin-resistant osteosarcoma cells. Neoplasma 2020, 67, 724–734. [Google Scholar] [CrossRef]
- Xie, C.; Liu, S.; Wu, B.; Zhao, Y.; Chen, B.; Guo, J.; Qiu, S.; Cao, Y.-M. miR-19 Promotes Cell Proliferation, Invasion, Migration, and EMT by Inhibiting SPRED2-mediated Autophagy in Osteosarcoma Cells. Cell Transplant. 2020, 29, 963689720962460. [Google Scholar] [CrossRef]
- Meng, C.Y.; Zhao, Z.Q.; Bai, R.; Zhao, W.; Wang, Y.X.; Sun, L.; Sun, C.; Feng, W.; Guo, S.B. MicroRNA-22 regulates autophagy and apoptosis in cisplatin resistance of osteosarcoma. Mol. Med. Rep. 2020, 22, 3911–3921. [Google Scholar] [CrossRef]
- Zhan, H.; Xiao, J.; Wang, P.; Mo, F.; Li, K.; Guo, F.; Yu, X.; Liu, X.; Zhang, B.; Dai, M.; et al. Exosomal CTCF Confers Cisplatin Resistance in Osteosarcoma by Promoting Autophagy via the IGF2-AS/miR-579-3p/MSH6 Axis. J. Oncol. 2022, 2022, 9390611. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Tabassum, G.; Dohare, R.; Syed, M.A. miR-495–3p regulates sphingolipid metabolic reprogramming to induce Sphk1/ceramide mediated mitophagy and apoptosis in NSCLC. Free Radic. Biol. Med. 2022, 189, 71–84. [Google Scholar] [CrossRef]
- Qi, S.; Xu, L.; Han, Y.; Chen, H.; Cheng, A. miR-29a-3p mitigates the development of osteosarcoma through modulating IGF1 mediated PI3k/Akt/FOXO3 pathway by activating autophagy. Cell Cycle 2022, 21, 1980–1995. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Z.-Q.; Guo, S.-B.; Yang, T.-Y.; Chang, Z.-Q.; Li, D.-H.; Zhao, W.; Wang, Y.-X.; Sun, C.; Wang, Y.; et al. Roles of microRNA-22 in Suppressing Proliferation and Promoting Sensitivity of Osteosarcoma Cells via Metadherin-mediated Autophagy. Orthop. Surg. 2019, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xiao, Y.; Ma, X.; He, W.; Kang, J.; Peng, Z.; Wang, L.; Li, Z. miR-193b Increases the Chemosensitivity of Osteosarcoma Cells by Promoting FEN1-Mediated Autophagy. OncoTargets Ther. 2019, 12, 10089–10098. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bao, F.; Teng, Y.; Li, Q.; Li, J. MicroRNA-506-3p initiates mesenchymal-to-epithelial transition and suppresses autophagy in osteosarcoma cells by directly targeting SPHK1. Biosci. Biotechnol. Biochem. 2019, 83, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K. Drug resistance mechanisms in cancers: Execution of pro-survival strategies. J. Biomed. Res. 2024, 38, 95–121. [Google Scholar] [CrossRef]
- Prudowsky, Z.D.; Yustein, J.T. Recent Insights into Therapy Resistance in Osteosarcoma. Cancers 2020, 13, 83. [Google Scholar] [CrossRef]
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Qujeq, D.; Hashemzadeh, M.S. Long noncoding RNAs as potential targets for overcoming chemoresistance in upper gastrointestinal cancers. Biomed. Pharmacother. 2024, 179, 117368. [Google Scholar] [CrossRef]
- Mandal, T.; Shukla, D.; Khan, M.M.A.; Ganesan, S.K.; Srivastava, A.K. The EXO1/Polη/Polι axis as a promising target for miR-3163-mediated attenuation of cancer stem-like cells in non-small cell lung carcinoma. Br. J. Cancer 2024, 131, 1668–1682. [Google Scholar] [CrossRef]
- Dong, Z.; Liao, Z.; He, Y.; Wu, C.; Meng, Z.; Qin, B.; Xu, G.; Li, Z.; Sun, T.; Wen, Y.; et al. Advances in the Biological Functions and Mechanisms of miRNAs in the Development of Osteosarcoma. Technol. Cancer Res. Treat. 2022, 21, 15330338221117386. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Zhu, W.; Ji, J. circ_0002060 Enhances Doxorubicin Resistance in Osteosarcoma by Regulating the miR-198/ABCB1 Axis. Cancer Biother. Radiopharm. 2023, 38, 585–595. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, Y.; Xie, J.; Pu, J.; Shen, Z.; Wang, A.; Li, T.; Wang, T.; Li, G.; Liu, Y.; et al. M7G modification of FTH1 and pri-miR-26a regulates ferroptosis and chemotherapy resistance in osteosarcoma. Oncogene 2023, 43, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Barathan, M.; Zulpa, A.K.; Vellasamy, K.M.; Ibrahim, Z.A.; Hoong, S.M.; Mariappan, V.; Venkatraman, G.; Vadivelu, J. Hyperforin-mediated anticancer mechanisms in MDA-MB-231 cell line: Insights into apoptotic mediator modulation and caspase activation. J. Taibah Univ. Sci. 2023, 17, 2237712. [Google Scholar] [CrossRef]
- Ganai, S.A.; Sheikh, F.A.; Baba, Z.A.; Mir, M.A.; Mantoo, M.A.; Yatoo, M.A. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 2021, 35, 3509–3532. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Qin, T.; Zhu, W.; Kan, X.; Li, L.; Wu, D. Luteolin attenuates the chemoresistance of osteosarcoma through inhibiting the PTN/β-catenin/MDR1 signaling axis by upregulating miR-384. J. Bone Oncol. 2022, 34, 100429. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating microRNA-217-KRAS Axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef]
- Chang, Y.; Zhao, Y.; Gu, W.; Cao, Y.; Wang, S.; Pang, J.; Shi, Y. Bufalin Inhibits the Differentiation and Proliferation of Cancer Stem Cells Derived from Primary Osteosarcoma Cells through Mir-148a. Cell. Physiol. Biochem. 2015, 36, 1186–1196. [Google Scholar] [CrossRef]
- Saldívar-González, F.I.; Aldas-Bulos, V.D.; Medina-Franco, J.L.; Plisson, F. Natural product drug discovery in the artificial intelligence era. Chem. Sci. 2021, 13, 1526–1546. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, S.-R.; Kim, H.; Wang, H.; Zhu, J.-J.; Yang, J.-K. Discovering novel Cathepsin L inhibitors from natural products using artificial intelligence. Comput. Struct. Biotechnol. J. 2024, 23, 2606–2614. [Google Scholar] [CrossRef]
- Qi, F.; Liu, Y.; Zhang, K.; Zhang, Y.; Xu, K.; Zhou, M.; Zhao, H.; Zhu, S.; Chen, J.; Li, P.; et al. Artificial Intelligence Uncovers Natural MMP Inhibitor Crocin as a Potential Treatment of Thoracic Aortic Aneurysm and Dissection. Front. Cardiovasc. Med. 2022, 9, 871486. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhang, K.; Pan, G.; Li, C.; Li, C.; Hu, X.; Yang, L.; Cui, H. Deoxyelephantopin Induces Apoptosis and Enhances Chemosensitivity of Colon Cancer via miR-205/Bcl2 Axis. Int. J. Mol. Sci. 2022, 23, 5051. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Fan, D.; Xu, Y.; Li, X.; Yuan, J.; Yang, Q.; Zhou, X.; Lu, J.; Zhang, C.; Han, J.; et al. Curcumol enhances the sensitivity of doxorubicin in triple-negative breast cancer via regulating the miR-181b-2-3p-ABCC3 axis. Biochem. Pharmacol. 2020, 174, 113795. [Google Scholar] [CrossRef] [PubMed]
- Terana, G.T.; Abd-Alhaseeb, M.M.; Omran, G.A.; Okda, T.M. Quercetin potentiates 5-fluorouracil effects in human colon cancer cells through targeting the Wnt/β-catenin signalling pathway: The role of miR-27a. Wspolczesna Onkol. Oncol. 2022, 26, 229–238. [Google Scholar] [CrossRef]
- Feng, M.; Fan, X.; Shi, J.; Shan, S.; Li, S.; He, S.; Ding, M.; Li, Z. Terpenoids from quinoa reverse drug resistance of colon cancer by upregulating miR-495-3p. J. Sci. Food Agric. 2024, 104, 8916–8927. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, G.; Hu, J.; Zhu, Y.; Lan, H.; Shen, X.; Lv, Y.; Huang, L. Rutin attenuates Sorafenib-induced Chemoresistance and Autophagy in Hepatocellular Carcinoma by regulating BANCR/miRNA-590-5P/OLR1 Axis. Int. J. Biol. Sci. 2021, 17, 3595–3607. [Google Scholar] [CrossRef]
- Fan, W.-H.; Wang, F.-C.; Jin, Z.; Zhu, L.; Zhang, J.-X. Curcumin Synergizes with Cisplatin to Inhibit Colon Cancer through Targeting the MicroRNA-137-Glutaminase Axis. Curr. Med. Sci. 2021, 42, 108–117. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Yadav, V.K.; Srivastava, P.; Wu, A.T.H.; Huynh, T.-T.; Wei, P.-L.; Huang, C.-Y.F.; Huang, T.-H. Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p. Biomolecules 2019, 9, 306. [Google Scholar] [CrossRef]
- Ge, T.; Zhang, Y. Tanshinone IIA reverses oxaliplatin resistance in colorectal cancer through microRNA-30b-5p/AVEN axis. Open Med. 2022, 17, 1228–1240. [Google Scholar] [CrossRef]
- Han, W.; Yin, H.; Ma, H.; Wang, Y.; Kong, D.; Fan, Z. Curcumin Regulates ERCC1 Expression and Enhances Oxaliplatin Sensitivity in Resistant Colorectal Cancer Cells through Its Effects on miR-409-3p. Evid.-Based Complement. Altern. Med. 2020, 2020, 8394574. [Google Scholar] [CrossRef]
- Huang, C.; Wang, S.-H.; Liu, T.-T.; Wu, M.-Y.; Cai, K.-N.; Xie, Z.-H.; Zhao, R.-Q.; Wen, Y. Epigallocatechin Gallate Derivative Y6 Reverses Oxaliplatin Resistance in Hepatocellular Carcinoma via Targeting the MiR-338-3p/HIF-1α/TWIST Axis to Inhibit EMT. Recent Pat. Anti-Cancer Drug Discov. 2024, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-M.; Li, M.; Luo, W.; Sun, H.-B. Curcumin attenuates Adriamycin-resistance of acute myeloid leukemia by inhibiting the lncRNA HOTAIR/miR-20a-5p/WT1 axis. Lab. Investig. 2021, 101, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, R.; Geng, L.; Yuan, J.; Fan, H. Ginsenoside Rg3 Alleviates Cisplatin Resistance of Gastric Cancer Cells Through Inhibiting SOX2 and the PI3K/Akt/mTOR Signaling Axis by Up-Regulating miR-429. Front. Genet. 2022, 13, 823182. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Zhang, J.-J.; Xu, Y.-P.; Shao, M.-M.; Wang, M.-C. Regulatory effects of natural products on N6-methyladenosine modification: A novel therapeutic strategy for cancer. Drug Discov. Today 2024, 29, 103875. [Google Scholar] [CrossRef]
- Yu, L.; Xu, H.; Xiong, H.; Yang, C.; Wu, Y.; Zhang, Q. The role of m5C RNA modification in cancer development and therapy. Heliyon 2024, 10, e38660. [Google Scholar] [CrossRef]
- Cai, M.; Li, X.; Luan, X.; Zhao, P.; Sun, Q. Exploring m6A methylation in skin Cancer: Insights into molecular mechanisms and treatment. Cell. Signal. 2024, 124, 111420. [Google Scholar] [CrossRef]
- Nguyen, T.K.H.; Kang, H. Reading m6A marks in mRNA: A potent mechanism of gene regulation in plants. J. Integr. Plant Biol. 2024, 66, 2586–2599. [Google Scholar] [CrossRef]
- Tang, Q.; Li, L.; Wang, Y.; Wu, P.; Hou, X.; Ouyang, J.; Fan, C.; Li, Z.; Wang, F.; Guo, C.; et al. RNA modifications in cancer. Br. J. Cancer 2023, 129, 204–221. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Fan, Z. Interactions between m6A modification and miRNAs in malignant tumors. Cell Death Dis. 2021, 12, 598. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Luo, G.; Chen, J.; Ren, Z. Regulation of Methylase METTL3 on Fat Deposition. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, T.; Zhang, X.; Miao, Y.; Tian, X.; Yu, K.; Xu, X.; Niu, Y.; Guo, S.; Zhang, C.; et al. The m6A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol. 2020, 38, 101801. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Wang, R.; Shi, X.; Xu, L.; Yilihamu, Y.; Sang, J. METTL14 promotes the migration and invasion of breast cancer cells by modulating N6-methyladenosine and hsa-miR-146a-5p expression. Oncol. Rep. 2020, 43, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, J.; Luo, X.; Nie, Z.; Lu, S.; Liu, H.; Liu, J. Interaction between m6A and ncRNAs and Its Association with Diseases. Cytogenet. Genome Res. 2022, 162, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, X.; Xu, Y.; Wu, S.; Li, Z.; Chen, R.; Huang, N.; Zhu, Z.; Xu, X. miR-145 suppresses colorectal cancer cell migration and invasion by targeting an ETS-related gene. Oncol. Rep. 2016, 36, 1917–1926. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Feng, G.; Gao, S.; Wang, Y.; Zhang, S.; Liu, Y.; Ye, L.; Li, Y.; Zhang, X. MicroRNA-145 Modulates N6-Methyladenosine Levels by Targeting the 3′-Untranslated mRNA Region of the N6-Methyladenosine Binding YTH Domain Family 2 Protein. J. Biol. Chem. 2017, 292, 3614–3623. [Google Scholar] [CrossRef]
- Li, J.; Wu, L.; Pei, M.; Zhang, Y. YTHDF2, a protein repressed by miR-145, regulates proliferation, apoptosis, and migration in ovarian cancer cells. J. Ovarian Res. 2020, 13, 111. [Google Scholar] [CrossRef]
- Deng, L.-J.; Deng, W.-Q.; Fan, S.-R.; Chen, M.-F.; Qi, M.; Lyu, W.-Y.; Qi, Q.; Tiwari, A.K.; Chen, J.-X.; Zhang, D.-M.; et al. m6A modification: Recent advances, anticancer targeted drug discovery and beyond. Mol. Cancer 2022, 21, 52. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Zhang, Y.; Huang, Q.; Feng, J.; Xing, H.; Fu, X.; Yan, X.; Zhang, Y.; Xu, Q.; et al. HA-DOPE-Modified Honokiol-Loaded Liposomes Targeted Therapy for Osteosarcoma. Int. J. Nanomed. 2022, 17, 5137–5151. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, I.; Rodríguez-Mañero, M.; Cebro-Márquez, M.; Vilar-Sánchez, M.E.; Serrano-Cruz, V.; Vidal-Abeijón, I.; Martínez-Monzonís, M.A.; Mazón-Ramos, P.; Pedreira, M.; González-Juanatey, J.R.; et al. Transforming Cardiotoxicity Detection in Cancer Therapies: The Promise of MicroRNAs as Precision Biomarkers. Int. J. Mol. Sci. 2024, 25, 11910. [Google Scholar] [CrossRef] [PubMed]
- Hackman, G.L.; Collins, M.; Lu, X.; Lodi, A.; DiGiovanni, J.; Tiziani, S. Predicting and Quantifying Antagonistic Effects of Natural Compounds Given with Chemotherapeutic Agents: Applications for High-Throughput Screening. Cancers 2020, 12, 3714. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Y.; Gao, Y.-R.; Bao, Y.-Q.; Zhao, J.; Liu, B.; Zhao, C.-W.; Zhang, Z.-Y. Is Ancient Medical Treatment an Option for Curating Osteosarcoma Combined with Chemotherapy? A Basic Analysis of Clinic Pharmacy. Comb. Chem. High Throughput Screen. 2024, 27, 2267–2277. [Google Scholar] [CrossRef]
- Kaplan, A. The nanocomposites designs of phytomolecules from medicinal and aromatic plants: Promising anticancer-antiviral applications. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 17. [Google Scholar] [CrossRef]
- Murtaza, G.; Ullah, N.; Mukhtar, F.; Nawazish, S.; Muneer, S. Mariam Phytotherapeutics: The Emerging Role of Intestinal and Hepatocellular Transporters in Drug Interactions with Botanical Supplements. Molecules 2017, 22, 1699. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Zhang, H. Evaluation of the toxicity of 5-fluorouracil on three digestive enzymes from the view of side effects. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117105. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Sicklick, J.K.; Kurzrock, R. At the right dose: Personalised (N-of-1) dosing for precision oncology. Eur. J. Cancer 2023, 194, 113359. [Google Scholar] [CrossRef]
- Gazola, A.A.; Lautert-Dutra, W.; Archangelo, L.F.; dos Reis, R.B.; Squire, J.A. Precision oncology platforms: Practical strategies for genomic database utilization in cancer treatment. Mol. Cytogenet. 2024, 17, 28. [Google Scholar] [CrossRef]
- Mei, Z.; Mou, Y.; Zhang, N.; Liu, X.; He, Z.; Gu, S. Emerging Mutual Regulatory Roles between m6A Modification and microRNAs. Int. J. Mol. Sci. 2023, 24, 773. [Google Scholar] [CrossRef]
- Rodriguez, G.F.; Cesaro, B.; Fatica, A. Multiple Roles of m6A RNA Modification in Translational Regulation in Cancer. Int. J. Mol. Sci. 2022, 23, 8971. [Google Scholar] [CrossRef] [PubMed]
- Dettweiler, M.; Marquez, L.; Bao, M.; Quave, C.L. Quantifying synergy in the bioassay-guided fractionation of natural product extracts. PLoS ONE 2020, 15, e0235723. [Google Scholar] [CrossRef] [PubMed]
- Erdagi, S.I.; Uyanik, C. Biological evaluation of bioavailable amphiphilic polymeric conjugate based-on natural products: Diosgenin and curcumin. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 73–84. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Sang, F.; Cao, W.; Qin, Z.; Zhang, P. Natural products targeting glycolytic signaling pathways-an updated review on anti-cancer therapy. Front. Pharmacol. 2022, 13, 1035882. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Ma, E.-S.; Wang, Z.-X.; Zhu, M.-Q.; Zhao, J. Immune evasion mechanisms and therapeutic strategies in gastric cancer. World J. Gastrointest. Oncol. 2022, 14, 216–229. [Google Scholar] [CrossRef]
- Lai, J.; Kong, W.; Fu, Q.; Jiang, Z.; Sun, B.; Ye, X.; Kong, J.; Wei, S.; Jiang, L. PSMD14 is a novel prognostic marker and therapeutic target in osteosarcoma. Diagn. Pathol. 2024, 19, 79. [Google Scholar] [CrossRef]
- Iida, M.; Hazama, S.; Tsunedomi, R.; Tanaka, H.; Takenouchi, H.; Kanekiyo, S.; Tokumitsu, Y.; Tomochika, S.; Tokuhisa, Y.; Sakamoto, K.; et al. Overexpression of miR-221 and miR-222 in the cancer stroma is associated with malignant potential in colorectal cancer. Oncol. Rep. 2018, 40, 1621–1631. [Google Scholar] [CrossRef]
- Alizamir, A.; Amini, M.A.; Karbasi, A.; Beyrami, M. MiR-4492, a New Potential MicroRNA for Cancer Diagnosis and Treatment: A Mini Review. Chonnam Med. J. 2024, 60, 21–26. [Google Scholar] [CrossRef]
- Li, F.; Xu, J.; Zhu, Y.; Sun, L.; Zhou, R. Analysis of Cells Proliferation and MicroRNAs Expression Profile in Human Chondrosarcoma SW1353 Cells Exposed to Iodine-125 Seeds Irradiation. Dose-Response 2020, 18, 1559325820920525. [Google Scholar] [CrossRef]
- Zhao, G.; Gu, W. Effects of miR-146a-5p on chondrocyte interleukin-1β-induced inflammation and apoptosis involving thioredoxin interacting protein regulation. J. Int. Med. Res. 2020, 48, 0300060520969550. [Google Scholar] [CrossRef]

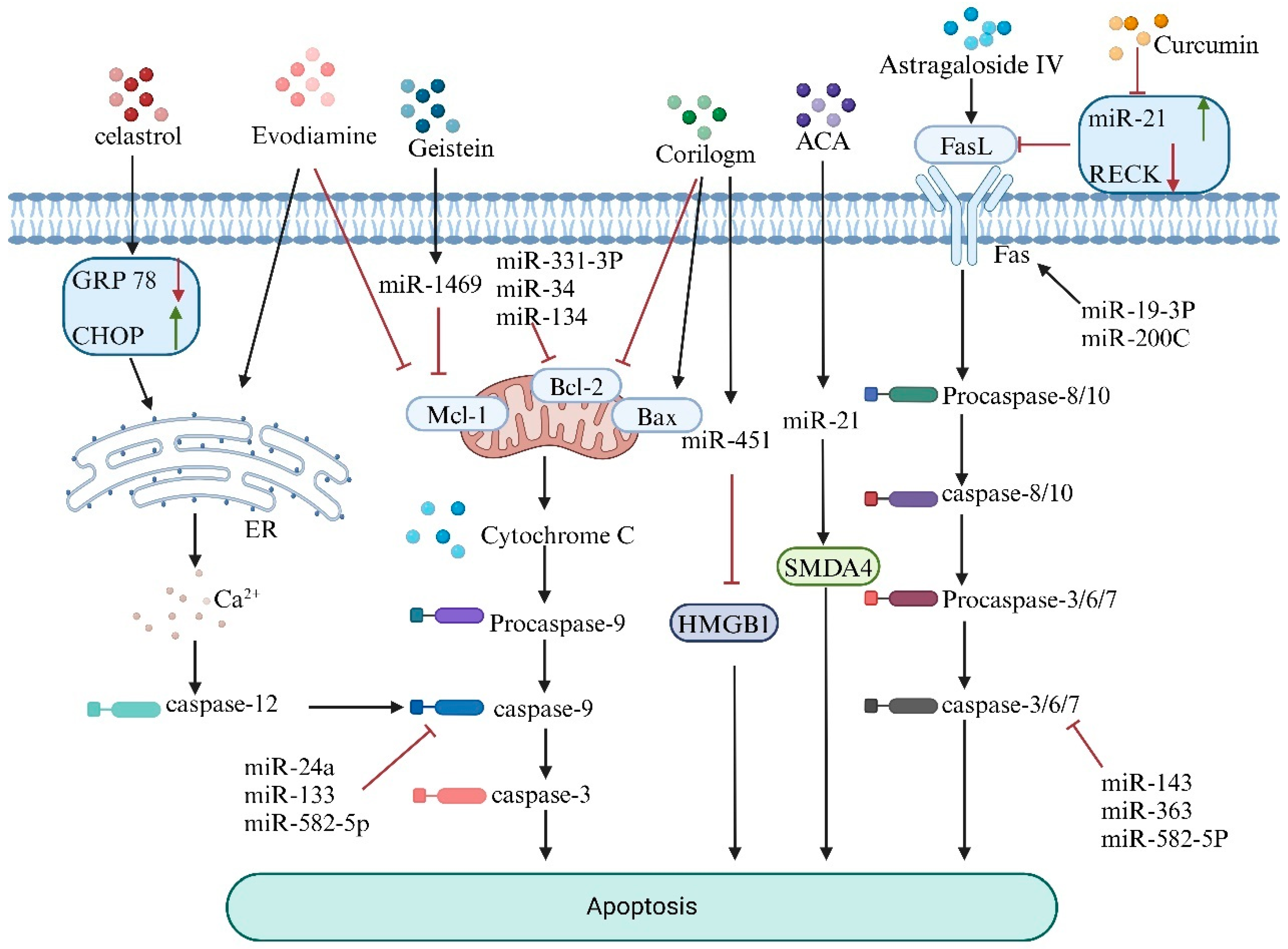

| Natural Product | Target Gene | Expression Effect | Type of Cell Line | Reference |
|---|---|---|---|---|
| Psoralidin | FAK PI3K/Akt | Inhibit Osteosarcoma Growth and Metastasis by Downregulating ITGB1 Expression | 143B MG63 | [31] |
| Bavachin | STAT3/P53/SLC7A11 | Induce Ferroptosis | MG63 HOS | [32] |
| Cantharidin | miR-214-3p/DKK3 | Inactivate β-catenin Nuclear Translocation and LEF1 Translation | U-2OS 143B Saos-2 MG-63 MNNG hFOB1.19 | [33] |
| Baicalein | ERK | Inhibit Cell Development, Metastasis, and EMT and Induce Apoptosis | MG-63 | [34] |
| Naringenin | STAT3-MGST2 | Induce Ferroptosis | HOS U2OS MG63 | [35] |
| Piperlongumine | ROS/PI3K/ Akt | Induce Apoptosis and G2/M Phase Arrest | MG63 U2OS | [36] |
| Metformin | PI3K/AKT/mTOR | Facilitate Osteoblastic Differentiation and M2 Macrophage Polarization | UC-MSC | [37] |
| Eldecalcitol | PI3K/Akt/mTOR | Accumulate ROS/Induce Apoptosis and Autophagy | MG-63 MC3T3-E1 MLO-Y4 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, X.; Lv, H.; Zhang, H.; Lin, R.; Xu, S.; Zhang, C.; Lou, S.; Qiu, Z.; Sun, C.; et al. Research Progress on Natural Products That Regulate miRNAs in the Treatment of Osteosarcoma. Biology 2025, 14, 61. https://doi.org/10.3390/biology14010061
Wang L, Liu X, Lv H, Zhang H, Lin R, Xu S, Zhang C, Lou S, Qiu Z, Sun C, et al. Research Progress on Natural Products That Regulate miRNAs in the Treatment of Osteosarcoma. Biology. 2025; 14(1):61. https://doi.org/10.3390/biology14010061
Chicago/Turabian StyleWang, Lin, Xinyu Liu, Haoze Lv, Han Zhang, Rimei Lin, Shan Xu, Chaojing Zhang, Shilei Lou, Zhidong Qiu, Cong Sun, and et al. 2025. "Research Progress on Natural Products That Regulate miRNAs in the Treatment of Osteosarcoma" Biology 14, no. 1: 61. https://doi.org/10.3390/biology14010061
APA StyleWang, L., Liu, X., Lv, H., Zhang, H., Lin, R., Xu, S., Zhang, C., Lou, S., Qiu, Z., Sun, C., & Cui, N. (2025). Research Progress on Natural Products That Regulate miRNAs in the Treatment of Osteosarcoma. Biology, 14(1), 61. https://doi.org/10.3390/biology14010061





